Abstract
Alginate is an acidic linear polysaccharide with immune-modulating activities. In this study, we found that enzymatically digested alginate oligomer (AO) with various degrees of polymerization (DP; 2–5) induced a higher level of nitric oxide (NO) production in RAW264.7 cells than undigested alginate polymer (AP). Reverse transcription-polymerase chain reaction and western blot analyses revealed that the expression level of inducible NO synthase in AO-treated RAW264.7 cells was higher than that in AP-treated cells. AO induced nuclear translocation of nuclear factor (NF)-κB p65 subunit in RAW264.7 cells to a greater extent than AP. Although AO and AP induced similar extents of phosphorylation in three mitogen-activated protein (MAP) kinases, c-Jun N-terminal kinase inhibitor exhibited the most potent inhibitory effect on NO induction in AO- and AP-treated RAW264.7 cells, among three MAP kinase inhibitors that were tested.
Graphical abstract
Alginate is composed of two forms of uronic acids, α-L-guluronate (G) and β-D-mannuronate (M), which in turn form three types of polymer blocks: G-block, M-block, and random block.
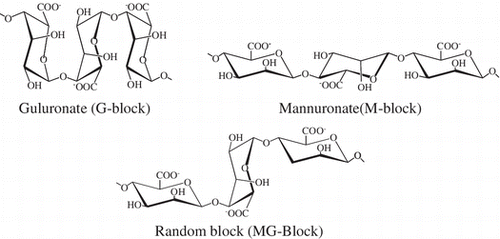
Alginate is an acidic linear polysaccharide often found in brown seaweeds such as Macrocystis pyrifera and Ascophyllum nodosum. Alginate is composed of two forms of uronic acids, α-L-guluronate (G) and β-D-mannuronate (M), which in turn form three types of polymer blocks: homopolymer of guluronate, homopolymer of manunuronate, and heteropolymer (mixed sequence of these residues). These block structures are called G-blocks, M-blocks, and MG-blocks, respectively.Citation1) Alginate has been used for a wide range of commercial applications, including as a thickening agent and dispersion stabilizer. Because alginate has gentle gelling properties in the presence of divalent cations, such as calcium, alginate is also used for live cell encapsulation in vitroCitation2) and in vivo,Citation3) and for several tissue engineering applications.Citation4,5)
Alginate has several biological activities. For instance, alginate can induce the secretion of cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1 from human monocytes.Citation6) Alginate also induces TNF-α production from mouse resident peritoneal monocytes,Citation7) and it enhances the cytotoxicity of natural killer cells in mouse.Citation8) Recently, we demonstrated that alginate induces TNF-α secretion from mouse macrophage cell line RAW264.7, and that its activity is significantly influenced by the molecular size and M/G ratio of alginate.Citation9) In addition to alginate polymer (AP), several low-molecular weight alginate oligomers (AOs) prepared by enzymatic degradation or acid hydrolysis of AP have various biological activities. In a recent study, we demonstrated that enzymatically depolymerized AOs exhibit higher activity in terms of TNF-α secretion from RAW264.7 cells than the original AP.Citation9)
Macrophages are one of the most important members of the immune system, and they are responsible for host defensive mechanisms against invaded pathogens. Stimulated macrophages produce reactive oxygen species and various cytokines. Stimulated macrophages also produce nitric oxide (NO) via the induction of inducible NO synthase (iNOS), which catalyzes the production of NO from L-arginine.Citation10,11) NO is a multifunctional molecule; it functions as a vascular relaxing agent, neurotransmitter, inflammatory mediator, and specific immunity regulator depending on the situation.Citation10) Although we had previously reported that enzymatically depolymerized AOs induced TNF-α secretion from RAW264.7 cells,Citation9) whether AO induces NO production in RAW264.7 cells has not been clarified. Therefore, in this study, we examined the effects of AO on NO production in RAW264.7 cells, and compared the results with those obtained for AP. We also analyzed the underlying intracellular signaling mechanism of action of AO.
Materials and methods
Reagents
Sodium alginate (1000-cps grade) was purchased from Nacalai Tesque Inc. (Kyoto, Japan). Alginate lyase was obtained from Nagase ChemteX Co. (Osaka, Japan). Mitogen-activated protein (MAP) kinase inhibitors (PD98059, SB202190, and SP600125, which are specific inhibitors for extracellular-regulated kinase (ERK), p38 MAP kinase, and c-jun N-terminal kinase (JNK), respectively) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). NG-monomethyl-L-arginine acetate (l-NMMA) was obtained from Dojindo Chemical Laboratories (Kumamoto, Japan). Other chemicals were of the highest grade commercially available.
Preparation of AO
AO was prepared from sodium alginate (AP) using the method described by Yokose et al. Citation12) In brief, 1% of AP in ultra-pure water was incubated with alginate lyase (final concentration, 1 μg/mL) at 40 °C for three days. The enzymatic reaction was stopped by adding HCl to reduce the pH to 4.0, and the reaction mixture was incubated for 10 min, and then neutralized with NaOH. To analyze the molecular sizes of AO, the enzyme-digested alginate was applied to gel filtration chromatography on a Bio-Gel P-6 column equilibrated with 50 mM phosphate buffer, pH 7.0. Before use, AO and AP were passed through an endotoxin removal column (Detoxi-gel™; Thermo Fisher Scientific Inc., Rockford, IL, USA) in order to eliminate endotoxin, a common contaminant in natural products.
Cell culture
RAW264.7 (mouse macrophage) cells were purchased from the American Type Culture Collection (Rockville, MD, USA) and were cultured at 37 °C in Dulbecco’s modified Eagle’s minimum essential medium (DMEM, high glucose) supplemented with 10% fetal bovine serum (FBS), penicillin (100 IU/mL), and streptomycin (100 μg/mL) in a humidified atmosphere with 5% CO2 and 95% air.
Enzyme-linked immunosorbent assay
Adherent RAW264.7 cells in a 96-well plate (5 × 104 cells/well) were treated with varying concentrations of samples (0–200 μg/mL) in the growth medium at 37 °C. After 24 h of incubation, the levels of TNF-α in the culture supernatants of treated cells were estimated by sandwich ELISA using two antibodies against two different epitopes on the TNF-α molecule.
Nitrite assay for the estimation of NO
To estimate NO production by RAW264.7 cells, nitrite (), a stable reaction product of NO with molecular oxygen, was analyzed by the Griess assay, as described previously.Citation13) In brief, adherent RAW264.7 cells in a 96-well plate (5 × 104 cells/well) were treated with the indicated concentrations of AO (0–200 μg/mL) or AP (0–200 μg/mL) for 24 h in the growth medium at 37 °C, and then the nitrite levels in the culture medium of treated cells were estimated. To examine the effects of three MAP kinase inhibitors, adherent RAW264.7 cells in a 96-well plate (5 × 104 cells/well) were pre-incubated with each inhibitor (concentration, 10 μM) for 1 h at 37 °C in the growth medium, and then, AO or AP (final concentration, 200 μg/mL) was added to the treated cells. After 24 h of incubation, nitrite levels were estimated.
RNA isolation, cDNA synthesis, and reverse transcription-polymerase chain reaction for iNOS mRNA
Adherent RAW264.7 cells in a 35-mm dish (2 × 106 cells/dish) were treated with each sample at a concentration of 100 μg/mL in the growth medium at 37 °C. After 4 h of incubation, total RNA was extracted from the treated cells using Sepasol®-RNA I Super (Nacalai Tesque). The extracted total RNA (2.5 μg) was reverse transcribed into a single-stranded cDNA using a PrimeScript® 1st strand cDNA Synthesis Kit (Takara Bio Inc., Shiga, Japan). PCR was performed using the GoTaq® Green Master Mix (Promega, Madison, WI, USA). The primers for the endogenous control (β-actin) and the target genes were as follows: β-actin, 5′-GGAGAAGATCTGGCACCACACC-3′ (forward) and 5′-CCTGCTTGCTGATCCACATCTGCTGG-3′ (reverse); iNOS, 5′-CAACCAGTATTATGGCTCCT-3′ (forward) and 5′-GTGACAGCCCGGTCTTTCCA-3′ (reverse). The PCR parameters for cycling were as follows: 1 cycle of 70 s at 95 °C, followed by 25 cycles of 55 s at 93 °C, 45 s at 61 °C, 40 s at 72 °C, and 1 cycle of 100 s at 72 °C. Each PCR product (10 μL) was run on 2% agarose gels containing 0.1 μg/mL ethidium bromide, and was observed by light capture (ATTO Co., Tokyo, Japan).
Western blot analysis
The assay used in this study was essentially identical to that described in our previous paper.Citation14) To estimate the expression level of iNOS protein, adherent RAW264.7 cells (3 × 106 cells/35-mm dish) were treated with AO (100 μg/mL) or AP (100 μg/mL) in the growth medium at 37 °C. After 6 h of incubation, the cells were lysed with extraction buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM ethylene glycol tetraacetic acid, 1% CHAPS, and 1% Triton™ X-100) containing 1% protease inhibitor cocktail (Nacalai Tesque) on ice. After 30 min, the cytosolic fraction was obtained by centrifugation at 15,000 × g at 4 °C for 10 min. To estimate the expression levels of MAP kinases, adherent RAW264.7 cells (4 × 106 cells/35-mm dish) were incubated with AO (200 μg/mL) or AP (200 μg/mL) in serum-free DMEM for 1 h. The cytosolic fraction was obtained as described above. To estimate the expression level of nuclear factor (NF)-κB, adherent RAW264.7 cells (5 × 106 cells/35-mm dish) were treated with AO (200 μg/mL) or AP (200 μg/mL) in serum-free DMEM at 37 °C. After 1 h of incubation, the cells were washed three times with ice-cold PBS and were incubated with 100 μL ice-cold cytosol extraction buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.2% Igepal® CA-630, 1 mM dithiothreitol, 20 mM β-glycerophosphate, 1 mM sodium orthovanadate, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg/mL leupeptin, and 1 μg/mL aprotinin) for 25 min on ice. The cytosolic extract was collected by centrifugation at 7000 × g for 5 min at 4 °C. The nuclear pellets were re-suspended in 20 μL ice-cold nuclear extraction buffer (20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 0.45 M NaCl, 25% glycerol, 0.2-mM ethylenediaminetetraacetic acid, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg/mL leupeptin, and 1 μg/mL aprotinin) and were incubated on ice for 25 min; nuclear extracts were obtained by centrifugation at 15,000 × g for 10 min at 4 °C. The extracts (containing 20 μg proteins) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide gel; Mini-PROTEAN® TGXTM Precast Gels, Bio-Rad Laboratories, Inc., CA USA). The proteins were then electrophoretically transferred to a polyvinylidene difluoride membrane using a Trans-Blot® TurboTM Transfer System (Bio-Rad). Each protein was analyzed by western blotting using specific primary antibodies, followed by anti-rabbit immunoglobulin G horseradish peroxidase-conjugated secondary antibodies (Merck), and were visualized with Amersham™ ECL™ Prime (GE Healthcare).
Statistical analysis
All statistical analyses were performed using GraphPad prism 6 software (GraphPad Software, San Diego, CA, USA). A p-value of <0.05 was considered statistically significant.
Results
Preparation of AO by enzymatic degradation
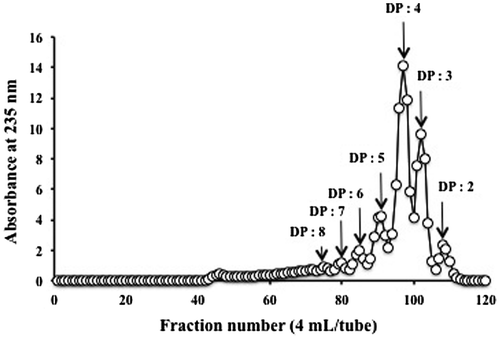
A mixture of AO with various molecular sizes was obtained by digesting AP with alginate lyase. The elution profile of AO on a Bio-Gel P-6 column suggested that the enzymatic product was mainly composed of oligomers with approximately 2–5 degrees of polymerization (DP; Fig. ). This result was consistent with those obtained previously.Citation9)
TNF-α-inducing activities of AO and AP in RAW264.7 cells
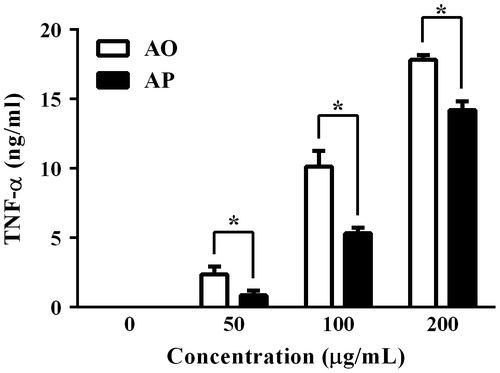
As shown in Fig. , the AO prepared in this study could induce TNF-α secretion from RAW264.7 cells to a greater extent than AP, suggesting that enzymatic digestion may be a reliable method to increase the macrophage-stimulating activity of alginate. Consistent with our previous results,Citation9) the AO prepared in this study induced TNF-α secretion from RAW264.7 cells to a greater level than AP, although the origin of alginate lyase used in this study for the preparation of AO was different.
Fig. 2. Secretion of TNF-α from RAW264.7 cells stimulated with AO or AP. Adherent RAW264.7 cells were incubated with the indicated concentrations of AO or AP for 24 h.
NO-inducing activities of AO and AP in RAW264.7 cells
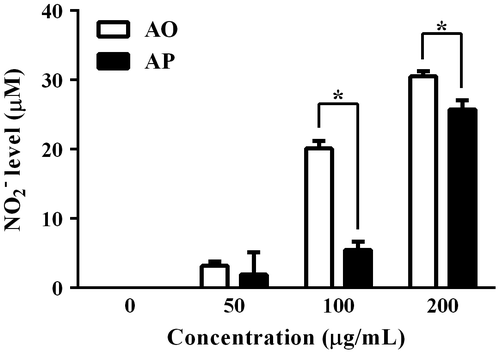
In addition to TNF-α, we investigated NO-inducing activities of AO and AP in RAW264.7 cells. As shown in Fig. , AO significantly induced NO production in RAW264.7 cells in a concentration-dependent manner. The activity of AO was much greater than that of the original polymer. Particularly, the NO level induced by AO at 100 μg/mL was nearly three times that induced by the same concentration of AP.
Fig. 3. NO levels in the cultured supernatant of RAW264.7 cells stimulated with AO or AP.
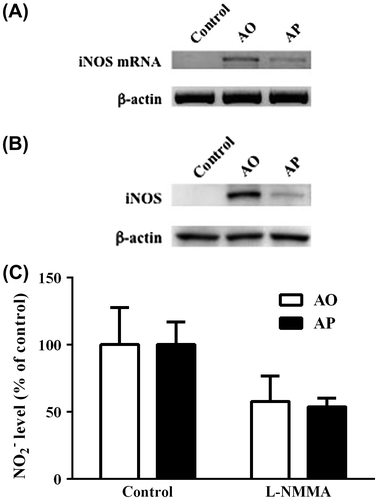
Effects of AO and AP on the expression level of iNOS mRNA and protein in RAW264.7 cells
In activated macrophages, iNOS is the main enzyme responsible for NO production. To estimate the iNOS expression level, we carried out RT-PCR and western blot analyses. The results clearly showed that the expression levels of both iNOS mRNA and protein were significantly higher in AO-treated RAW264.7 cells than in AP-treated cells (Fig. (A) and (B)). Furthermore, AO- and AP-induced NO production in RAW264.7 cells was inhibited by l-NMMA, a specific iNOS inhibitor (Fig. (C)). These results indicate that the enhanced ability of AO to induce NO production in RAW264.7 cells relative to that of the original polymer may be attributed to stronger induction of iNOS expression by AO.
Fig. 4. Levels of iNOS mRNA and protein in RAW264.7 cells stimulated with AO or AP.
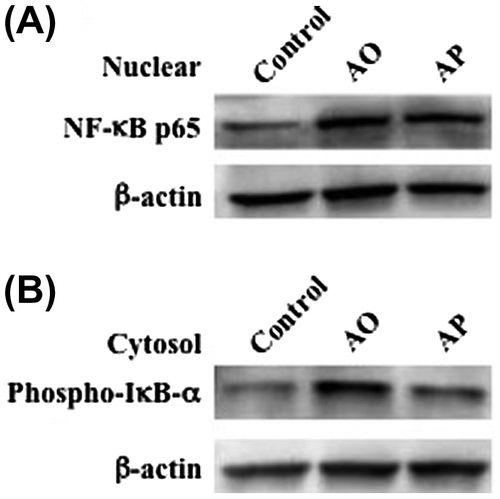
Effects of AO and AP on the activation of NF-κB in RAW264.7 cells
We investigated nuclear translocation of NF-κB in RAW264.7 cells stimulated with AO and AP. Nuclear translocation of NF-κB p65 subunit in RAW264.7 cells stimulated with AO or AP was investigated by western blotting. NF-κB is a transcription factor that regulates many important biological and pathological processes, including immune and inflammatory responses.Citation15–17) Translocation of NF-κB from the cytosol to the nucleus is usually prevented by the binding of IκB-α subunit, which is phosphorylated and subsequently degraded upon stimulation.Citation15) As shown in Fig. (A), the nuclear level of the p65 subunit was slightly higher in AO-treated RAW264.7 cells than that in AP-treated cells. In addition, the phosphorylation level of IκB-α subunit induced by AO was evidently higher than that induced by AP (Fig. (B)). These results suggest that AO activates NF-κB more potently than AP.
Fig. 5. The effects of AO and AP on nuclear translocation of NF-κB p65 subunit and phosphorylation of IκB-α subunit in RAW264.7 cells.
Effects of AO and AP on MAP kinases in RAW264.7 cells
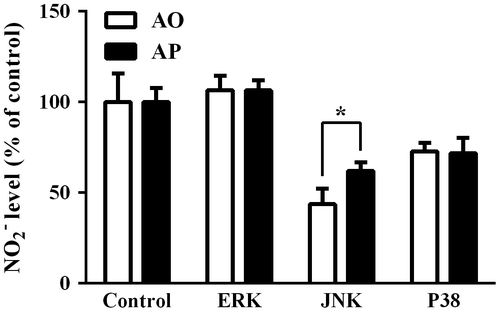
MAP kinases are known to be involved in intracellular signaling pathways linked with iNOS expression.Citation18) We examined the activation of the MAP kinase system in RAW264.7 cells treated with AO and AP by western blotting analysis. AO and AP activated ERK, JNK, and p38 kinases with different extents, and slight differences in the phosphorylation levels of these MAP kinases were observed between AO- and AP-treated cells (Fig. ). The phosphorylated ERK level in AP-treated RAW264.7 cells was slightly higher than that of AO-treated cells, whereas phosphorylated p38 kinase level in AO-treated cells was slightly higher than that of AP. On the other hand, expression of ERK was suppressed in AP-treated cells. To further investigate the involvement of MAP kinase signaling pathways leading to NO production in AO- and AP-treated RAW264.7 cells, we examined the effects of specific MAP kinase inhibitors on the NO-inducing activities of AO and AP in RAW264.7 cells. We used PD98059, SB202190, and SP600125, which are specific inhibitors for ERK, JNK, and p38, respectively. Among these inhibitors, JNK inhibitor exhibited the most potent inhibitory effect on AO- and AP-induced NO production, suggesting that JNK might be involved in the intracellular signaling pathway leading to NO production in AO- and AP-stimulated RAW264.7 cells (Fig. ).
Fig. 6. Effects of AO and AP on the MAP kinase system in RAW264.7 cells.
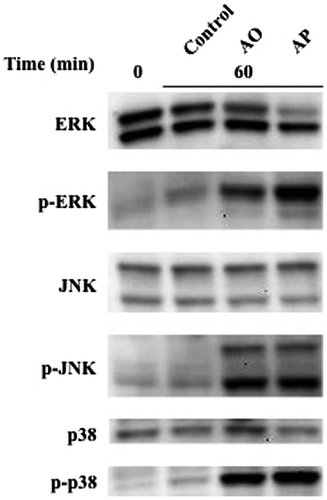
Fig. 7. Effects of MAP kinase inhibitors on AO- or AP-induced NO production from RAW264.7 cells.
Discussion
Our previous studies have demonstrated that, compared to the original AP, enzymatically depolymerized AO exhibits increased macrophage activation activities such as induction of various cytokines.Citation9) The gel filtration profile of AO suggested that the enzymatic digestion products were oligomers with approximately 2–5 DP, similar to the results reported previously.Citation9) In this study, AO induced RAW264.7 cells to produce NO to a greater extent than the original AP (Fig. ). Significant increase in iNOS mRNA and protein levels were observed in AO-treated RAW264.7 cells, and the levels were much higher than those induced by AP (Fig. ). Involvement of iNOS in NO production by AO-stimulated RAW264.7 cells was further confirmed by the fact that l-NMMA inhibited NO production in AO-treated RAW264.7 cells. Slightly higher levels of nuclear translocation of NF-κB and phosphorylation of IκB were observed in AO-treated RAW264.7 cells than those in AP-treated cells (Fig. ). These results suggest that the enhanced ability of AO to activate NF-κB may partly explain increased induction of iNOS expression and NO production by AO. Phosphorylation of ERK, JNK, and p38 MAP kinases were observed in AO- and AP-treated cells, but with different extents. Among these MAP kinases, the phosphorylation level of ERK in AP-treated RAW264.7 cells was slightly higher than that of AO-treated cells. No significant difference in the phosphorylation level of JNK and p38 MAP kinase was observed between AO- and AP-treated cells. On the other hand, expression of ERK was suppressed in AP-treated cells. Although the exact reason and underlying mechanisms for the different effects of AO and AP on the MAP kinase system are still unclear, these results suggest that MAP kinase systems activated with AO and AP might be slightly different. Probably these differences may lead to different effects of AO and AP on other activities of RAW264.7 cells. It is supposed that certain cell surface receptors, which recognize AP, are different from those of AO. Further studies are obviously required to find the different effects of AP and AO on the bioactivities or behaviors of RAW267.4 cells. To further analyze the involvement of MAP kinases in terms of NO production, we investigated the effects of three specific MAP kinase inhibitors, PD98059, SB202190, and SP600125. Among these inhibitors, JNK inhibitor exhibited the most potent inhibitory effect on NO production by AO- and AP-stimulated RAW264.7 cells, and the inhibitory effects of p38 inhibitor were less than that of JNK inhibitor. ERK inhibitor had no inhibitory effect under the same conditions. These results suggest that, among the activated MAP kinases, JNK MAP kinase may be involved in the pathway leading to NO production in AO- and AP-treated RAW264.7 cells. However, whether other activated MAP kinases play a role in NO production in AO- or AP-stimulated RAW264.7 cells needs to be clarified in future studies.
The increased macrophage stimulation activity of AO may be attributed to alteration of the physicochemical properties of AOs upon enzymatic degradation. In general, AP has high viscosity in aqueous solution, and it can form gels in the presence of divalent ions such as calcium ion. After enzymatic oligomerization, the viscosity of AO decreases dramatically and its gel-forming property disappears. Therefore, AO molecules may be able to move more freely in aqueous solution than AP molecules, and they might be able to encounter specific receptors on the cell surface of macrophages more frequently. Additionally, the increased molarity of AO relative to the original polymer may lead to enhanced ability to stimulate RAW264.7 cells. Similarly, a previous report has suggested that hyaluronan oligosaccharides activate dendritic cells via toll-like receptor (TLR) 4 to induce TNF-α, and the activity of hyaluronan oligosaccharides is greater than that of hyaluronan polysaccharides.Citation19,21) Thus, oligomerization might represent a promising strategy to increase the bioactivities of these polysaccharides.
Previous studies on the structure–activity relationships of AOs have demonstrated that the TNF-α-inducing activities of enzymatically depolymerized AOs in RAW264.7 cells vary with the size and composition of the oligomer.Citation22) Among the oligomers tested, G8 and M7 have the optimal molecular size to function as stimulants for the induction of TNF-α expression in macrophages, although the yields of G8 and M7 were lower than those of other oligomers.Citation22) Oligomers with DP of 3 and 4 also exhibited relatively high activities.Citation22) The procedure used in this study mainly resulted in oligomers with DP of 3 or 4 (Fig. ). Hence, the relatively high content of AOs of appropriate molecular sizes may partly explain the enhanced macrophage-stimulating activity of AO in this study.
Although the exact mechanism underlying the ability of AOs to stimulate macrophages, especially specific receptors on the macrophage cell surface, are unclear, previous studies using antibodies against TLR2 and TLR4 have suggested that both TLR2 and TLR4 are responsible for AO-induced TNF-α production from RAW264.7 cells. Additionally, TLR2 and TLR4 are involved in mannuronate-rich AP-induced cytokine production from human monocytes.Citation23) TLRs can recognize specific pathogen-associated molecular patterns without apparent structural similarity.Citation24) TLRs play important roles in signal transduction for the initiation of mammalian macrophage responses, including cytokine production.Citation24) In addition to bacterial products such as lipopolysaccharides, TLRs seem to be responsible for oligosaccharide-mediated stimulation processes.Citation25) For instance, oligosaccharides of hyaluronan activated dendritic cells via TLR4 to induce TNF-αCitation19) and they induced inflammation in chondrocytes through TLR4 and CD44 receptors.Citation21) These findings support the involvement of TLRs in the macrophage-stimulating activities of AOs.
In conclusion, our results suggest that AO prepared by enzymatic digestion stimulates NO production in RAW264.7 cells through activation of NF-κB and subsequent expression of iNOS. These effects are more marked in AO-treated cells than in cells treated with the original polymer. The procedure used in this study for the preparation of AO may be a promising strategy to increase the bioactivities of AP.
Authors’ contribution
MU and ST conceived the experiments. MU performed the experiments, and analyzed the data together with SN, SI, and RA. KC and KY provided valuable advice. DK and TO co-wrote the paper. All authors discussed the results and commented on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a fund from Nagasaki University Major Research Project (Research Initiative for Adaptation to Future Ocean Change). This work was also supported by Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) Fellows to M. Ueno.
Notes
Abbreviations: AO, alginate oligomer; AP, alginate polymer; NO, nitric oxide; TNF-α, tumor necrosis factor-α; RT-PCR, reverse transcription-polymerase chain reaction; iNOS, inducible nitric oxide synthase; ELISA, enzyme linked-immunosorbent assay; NF-κB, nuclear factor-kappa B; MAP kinase, mitogen-activated protein kinase; ERK, extracellular-regulated kinase; JNK, c-jun N-terminal kinase; DMEM, Dulbecco’s modified Eagle’s minimum essential medium; FBS, fetal bovine serum; L-NMMA, NG-monomethyl-L-arginine acetate.
References
- Haug A. Isolation and fractionation with potassium chloride and manganous ions. Methods Carbohydr. Chem. 1965;5:69–73.
- Fremond B, Malandain C, Guyomard C, et al. Correction of bilirubin conjugation in the Gunn rat using hepatocytes immobilized in alginate gel beads as an extracorporeal bioartificial liver. Cell Transplant. 1993;2:453–460.
- Chang SCN, Rowley JA, Tobias G, et al. Injection molding of chondrocyte/alginate constructs in the shape of facial implants. J. Biomed. Mater. Res. 2009;55:503–511.
- Atala A, Kim W, Paige KT, et al. Endoscopic treatment of vesicoureteral reflux with a chondrocyte-alginate suspension. J. Urol. 1994;152:641–643.
- Hauselmann HJ, Masuda K, Hunziker EB, et al. Adult human chondrocytes cultured in alginate from a matrix similar to native human articular cartilage. Am. J. Physiol. 1996;271:C742–C752.
- Otterlei M, Østgaard K, Skjåk-Bræk G, et al. Induction of Cytokine Production from Human Monocytes Stimulated with Alginate. J. Immunother. 1991;10:286–291.10.1097/00002371-199108000-00007
- Pasquali P, Zalcman A, Murtas S, et al. In vitro stimulation of murine peritoneal monocytes induced by alginates. Arch. Pharm. Res. 2005;28:936–941.10.1007/BF02973880
- Son EW, Yang KH, Rhee DK, et al. Immunomodulatory function of murine nk cell activity by alginate. Arch. Pharm. Res. 2005;28:1282–1286.10.1007/BF02978213
- Kurachi M, Nakashima T, Miyajima C, et al. Comparison of the activities of various alginates to induce TNF-α secretion in RAW264.7 cells. J. Infect. Chemother. 2005;11:199–203.10.1007/s10156-005-0392-0
- Coleman JW. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001;1:1397–1406.10.1016/S1567-5769(01)00086-8
- Karpuzoglu E, Ahmed SA. Estrogen regulation of nitric oxide and inducible nitric oxide synthase (iNOS) in immune cells: Implications for immunity, autoimmune diseases, and apoptosis. Nitric Oxide. 2006;15:177–186.10.1016/j.niox.2006.03.009
- Yokose T, Yamasaki Y, Nishikawa T, et al. Effects of alginate oligosaccharides on the growth of various mammalian cell lines, unicellular phytoplankters, and marine bactirea. Jpn. J. Food Chem. Saf. 2010;17:27–35. Japanese.
- Ueno M, Hiroki T, Takeshita S, et al. Comparative study on antioxidative and macrophage-stimulating activities of polyguluronic acid (PG) and polymannuronic acid (PM) prepared from alginate. Carbohydr Res. 2012;352:88–93.
- Jiang Z, Okimura T, Yamaguchi K, et al. The potent activity of sulfated polysaccharide, ascophyllan, isolated from Ascophyllum nodosum to inuce nitric oxide and cytokine production from mouse macrophage RAW264.7 cells: Comparison between ascophyllan and fucoidan. Nitric Oxide. 2012;25:407–415.
- Fujioka S, Niu J, Schmidt C, et al. NF-kB and AP-1 connection: mechanism of NF-kB-dependent regulation of AP-1 activity. Mol. Cell. Biol. 2004;24:7806–7819.10.1128/MCB.24.17.7806-7819.2004
- Liang YC, Huang YT, Tsai SH, et al. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis. 1999;20:1945–1952.10.1093/carcin/20.10.1945
- Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four κB-like motifs and of constitutive and inducible forms of NF-κB. Mol. Cell. Biol. 1990;10:1498–1506.
- Korhonen R, Lahti A, Kankaanranta H, et al. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy. 2005;4:471–479.10.2174/1568010054526359
- Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of hyaluronan activate dendric cells via toll-like receptor 4. J. Exp. Med. 2002;195:99–111.10.1084/jem.20001858
- Termeer CC, Hennies J, Voith U, et al. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J. Immunol. 2000;165:1863–1870.10.4049/jimmunol.165.4.1863
- Campo GM, Avenoso A, Campo S, et al. Small hyaluronan oligosaccharides induce inflammation by engaging both toll-like-4 and CD44 receptors in human chondrocytes. Biochem. Pharmacol. 2010;80:480–490.10.1016/j.bcp.2010.04.024
- Iwamoto M, Kurachi M, Nakashima T, et al. Structure-activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264.7 cells. FEBS Lett. 2005;579:4423–4429.10.1016/j.febslet.2005.07.007
- Flo TH, Ryan L, Latz E, et al. Involvement of toll-like receptor (TLR) 2 and TLR4 in cell activation by mannuronic acid polymers. J. Biol. Chem. 2002;277:35489–35495.10.1074/jbc.M201366200
- Hirschfeld M, Ma Y, Weis JH, et al. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 2000;165:618–622.10.4049/jimmunol.165.2.618
- Shoham S, Huang C, Chen JM, et al. Toll-like receptor 4 mediates intracellular signaling without TNF-α release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 2001;166:4620–4626.10.4049/jimmunol.166.7.4620