Graphical abstract
Iodide oxidation by Roseovarius sp. strain A-2 grown on Marine Agar 2216 medium supplemented with iodide, starch and Cu2+.
Abstract
Roseovarius sp. strain A-2 is an aerobic heterotrophic bacterium with a capacity for oxidizing iodide ion (I−) to form molecular iodine (I2). In this study, iodide-oxidizing enzyme of strain A-2 was characterized. The enzyme was an extracellular protein, and Cu2+ ion significantly enhanced the enzyme activity in the culture supernatant. When iodide was used as the substrate, the crude enzyme showed Km and Vmax values of 4.78 mM and 25.1 U mg−1, respectively. The enzyme was inhibited by NaN3, EDTA, KCN, and o-phenanthroline, and also had significant activities toward p-phenylenediamine and hydroquinone. Tandem mass spectrometric analysis of an active band excised from SDS–PAGE gel revealed that at least two proteins are involved in the enzyme. One of these proteins was closely related with IoxA, a multicopper oxidase previously found as a component of iodide-oxidizing enzyme of Alphaproteobacterium strain Q-1. Furthermore, a terrestrial bacterium Rhodanobacter denitrificans 116-2, which possesses an ioxA-like gene in its genome, was found to oxidize iodide. These results suggest that IoxA catalyzes the oxidation of iodide in phylogenetically distinct bacteria.
Iodine is one of halogens, and distributes widely in the environment mainly in the form of iodide (I−), iodate (IO3−), and organically bound iodine.Citation1–3) Japan is the world’s second largest iodine producer, since ancient underground seawater called “brine” often contains very high concentration of iodide. For example, brine water in Chiba prefecture contains approximately 1 mM iodide, which is 2000 times higher than that in natural seawater.Citation4) In the production of iodine, brine is first acidified by sulfuric acid, and then iodide is oxidized to molecular iodine (I2) by using chlorine gas.Citation5) Iodine has a number of applications such as X-ray contrast media, biocides (iodophors), industrial catalyst, feed additive, and pharmaceutical.
Iodide-oxidizing bacteria are aerobic heterotrophs capable of oxidizing iodide to I2, and isolated from brine water and seawater supplemented with exogenous iodide.Citation6–11) They are phylogenetically divided into two groups (groups A and B) within the class Alphaproteobacteria. Group A is included in the genus Roseovarius, while group B is distantly related to Rhodothalassium salexigens.Citation7) Although I2 is known as an effective microbiocidal agent, iodide-oxidizing bacteria have much higher I2 tolerance than the other bacteria in seawater.Citation8) Thus, they become predominant in iodide-enriched environments, and sometimes participate in microbial clogging of well pipes in iodine-producing facilities.Citation10,11)
Recently, Suzuki et al.Citation12) characterized an iodide-oxidizing enzyme (IOE)-II of a group B iodide-oxidizing bacterium strain Q-1. The enzyme was an extracellular oxidase, and Cu2+ ion significantly enhanced the enzyme production by strain Q-1. The enzyme did not oxidize other halogen ions tested except for iodide ion, but oxidized phenolic compounds such as p-phenylenediamine, hydroquinone, syringaldazine, 2,6-dimethoxy phenol, and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS). Comparison of several internal amino acid sequences of the purified IOE-II with a draft genome sequence of strain Q-1Citation13) revealed that at least two proteins, IoxA and IoxC, were involved in the enzyme. IoxA was a putative multicopper oxidase with four conserved copper-binding regions, but it was phylogenetically distinct from other known bacterial multicopper oxidases such as CueO, CumA, CopA, and CotA.Citation12) IoxC is not a multicopper oxidase and its function in iodide oxidation is still unclear.
Since IOEs can efficiently produce I2 from iodide, various industrial and medicinal applications, including a new generation system of disinfectant I2 as well as eco-friendly production of iodine from brine, are expected.Citation14) However, to date, IOE-II purified from strain Q-1 has only been characterized its biochemical properties. In this study, we characterized iodide-oxidizing enzyme of Roseovarius sp. strain A-2, a member of group A iodide-oxidizing bacteria, and found that proteins closely related to IoxA and IoxC are also involved in iodide oxidation by this strain.
Materials and methods
Bacterial strains and culture conditions
Twenty-one strains of group A iodide-oxidizing bacteria were previously isolated from brine water collected in Japan.Citation7) They were grown aerobically in Marine Broth 2216 medium (Becton Dickinson, Sparks, MD) supplemented with 40 μM CuCl2·2H2O at 30 °C with shaking at 120 rpm. Rhodanobacter denitrificans strains 2APBS1T (JCM 17641T) and 116-2 (JCM 17642)Citation15) were purchased from Japan Collection of Microorganisms (Tsukuba, Ibaraki, Japan). They were grown aerobically in R2A mediumCitation16) at 30 °C with shaking at 160 rpm. When iodide-oxidizing capacity of R. denitrificans was determined, the strains were grown on R2A solid medium supplemented with 40 μM Cu2+ ion and 1 g L−1 potassium iodide. In this case, the pH of the medium was adjusted to 5.0.
Sequencing and phylogenetic analysis of 16S rRNA gene
Genomic DNA of strain A-2 was isolated as described previously.Citation17) The 16S rRNA gene was amplified by PCR using the bacterial consensus primers 8F (5′-AGAGTTTGATCCTGGCTCAG-3′, Escherichia coli positions 8–27) and 1491R (5′-GGTTACCTTGTTACGACTT-3′, E. coli positions 1509–1491). PCR products were purified using a QIAquick PCR Purification kit (Qiagen, Hilden, Germany) and sequenced using a BigDye Terminator Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA) and an ABI Prism 3100 Genetic Analyzer (Applied Biosystems) using appropriate sequencing primers.Citation18) The obtained 16S rRNA gene sequences were subjected to a blast search (http://www.ncbi.nlm.nih.gov/BLAST/) to determine sequence similarity to phylogenetically related bacteria. The retrieved sequences were aligned using the Clustal X program, version 2.0. The phylogenetic tree was constructed using the neighbor-joining method.Citation19) Bootstrap values were obtained for 1000 replicates to estimate the confidence of tree topologies.
Enzyme assays
In the presence of high concentration of iodide, I2 forms triiodide (I3−), which has a high extinction coefficient of 25.5 mM−1 cm−1 at 353 nm. In this study, IOE activity was determined by measuring I3− formation.Citation7,12) In most cases, strain A-2 was grown in Marine broth 2216 supplemented with 100 μM Cu2+ ion for 24 h for preparation of crude enzyme solution. In some cases, the strain was cultured for 5 days together with other 20 strains, and their IOE activities were determined by every 24 h. When Cu2+ dependency of IOE was determined, strain A-2 was cultured in the absence or presence of 10–400 μM Cu2+ ion. The culture was centrifuged at 10,000 × g at 4 °C for 10 min, and the supernatant was used as the crude enzyme solution. The reaction mixture (1.5 mL) contained 20 mM Tris-HCl buffer (pH 7.0), 10 mM potassium iodide, and crude enzyme solution (100 μL). The reaction was started by the addition of crude enzyme. After 20 min to 24 h of incubation at 30 °C, I3− formation was measured spectrophotometrically. One unit of enzyme activity was defined as the amount of enzyme required to form 1 μmol of I3− per minute. The detection limit of this method was 0.4 mU mL−1. Protein concentrations were determined by BCA protein assay (Thermo Scientific, Rockford, IL, USA) with bovine serum albumin as the standard.
All assays to determine the oxidation of various substrates were performed at 30 °C in 20 mM Tris-HCl buffer (pH 7.0). One unit was defined as the amount of enzyme that catalyzed the formation of 1 μmol of the appropriate product per minute in all cases. The molar absorption coefficients used for these substrates were as follows: p-phenylenediamine, ε487 = 14.7 mM−1 cm−1; hydroquinone, ε247 = 21.0 mM−1 cm−1; syringaldazine, ε525 = 65.0 mM−1 cm−1; ABTS, ε420 = 36.0 mM−1 cm−1; catechol, ε392 = 1.46 mM−1 cm−1; and 2,6-dimethoxy phenol, ε468 = 27.5 mM−1 cm−1. The Mn2+-oxidizing activity was determined with the addition of 20 mM MnCl2 in 20 mM Tris-HCl buffer (pH 7.0) and the redox dye Leucoberbelin blue as described previously.Citation20)
The effect of pH on the activity of IOE was examined with 20 mM sodium acetate buffer (pH 3.5–6.0), 20 mM potassium phosphate buffer (pH 6.0–8.0), 20 mM Tris-HCl buffer (pH 7.0–9.0), and 20 mM glycine-NaOH buffer (pH 9.0–10.0). The pH stability was determined by measuring the activity after 30 min treatment at various pHs at 4 °C. The temperature stability was determined by measuring the activity after 30 min treatment at various temperatures at pH 7.0.
Electrophoresis
The enzyme was visualized and identified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Culture broth after 24 h was centrifuged at 6000 × g for 20 min at 4 °C, and the supernatant was concentrated and desalted by ultrafiltration with an Amicon Ultra centrifugal filter (50 K; Millipore, Bedford, MA, USA). After the concentrated supernatant was boiled for denaturation with 2% SDS and 5% 2-mercaptoethanol for 1–3 min, electrophoresis was performed using 7% polyacrylamide gel in 25 mM Tris-glycine buffer (pH 8.3) containing 0.1% SDS by the method described by Laemmli.Citation21) In some cases, electrophoresis was performed without boiling but with SDS and 2-mercaptoethanol for activity staining (see below). CLEARLY Stained Protein Ladder (Takara Bio, Otsu, Japan) was used as standard marker proteins. Proteins were visualized by staining with Coomassie brilliant blue R-250. Activity staining was performed by incubating the gels with 100 mM potassium iodide and 1% soluble starch. The purple band corresponding to IOE was excised and used for liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis as described below.
LC–MS/MS analysis
Protein sequencing using mass spectrometry was carried out as described by Shevchenko et al.Citation22) The tryptic digest of IOE was directly analyzed by nanoscale high-performance liquid chromatography (Paradigm MS2; Michrom Bioresources, Auburn, CA, USA) with an L-column ODS (0.1 × 50 mm; Chemical Evaluation and Research Institute, Tokyo, Japan). LC was coupled to a tandem mass spectrometer (Q-Tof2; Waters Micromass, Manchester, UK) equipped with a nanoelectrospray ionization source. Positive ion tandem mass spectra were measured.
Amplification of putative ioxA gene
Putative ioxA gene sequences of strain Q-1 (AB693882), Roseovarius sp. 217 (EAQ25965), and R. denitrificans 116-2 (EIM00854) were aligned using the Clustal X program. Regions unique to ioxA gene were identified, and a set of PCR primers was designed: forward primer ioxAF (5′-ACSYTSTGGTAYCAYGAYCAY-3′) and reverse primer ioxAR (5′-ACSACRTTRTGRCARTGCAT-3′). These primers were used to amplify putative ioxA genes from strain A-2 as well as from other strains including A-4, E-4, Ka-4, and RB-2C. The amplification cycle consisted of an initial touchdown step in which the annealing temperature was lowered from 55 to 50 °C in intervals of 0.5 °C, and 19 additional cycles were performed at an annealing temperature of 50 °C. Denaturation was performed at 95 °C for 10 s; primer annealing was performed for 45 s, and the extension was performed at 72 °C for 75 s. A final extension at 72 °C for 7 min was also performed. TaKaRa Ex Taq (Takara Bio) was used as the DNA polymerase. The amplified product (approximately 1100 bp) was confirmed on 1% agarose gel by electrophoresis, followed by purification and sequencing as described above. The obtained gene sequences were translated to amino acid sequences, and phylogenetic analysis was carried out as described in the Sequencing and phylogenetic analysis of 16S rRNA gene section.
Nucleotide sequence accession number
The gene sequences identified in this study have been deposited in the DDBJ/EMBL/GenBank databases under accession numbers of LC010105 through LC010112.
Results
Iodide-oxidizing enzyme activity of group A strains
As shown in Table S1, 15 of 21 strains showed slight enzyme activity (less than 2.0 mU mL−1), despite all of these strains demonstrated a significant iodide-oxidizing phenotype (purple bacterial colony formation) on a solid medium containing iodide and starch.Citation7) On the other hand, strains A-2, A-4, and A-6, all of which were originally isolated from brine water in Chiba prefecture, showed relatively high enzyme activities of more than 50 mU mL−1. Among these, strain A-2 demonstrated the highest enzyme activity of 161 mU mL−1, and thus it was used in the following experiments.
Phylogenetic analysis of strain A-2
Since only 400-bp of 16S rRNA gene of strain A-2 has been sequenced so far, a nearly full length of the gene was sequenced in this study. blast and similarity analysis of the 16S rRNA gene sequence showed that strain A-2 was most similar to an iodide-oxidizing bacterium strain A-6Citation7) with the sequence similarity of 99.8%. The phylogenetic tree of the 16S rRNA gene sequence using approximately 1430 bases indicated that group A iodide-oxidizing bacteria, including strain A-2, are most closely related to Roseovarius mucosusCitation23) in the family Rhodobacteraceae (Fig. S1).
Cu2+ ion dependency of IOE
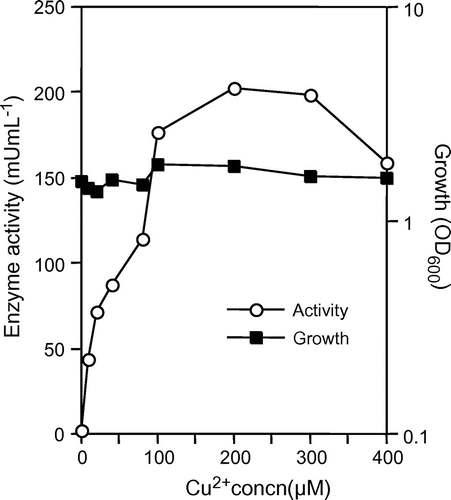
The IOE activity was observed in the crude enzyme solution of strain A-2 cultured in the presence of 0–400 μM Cu2+ ion. As shown in Fig. , The IOE activity was very low (2.5 mU mL−1) in the crude enzyme solution of strain A-2 cultured in the absence of Cu2+ ion, but was approximately 70–80 times higher in that in the presence of 100–300 μM Cu2+ ion. The activity in the crude enzyme solution of strain A-2 cultured in the presence of 400 μM Cu2+ ion was low rather than that in the presence of 300 μM Cu2+ ion. No other metal ions (100 μM each of Fe2+, Mn2+, Zn2+, Co2+, and Ni2+) enhanced the enzyme production.
Fig. 1. Enhancement of IOE activity of strain A-2 cultured in various concentration of Cu2+ ion.
Notes: Strain A-2 was cultured aerobically in Marine Broth 2216 with or without Cu2+ ion. Turbidity of the culture (closed squares) and IOE activity in the culture supernatant (open circles) are shown. Symbols represent the mean values obtained for triplicate determinations. Standard deviations are small, and not displayed.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
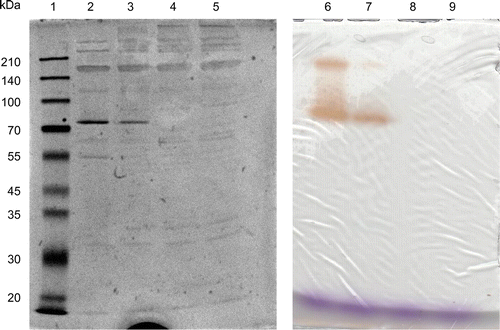
We first attempted to analyze the presence of IOE by native PAGE. However, the crude enzyme solution concentrated in the stacking gel was not migrated in the resolving gel (data not shown). Thus, the supernatant was analyzed by SDS–PAGE without heat treatment (but with 2-mercaptoethanol). As shown in Fig. , strain A-2 secreted mainly two proteins (or protein complexes) with apparent molecular masses of approximately 80 and 170 kDa. Activity staining on the SDS–PAGE gel with iodide and starch revealed that both of these proteins were IOE (Fig. , lanes 2 and 6). By heat treatment (96 °C, 2–3 min), the 80-kDa band disappeared but the 170-kDa band was still observed. Under this condition, faint bands with low molecular masses (less than 35 kDa) as well as with high molecular masses (more than 210 kDa) appeared.
Fig. 2. SDS–PAGE analysis of IOE.
Notes: Concentrated culture supernatant was run on SDS–PAGE gel with or without heat treatment, and the gels were stained with Coomassie brilliant blue (left) or with iodide and starch for activity assay (right). The crude enzyme solution was mixed with SDS and 2-mercaptoethanol (all lanes), and the mixture was applied on the gels without heat treatment (lanes 2 and 6); the mixture was heated at 96 °C for 1 min before application (lanes 3 and 7); at 96 °C for 2 min (lanes 4 and 8); at 96 °C for 3 min (lanes 5 and 9). Lane 1 represents the standard marker proteins.
Characteristics of IOE
Since the maximum activity of IOE by strain A-2 (200 mU mL−1) was still 15 times lower than that by strain Q-1 (approximately 3000 mU mL−1), it was difficult to separate two iodide-oxidizing isozymes (80 and 170 kDa) and to purify one of the isozymes by electroelution. Thus, we determined characteristics of IOE by using crude enzyme solution. The IOE activity was significantly inhibited by potassium cyanide, sodium azide, EDTA, and the copper chelator o-phenanthroline (Table ). The optimum pH was 7.0 in Tris-HCl buffer, and the activity was relatively stable under alkaline conditions (Fig. ). The activity of iodide-oxidizing enzyme gradually decreased at temperature higher than 50 °C, while the optimum temperature was 20 °C (Fig. ). The IOE activity was observed toward p-phenylenediamine, hydroquinone, catechol, and 2,6-dimethoxyphenol but not for ABTS, syringaldazine, and manganese ion (Table ). When iodide was used as the substrate, the IOE activity showed Km and Vmax values of 4.78 mM and 25.1 U mg−1, respectively (Fig. S2).
Table 1. Effect of various inhibitors on IOE from strain A-2.
Fig. 3. Effects of pH and temperature on the activity and stability of IOE.
Notes: (A) Effect of pH on the IOE activity. (B) Effect of pH on the stability of IOE. (C) Effect of temperature on the activity (open symbols) and stability (closed symbols) of IOE. Symbols represent the averages of duplicate experiments, and error bars show the range of data. Ranges smaller than the symbols are not displayed.
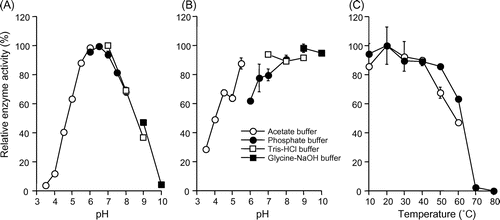
Table 2. Substrate specificity of IOE from strain A-2.
LC–MS/MS analysis
The peptides of the activity-stained 80-kDa band (Fig. ) digested with trypsin were separated by nanoscale reverse-phase chromatography, and directly analyzed by nanoelectrospray ionization–tandem mass spectrometry. A database search of tandem mass spectra with a Mascot Search Program revealed that two different peptides (IEQGSAEIWTFR and WRDFFGK) were recovered from the active IOE band, showing high Mascot scoring against a putative multicopper oxidase of Roseovarius sp. 217 (EAQ25965) (Fig. S3). In addition, a peptide (EYEGFPLLHYLGGLK) with a high Mascot scoring with the hypothetical protein of Roseovarius sp. 217 (EAQ25963) was also recovered from the band. As reported previously, IoxA and IoxC of the group B iodide-oxidizing bacterium strain Q-1 are closely related with these two proteins (EAQ25965 and EAQ25963), respectively.Citation12)
Phylogenetic analysis of putative IoxA proteins
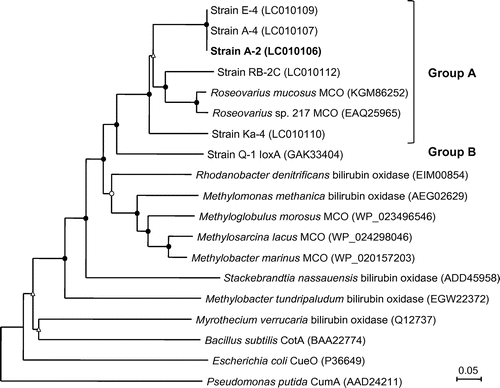
The putative ioxA gene was successfully amplified from genomic DNA of strains A-2, A-4, E-4, Ka-4, and RB-2C by the PCR with primers ioxAF and ioxAR. Phylogenetic analysis of the translated amino acid sequences (approximately 330–370 residues) revealed that all of these proteins were closely related to putative multicopper oxidases of Roseovarius sp. 217 (EAQ25965) and R. mucosus DSM 17069 (KGM86252) (Fig. ). These proteins had significant sequence similarities of 59–62% to IoxA of strain Q-1.
Fig. 4. Neighbor-joining phylogenetic tree of IoxA found in various iodide-oxidizing bacteria and related proteins in other bacteria.
Notes: Circles and triangles at the branch nodes represent bootstrap percentages (1000 replicates): filled circles, 90–100%; open circles, 70–89%; open triangles, 50–69%. Values <50% are not shown. The scale bar represents the estimated number of substitutions per site. The GenBank accession number for each reference protein is shown in parentheses.
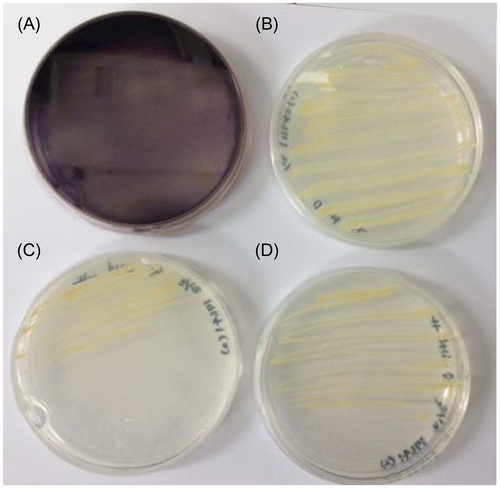
Iodide oxidation by R. denitrificans 116-2
A capacity for iodide oxidation was determined from R. denitrificans 116-2Citation15) because an ioxA-like gene is found in a genome of this strain (EIM00854, Fig. ). When the strain grew on a R2A solid medium containing Cu2+ ion and iodide (pH 7.2), the oxidation of iodide was not observed. However, significant iodide oxidation occurred when the bacterium grew on the same medium whose pH was adjusted to 5.0 (Fig. (A)). On the other hand, R. denitrificans 2APBS1,Citation15) which does not possess ioxA-like gene in its genome, did not oxidize iodide under the same growth condition (Fig. (B)). In the absence of Cu2+ ion, neither strain oxidized iodide (Fig. (C) and (D)).
Fig. 5. Iodide oxidation by Rhodanobacter denitrificans strains 116-2 (A and C) and 2APBS1 (B and D). The strains were grown on R2A solid medium containing 1 g L−1 iodide (pH 5.0) in the presence (A and B) or absence (C and D) of 40 μM Cu2+ ion. In strain 116-2 with Cu2+ ion, the purple iodine–starch complex was observed due to I2 formation.
Discussion
The enzymatic oxidation of iodide is a promising reaction for various industrial and medicinal applications.Citation14) For example, I2 might be produced from brine water at industrial scale without using chemical oxidants such as chlorine gas and nitrite. It is also possible to construct a new enzyme-based disinfection system, in which I2 is generated in situ by mixing the enzyme and iodide. Although there have been several reports on the peroxidase-based iodide-oxidizing systems, it is necessary to use toxic hydrogen peroxide in the systems.Citation24,25) On the other hand, multicopper oxidases do not need hydrogen peroxide but molecular oxygen as the electron acceptor. Thus, the multicopper oxidase-based iodide-oxidizing system is considered to be much more advantageous over the peroxidase-based system in views of safety, simplicity, and eco-friendliness. Previously, we found that the IOE-II purified from the group B iodide-oxidizing bacterium strain Q-1 is the most efficient IOE among the multicopper oxidases reported thus far, since its catalytic efficiency (kcat/Km) for iodide was 3–5 orders higher than those of other enzymes.Citation12) In this study, we found that IOE of the group A bacterium strain A-2 is also one of multicopper oxidases. LC–MS/MS analysis revealed that the active IOE of strain A-2 involved IoxA and IoxC, both of which are known as components of IOE in strain Q-1.
The IOE activity of strain A-2 that grew in the presence of 200 μM Cu2+ ion was more than 80 times higher than that without Cu2+ ion, despite their growth being almost same (Fig. ). As reported previously, the IOE activity of strain Q-1 that grew in the presence of 40 μM Cu2+ ion was also 20 times higher than that in the absence of Cu2+ ion.Citation12) The Cu2+ ion-dependent enzyme production or enzyme activity was reported in other bacterial multicopper oxidases, including CueO,Citation26) CumA,Citation27) MnxGCitation28), and YacK.Citation29) The inhibition of the IOE activity by the copper chelator o-phenanthroline and EDTA (Table ) also indicated the involvement of Cu2+ ion.
On the SDS–PAGE gel, at least two isoforms with apparent molecular masses of 80 and 170 kDa were observed (Fig. ), and they showed in-gel activity even in the presence of SDS and reducing reagent. Such high resistance to denaturation has been observed in other bacterial multicopper oxidases.Citation12,30) Under fully denatured conditions with reducing reagent and boiling, the 80-kDa band disappeared while the 170-kDa band was still observed (Fig. ). Considering that molecular masses of IoxA and IoxC are generally 60–70 kDa, the disappearance of the 80-kDa band under fully denatured conditions seems to be unreasonable. Similar to the 80-kDa band of strain A-2, when IOE purified from strain Q-1 with an apparent molecular mass of 51 kDa was fully denatured on the SDS–PAGE gel, two bands with much higher molecular masses of 150 and 230 kDa were reported to newly appear.Citation12) It was speculated that these bands might represent native enzyme and its aggregated form, respectively, since bacterial multicopper oxidases are often found in high-molecular mass complexes.Citation31)
IOE of strain A-2 hardly showed activity toward ABTS and syringaldazine (Table ), although both compounds are typical substrates for common multicopper oxidases and are also superior substrates for the enzyme of strain Q-1.Citation12) On the other hand, both IOEs of strains A-2 and Q-1 had similar pH optima of near neutral, alkaline stability, and heat stability up to 50 °C (Fig. ). These common biochemical characteristics may reflect original brine environments from which strains A-2 and Q-1 were isolated.Citation4) Kinetic determinations revealed that Km and Vmax values were similar to those of strain Q-1.
Tandem mass spectrometric analysis of the active enzyme detected peptides from two proteins, putative multicopper oxidase (EAQ25965), and the hypothetical protein (EAQ25963) of Roseovarius sp. 217, although sequence coverage was very low (2–3%). Considering that IOE of strain A-2 is highly resistant to denaturation (Fig. ), this low sequence coverage might be due to its compact structure and poor water solubility, which lead to the enzyme to be resistant to proteolysis.Citation32) Our previous study showed that these two proteins were closely related to IoxA and IoxC of strain Q-1, respectively.Citation12) Thus, it is strongly suggested that IOE of strain A-2 comprises at least two proteins, IoxA and IoxC. PCR amplification of putative ioxA gene fragment revealed that IoxA of strain A-2 had high sequence similarity to that of Roseovarius sp. 217, and conserved amino acid residues corresponding to potential copper-binding site (Fig. S3). Putative ioxA gene was amplified from all strains tested in the group A iodide-oxidizing bacteria. Phylogenetic analysis suggested that IoxA proteins from group A iodide-oxidizing bacteria form a well supported monophyletic group (Fig. ). In addition, it seems likely that IoxA of both groups A and B iodide-oxidizing bacteria is distinct from other multicopper oxidases, suggesting the existence of a shared ancestry.
It is interesting that R. denitrificans 116-2 isolated from subsurface sediment showed an iodide-oxidizing phenotype when grown with iodide and starch (Fig. ). As shown in Fig. , putative multicopper oxidase of R. denitrificans 116-2 is closely related with IoxA of strain Q-1 and Roseovarius spp. with amino acid sequence similarities of 62–63%. In addition, R. denitrificans 2APBS1, which does not possess the homologous gene of ioxA in its genome, did not oxidize iodide. Furthermore, both strains did not oxidize iodide when grown without Cu2+ ion. Therefore, it is possible that IoxA catalyzes the oxidation of iodide not only in marine iodide-oxidizing bacteria, but also in iodide-oxidizing terrestrial bacteria including R. denitrificans 116-2. The oxidation of iodide and its subsequent sorption to natural soil organic matter is of great importance when we predict the fate of long-lived radioactive 129I (half-life: 1.6 × 107 years) released from nuclear facilities.Citation1,2) Seki et al.Citation33) recently found that the partition coefficient for iodide and specific activity of multicopper oxidase (laccase) in soils showed significant positive correlation. In addition, addition of iodide-oxidizing enzyme from strain Q-1 strongly enhanced the sorption of iodide to soils.Citation33) These results suggest that IoxA and related proteins may participate in iodide oxidation and subsequent sorption to soils in terrestrial environments, although several other microbial mechanisms have also been proposed.Citation34,35)
In conclusion, we demonstrated that IOE of Roseovarius sp. A-2 is one of multicopper oxidases, comprising at least two proteins IoxA and IoxC. All iodide-oxidizing bacterial strains tested in this study possessed homologous genes of ioxA, indicating the importance of IoxA in the oxidation of iodide. Furthermore, an iodide-oxidizing phenotype was found in the terrestrial bacterium R. denitrificans 116-2, which also has an ioxA-like gene in its genome. Our results suggest a possibility that the multicopper oxidase IoxA catalyzes the oxidation of iodide in phylogenetically distinct bacteria. Further study is clearly needed to fully understand structural arrangement and subunit components of IOE. Considering possible applications of the IOE in the future, it is also required to elucidate the mechanism by which the IOE becomes highly resistant to denaturation and toxic I2.
Author’s contribution
Seigo Amachi has designed this experiment. Kanna Shiroyama and Seigo Amachi prepared this manuscript. Kanna Shiroyama and Yasutaka Kawasaki carried out most of experiments, while Yusuke Unno designed PCR primers. All authors have read and approved the final manuscript.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.10.1080/09168451.2015.1052767
Funding
This work was supported by the JSPS KAKENHI [grant number 20780049]; Institute of Fermentation, Osaka, Japan to S.A.
Supplemental Materials
Download Zip (327.8 KB)References
- Kaplan DI, Denham ME, Zhang S, et al. Radioiodine biogeochemistry and prevalence in groundwater. Crit. Rev. Environ. Sci. Technol. 2014;44:2287–2335.
- Schwehr KA, Santschi PH, Kaplan DI, et al. Organo-iodine formation in soils and aquifer sediments at ambient concentrations. Environ. Sci. Technol. 2009;43:7258–7264.
- Otosaka S, Schwehr KA, Kaplan DI, et al. Factors controlling mobility of 127I and 129I species in an acidic groundwater plume at the Savannah River Site. Sci. Total Environ. 2011;409:3857–3865.
- Muramatsu Y, Fehn U, Yoshida S. Recycling of iodine in fore-arc areas: evidence from the iodine brines in Chiba, Japan. Earth Planet Sci. Lett. 2001;192:583–593.
- Sakuma A. Manufacturing, recovery and recycling of iodine, p. 15–27. In: Yokoyama M., editor. Function & Application of Iodine Compounds. CMC Publishing: Tokyo; 2005. ( In Japanese).
- Amachi S. Microbial contribution to global iodine cycling: volatilization, accumulation, reduction, oxidation, and sorption of iodine. Microb. Environ. 2008;23:269–276.
- Amachi S, Muramatsu Y, Akiyama Y, et al. Isolation of iodide-oxidizing bacteria from iodide-rich natural gas brines and seawaters. Microbial. Ecol. 2005;49:547–557.
- Arakawa Y, Akiyama Y, Furukawa H, et al. Growth stimulation of iodide-oxidizing α-Proteobacteria in iodide-rich environments. Microb. Ecol. 2012;63:522–531.
- Fuse H, Inoue H, Murakami K, et al. Production of free and organic iodine by Roseovarius spp. FEMS Microbiol. Lett. 2003;229:189–194.
- Sugai Y, Sasaki K, Wakizono R, et al. Considerations on the possibility of microbial clogging of re-injection wells of the wastewater generated in a water-dissolved natural gas field. Int. Biodeterior. Biodegrad. 2013;81:35–43.
- Wakai S, Ito K, Iino T, et al. Corrosion of iron by iodide-oxidizing bacteria isolated from brine in an iodine production facility. Microb. Ecol. 2014;68:519–527.
- Suzuki M, Eda Y, Ohsawa S, et al. Iodide oxidation by a novel multicopper oxidase from Alphaproteobacterium strain Q-1. Appl. Environ. Microbiol. 2012;78:3941–3949.
- Ehara A, Suzuki H, Kanesaki Y, et al. Draft genome sequence of strain Q-1, an iodide-oxidizing Alphaproteobacterium isolated from natural gas brine water. Gen. Announcements. 2014;2:e00659–14.
- Xu F. Catalysis of novel enzymatic iodide oxidation by fungal laccase. Appl. Biochem. Biotechnol. 1996;59:221–230.
- Prakash O, Green SJ, Jasrotia P, et al Rhodanobacter denitrificans sp. nov., isolated from nitrate-rich zones of a contaminated aquifer. Int. J. Sys. Evol. Microbiol. 2012;62:2457–2462.
- Reasoner DJ, Geldreichi EE. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 1985;49:1–7.
- Hiraishi A. Direct automated sequencing of 16S rDNA amplified by polymerase chain reaction from bacterial cultures without DNA purification. Lett. Appl. Microbiol. 1992;15:210–213.
- Weisburg WG, Barns SM, Pelletier DA, et al. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991;173:697–703.
- Saito N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425.
- Su J, Deng L, Huang L, et al. Catalytic oxidation of manganese(II) by multicopper oxidase CueO and characterization of the biogenic Mn oxide. Water Res. 2014;56:304–313.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.
- Shevchenko A, Wilm M, Vorm O, et al. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858.
- Biebl H, Allgaier M, Lünsdorf H, et al. Roseovarius mucosus sp. nov., a member of the Roseobacter clade with trace amounts of bacteriochlorophyll a. Int. J. Sys. Evol. Microbiol. 2005;55:2377–2383.
- Hickey J, Panicucci R, Duan Y, et al. Control of the amount of free molecular iodine in iodine germicides. J. Pharm. Pharmacol. 1997;49:1195–1199.
- Hansen EH, Albertsen L, Schäfer T, et al. Curvularia haloperoxidase: antimicrobial activity and potential application as a surface disinfectant. Appl. Environ. Microbiol. 2003;69:4611–4617.
- Grass G, Rensing C. CueO is a multi-copper oxidase that confers copper tolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 2001;286:902–908.
- Brouwers G-J, de, Vrind JPM, Corstjens PLAM, et al. cumA, a gene encoding a multicopper oxidase, is involved in Mn2+ oxidation in Pseudomonas putida GB-1. Appl. Environ. Microbiol. 1999;65:1762–1768.
- van Waasbergen LG, Hildebrand M, Tebo BM. Identification and characterization of a gene cluster involved in manganese oxidation by spores of the marine Bacillus sp. strain SG-1. J. Bacteriol. 1996;178:3517–3530.
- Kim C, Lorenz WW, Hoopes JT, et al. Oxidation of phenolate siderophores by the multicopper oxidase encoded by the Escherichia coli yacK gene. J. Bacteriol. 2001;183:4866–4875.
- Okazaki M, Sugita T, Shimizu M, et al. Partial purification and characterization of manganese-oxidizing factors of Pseudomonas fluorescens GB-1. Appl. Environ. Microbiol. 1997;63:4793–4799.
- Claus H. Laccases and their occurrence in prokaryotes. Arch. Microbiol. 2003;179:145–150.
- Lin Y, Zhou J, Bi D, et al. Sodium-deoxycholate-assisted tryptic digestion and identification of proteolytically resistant proteins. Anal. Biochem. 2008;377:259–266.
- Seki M, Oikawa J, Taguchi T, et al. Laccase-catalyzed oxidation of iodide and formation of organically bound iodine in soils. Environ. Sci. Technol. 2013;47:390–397.
- Li H-P, Yeager CM, Brinkmeyer R, et al. Bacterial production of organic acids enhances H2O2-dependent iodide oxidation. Environ. Sci. Technol. 2012;46:4837–4844.
- Li H-P, Daniel B, Creeley D, et al. Superoxide production by a manganese-oxidizing bacterium facilitates iodide oxidation. Appl. Environ. Microbiol. 2014;80:2693–2699.
