Abstract
MUC5B mucin is a principal component of airway mucus and plays a key role in biodefense. We investigated the regulation of MUC5B production using the signals from extracellular matrix (ECM) components in NCI-H292 human lung epithelial cells. We found that MUC5B production in NCI-H292 cells cultured on fibronectin or laminin increased by 4–5-fold, with the increase occurring in a dose- and time-dependent manner. In contrast, MUC5B production was unchanged on type-IV collagen. Inhibition of integrin β1 induced upregulation of MUC5B and MUC5AC; however, inhibition of p38 MAPK did not show any remarkable change in overproduced MUC5B. Inhibition of extracellular signal-regulated kinase (ERK) pathway or the transcription factor NF-κB induced the recovery of overproduced MUC5B on fibronectin and laminin. These results suggest that MUC5B production can be regulated by ECM components and that MUC5B is upregulated by fibronectin and laminin via the integrin, ERK, and NF-κB dependent pathway.
MUC5B mucin plays a key role in biodefense. Our results suggest that a signal from fibronectin and laminin induce upregulation of MUC5B, mediated by integrin pathway and ERK pathway.
Key words:
The mucus layer in the human airway is produced from secretory epithelial cells and is a component of primary host defense. The mucus layer traps inhaled foreign particulates and transports them out of the airway through mucociliary clearance (MCC) by mucus flow and cough.Citation1,2) Although deficiency of the mucus layer causes injury of the airway, excessive mucus production leads to temporary narrowing of the airway and deterioration in some respiratory diseases.Citation3,4) Hypersecretion of airway mucus is a primary feature of certain common respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). Hence, regulation of mucus secretion is important in the treatment of respiratory diseases.
Mucus is mainly composed of water and mucin, a highly glycosylated protein. To date, 17 mucin genes have been identified in the human genome.Citation5) In airway mucus, the principal mucin subtypes are MUC5AC and MUC5B. In the airway of patients with asthma or COPD, MUC5AC mucin is overproduced (40–200 times than that under normal conditions) and causes airway narrowing.Citation5–7) MUC5B mucin is also a major component of mucus. It shows a small increase in the airway of patients with respiratory diseases, but it is required for biodefense of the airway, with a lack of MUC5B inducing inefficient MCC.Citation8–10)
MUC5AC production is regulated in various ways, such as by inflammatory cytokines (interleukin-1β, tumor necrosis factor-α), cell–cell adhesion molecules, and EGF receptors.Citation11,12) However, little is known about the regulation of MUC5B production. In a previous study, we reported that several extracellular matrix (ECM) components are involved in the regulation of MUC5AC production.Citation13) ECM components are a complex of substances including laminin, fibronectin, type-IV collagen, and elastin. ECM components not only provide mechanical support for organ structure but also stimulate cell functions in vivo.Citation14) ECM components in asthmatic airway show marked qualitative changes. It has been reported that types I, III, and V collagen, fibronectin, tenascin C, and laminin are present in increased amounts in asthmatic airway; in contrast, type-IV collagen and elastin are decreased.Citation15–17) Despite marked changes in ECM components in asthma, the relationship between ECM components and MUC5B production is not yet well understood.
ECM components relay signals to cells via key molecules called integrins.Citation18) Integrins are major adhesion receptors that recognize and interact with the ECM and cells.Citation19,20) Integrins are composed of two distinct chains, α and β subunits. A specific combination of integrin α- and β-subunits mediates the binding of cells to collagen, fibronectin, or laminin.Citation21) In the ECM-mediated integrin signaling pathway, signals from integrins activate numerous kinases, such as extracellular signal-regulated kinase (ERK), p38, and thus influence gene expression in cells.Citation22)
ERK, a member of the MAPK family, is a serine/threonine kinase that plays key roles in the integrin pathway and regulates both cell growth and proliferation.Citation23) Integrins can act as positive mediators of ERK by phosphorylating specific sites and stimulating the activation pathway. A distinct MAPK group, p38, is also activated by integrin and influences inflammation, apoptosis, and cell proliferation.Citation24)
We have previously reported that certain ECM components are involved in the regulation of MUC5AC production. MUC5AC production was upregulated on laminin and Matrigel, a complex of ECM components, whereas it was downregulated on type-IV collagen and unchanged on fibronectin in NCI-H292 human airway epithelial cells.Citation13) In addition, we suggested that integrin β1 downregulates MUC5AC production.Citation25) Although ECM components and activation of the integrin signaling pathway are involved in the regulation of MUC5AC production, the regulation of MUC5B production by ECM components is not yet understood. In the current study, we investigated a new mechanism of regulation of MUC5B production by ECM components involving integrin pathways. Our results suggest that ECM components have unique functions during the regulation of MUC5B production via integrin, ERK, and NF-κB dependent pathway.
Materials and methods
Cell culture
Human airway cancer cell line NCI-H292 was purchased from the American Type Culture Collection (Manassas, VA, USA). NCI-H292 cells were cultured in RPMI-1640 (Sigma-Aldrich, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS, Cansera International, Etobicoke, Ontario, Canada), 100 units/mL of penicillin (Gibco Oriental, Tokyo, Japan), and 100 μg/mL streptomycin (Gibco Oriental) in a 5% CO2 incubator at 37 °C. Adherent cells were subcultured every 3–4 days by treatment with a trypsin–EDTA solution (Gibco Oriental).
Plate coating with ECM proteins
A 96-well plate polystyrene (MS8096F, Sumilon, Tokyo, Japan) was coated with 100 μL of type-IV collagen (33, 100, 500 μg/mL, Sigma, Tokyo, Japan), fibronectin (33, 100, 500 μg/mL, Asahi Techno Glass, Tokyo, Japan), and laminin (33, 100, 500 μg/mL, BD, Bedford, MA, USA) in phosphate-buffered saline (PBS; 0.01 mM phosphate buffer, 0.138 mM NaCl, 0.0027 mM KCl, pH 7.4) over 10 h at 4 °C. As control, the plate was pretreated with PBS.
Reagents
NF-κB activation inhibitor III (Merck, Darmstadt, Germany) was added to the cell culture medium to a final concentration of 2.5 μM. TC-I 15 (Karebay Biochem, Deerpark, NJ, USA), an integrin β1 inhibitor was added to the cell culture medium to a final concentration of 200 μM. U0126 (Wako, Tokyo, Japan), an inhibitor of the ERK activator MAPK/ERK kinase 1/2 and inhibitis ERK pathway was added to the cell culture medium to a final concentration of 10 μM. PH-797804 (A11078, adooq bioscience, irvine, CA, USA), a p38 inhibitor was added to the cell culture medium to a final concentration of 10 μM. All reagents were dissolved to the appropriate concentration in dimethylsulfoxide (DMSO). The same concentration of DMSO was added to the controls.
MUC5B and MUC5AC protein assay
NCI-H292 cells were washed once with culture medium and suspended in the medium by means of syringe with a 26G needle to create a single-cell suspension. Diluted cells (2 × 104 cells per 100 μL) were added to the wells of coated 96-well plate and incubated at 37 °C. After removal and storage of the culture media (100 μL), the cells were harvested by lysis in 100 μL of Tris-buffered saline (TBS; 100 mM NaCl and 10 mM Tris pH 7.5) containing 0.1% SDS at the indicated time points. A total of 100 μL (2 × 104 cells) of the solution was blotted onto an Immobilon membrane (Millipore, Bedford, MA, USA) by Dot Blot Hybridization Manifold (48 wells; SCIE-PLAS, Cambridge, UK). The membrane was treated with 4% skim milk (Gibco Oriental) in 0.1% Tween 20-TBS (TBS-T) for 12 h at 4 °C, and then incubated with mouse anti-human MUC5B antibody (1:2000 in 4% skim milk, ab77995, abcam, Tokyo, Japan) or with mouse anti-human MUC5AC antibody (1:2000 in 4% skim milk, MS145-P1, Thermo Scientific, Kanagawa, Japan) for 1 h. The membrane was washed five times for 5 min each with TBS-T and then incubated with rabbit anti-mouse IgG (H + L) (1:2000 in 4% skim milk, NA931V, GE Healthcare, Buckinghamshire, UK) for 1 h. After washing the membrane for five times, enzyme reactions were detected with a Luminata Forte western HRP substrate (WBLUF0500, Millipore) and a LAS-4000 image analyzer (Fujifilm, Tokyo, Japan).
Western blot analysis
The cells cultured on the ECM-coated plate were lysed in a conventional SDS sample buffer (62.5 mM Tris, 10% glycerol, 2% SDS, 0.01% bromophenol blue, pH 6.8). The culture media (20 μL) were sampled and lysed in a conventional SDS sample buffer. The samples were electrophoresed on 10% of acrylamide gels or on 4–15% Mini-PROTEAN TGX stain free precast gels for the detection of MUC5B and MUC5AC (BioRad, Tokyo, Japan) with a CM-1005 gel apparatus (Cima Biotech, Tokyo, Japan), and then blotted onto a Hybond ECL nitrocellulose membrane (GE Healthcare) with a M3001 transfer apparatus (Cima Biotech). The membrane was treated with 4% skim milk (Gibco Oriental) in TBS-T (0.1% tween-20, 150 mM NaCl and 10 mM Tris pH 7.5) for 12 h at 4 °C and then incubated with a rabbit anti-p38 MAPK (9212S, Cell Signaling Technology, Tokyo, Japan), a rabbit anti-phospho p38 MAPK (AF869, R&D systems, Minneapolis, MN, USA), a rabbit anti-phospho ERK 1/2 antibody (sc-101760, Santa Cruz, Tokyo, Japan), a rabbit anti-ERK 1/2 antibody (V1141, Promega, Madison, WI, USA), a mouse anti-human MUC5B antibody (ab77995, abcam), or a mouse anti-human MUC5AC antibody (MS145-P1, Thermo Scientific) at a dilution of 1:2000 in 4% skim milk for 1 h. The membrane was washed five times for 5 min each with TBS-T and then incubated with an anti-rabbit IgG antibody conjugated with horseradish peroxidase (W4011, Promega, Madison, WI, USA) or an anti-mouse IgG (H + L) conjugated with horseradish peroxidase (NA931V, GE Healthcare) at a dilution of 1:2000 in 4% skim milk for 1 h. After washing the membrane for five times, the enzyme reaction was detected with a Luminata Forte western HRP substrate (WBLUF0500, Millipore) and a LAS-4000 image analyzer (Fujifilm). Cellular G3PDH and β-actin was detected as control using a rabbit anti-G3PDH antibody (ab9485, abcam, Tokyo, Japan) or a mouse anti-β-actin antibody (A5316, Sigma) at a dilution of 1:2000 and an anti-rabbit IgG antibody conjugated with horseradish peroxidase (Promega) or an anti-mouse IgG (H + L) (GE Healthcare) at a dilution of 1:2000 in 4% skim milk for 1 h in the same way. After detection, a blot membrane was incubated with Restore Western blot Stripping buffer (21059, Thermo Scientific, Rockford, IL, USA) for 15 min at room temperature with shaking. The membrane was washed five times for 5 min each with TBS-T and then treated with 4% skim milk (Gibco Oriental) in TBS-T for 12 h at 4 °C for reblocking.
Cell proliferation assay
Cell proliferation was assessed by a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). NCI-H292 cells (2 × 104 cells in 0.1 mL) were cultured on a 96-well plate (Sumilon, Tokyo, Japan) at 37 °C. The reagent of the kit (0.01 mL) was added to each well, and then the plate was incubated for 2 h at 37 °C. Cell growth was assessed by measuring the absorbance at 450 nm with a microplate spectrophotometer Benchmark plus (BioRad).
Statistics
Analysis of variance (ANOVA) was used for comparisons among more than two groups. For other statistics, Student’s t-test was performed. *p < 0.05 was considered significant.
Results
MUC5B production was upregulated on fibronectin and laminin
In our former report, the production of MUC5AC was upregulated on laminin, but it was downregulated on type-IV collagen and unchanged on fibronectin in NCI-H292 human cells.Citation13) To examine the effect of these three ECM components on MUC5B and MUC5AC production, we used NCI-H292 human lung epithelial cells, which produce both subtypes of mucin. To evaluate the regulation of MUC5B and MUC5AC production, we measured the quantity of both subtypes of mucins in the cells and in the medium separately. The amount of secreted MUC5B in the medium was 6-fold higher than that in the cells, whereas most MUC5AC was detected in the cells (Fig. ). MUC5B production in the cells and in the medium was upregulated in the cells cultured on 500 μg/mL of fibronectin and laminin but unchanged on type-IV collagen after culture for 30 h (Fig. (A), (B), and (E)). The G3PDH protein was detected in the corresponding cells as a standard of the seeded cells (Fig. (E)). In contrast, MUC5AC production was unchanged on 500 μg/mL of fibronectin but decreased on type-IV collagen and increased in the cells on laminin after culture for 30 h (Fig. (C) and (E)). The secreted MUC5AC in the medium was also decreased on type-IV collagen but was unchanged on fibronectin or on laminin (Fig. (D)). Based on these results, to evaluate the major part of MUC5B and MUC5AC production, we measured the amount of secreted MUC5B in the medium and MUC5AC in the cells (Figs. ). The production of MUC5AC was regulated by ECM components in time- and dose-dependent manner.Citation13) The secreted MUC5B was unchanged on type-IV collagen after 24 and 30 h of culture (Fig. ). The secreted MUC5B on fibronectin was upregulated after 24 and 30 h of culture in a dose-dependent manner (Fig. ). The secteted MUC5B on laminin was upregulated in a dose-dependent manner after 30 h of culture (Fig. ). These results suggest that MUC5B and MUC5AC production is regulated differently in cells cultured on type-IV collagen and fibronectin, whereas both are upregulated on laminin.
Fig. 1. Evaluation of MUC5B and MUC5AC protein levels in the cells and in the medium.
Notes: NCI-H292 cells (2 × 104 cells/well) were cultured in 96-well plates pretreated with PBS. (A) The cells were cultured for 30 h and sampled. Cells (cells) and culture media (medium) were sampled. The samples were analyzed using the mucin protein assay to detect the levels of MUC5B protein. Fold changes were based on MUC5B levels in the cells (mean ± SD, n = 5, one-way ANOVA). (B) The cells were cultured for 30 h and sampled. Cells (cells) and culture media (medium) were sampled. The samples were analyzed using the mucin protein assay to detect the levels of MUC5AC protein. Fold changes were based on MUC5AC levels in the cells (mean ± SD, n = 5, one-way ANOVA). Fold changes were normalized to cell numbers. Asterisks indicate statistical probability, *p < 0.05 (ANOVA). The representative results of three independent experiments are shown.
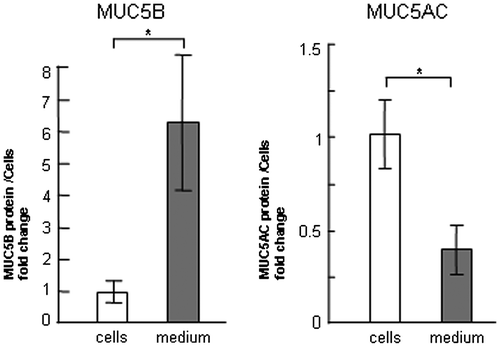
Fig. 2. Evaluation of MUC5B and MUC5AC protein in NCI-H292 cells on ECM components.
Notes: NCI-H292 cells (2 × 104 cells/well) were cultured in 96-well plates precoated with PBS (CNTL), 500 μg/mL of type-IV collagen (C4), fibronectin (FN), or laminin (LM). (A) The cells were cultured on ECM components for 30 h and sampled. The samples were analyzed using the mucin protein assay to detect the levels of MUC5B protein in cells. Fold changes were based on CNTL level of MUC5B in the cells (mean ± SD, n = 5, one-way ANOVA). (B) The cells were cultured on ECM components for 30 h and their culture media (medium) were sampled. The samples were analyzed using the mucin protein assay to detect the levels of MUC5B protein in medium. Fold changes were based on CNTL level of MUC5B (mean ± SD, n = 5, one-way ANOVA). (C) The cells were cultured on ECM components for 30 h and sampled. The samples were analyzed using the mucin protein assay to detect the levels of MUC5AC protein in cells. Fold changes were based on CNTL level of MUC5AC in the cells (mean ± SD, n = 5, one-way ANOVA). (D) The cells were cultured on ECM components for 30 h and their culture media (medium) were sampled. The samples were analyzed using the mucin protein assay to detect the levels of secreted MUC5AC protein in medium. Fold changes were based on CNTL level of MUC5AC (mean ± SD, n = 5, one-way ANOVA). (E) The cells were cultured on ECM components for 30 h and the cells and their culture media (medium) were sampled. The samples were analyzed using the western blot analysis to detect the levels of MUC5B protein in culture media. The G3PDH protein was detected in the corresponding cells to count the seeded cells. The samples were analyzed using the western blot analysis to detect the levels of MUC5AC and G3PDH protein in cells. The representative results of three independent experiments are shown.
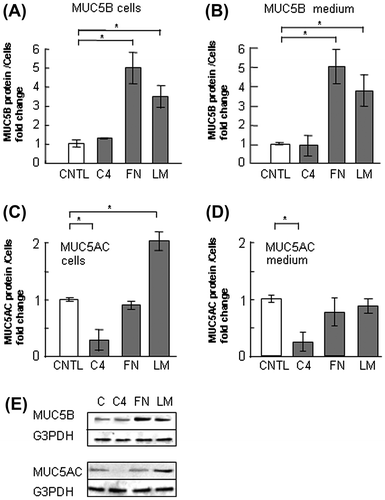
Fig. 3. Evaluation of MUC5B protein in NCI-H292 cells on type-IV collagen.
Notes: NCI-H292 cells (2 × 104 cells/well) were cultured in 96-well plates precoated with PBS (CNTL), or with 33, 100, 500 μg/mL of type-IV collagen (C4). The cells were cultured for 24 or 30 h and their culture medium were sampled. The samples were analyzed using the mucin protein assay to detect the levels of MUC5B protein in culture media. Fold changes were based on CNTL level of MUC5B in culture media (mean ± SD, n = 5, one-way ANOVA). The representative results of three independent experiments are shown.
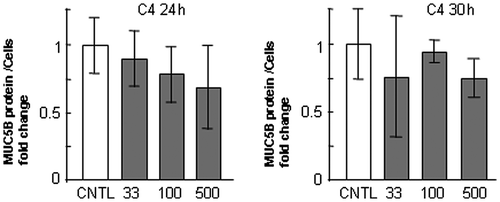
Fig. 4. Evaluation of MUC5B protein in NCI-H292 cells on fibronectin.
Notes: NCI-H292 cells (2 × 104 cells/well) were cultured in 96-well plates precoated with PBS (CNTL), or with 33, 100, 500 μg/mL of fibronectin (FN). The cells were cultured for 24 or 30 h and their culture media were sampled. The samples were analyzed using the mucin protein assay to detect the levels of MUC5B protein in culture media. Fold changes were based on CNTL level of MUC5B in culture media (mean ± SD, n = 5, one-way ANOVA). The representative results of three independent experiments are shown.
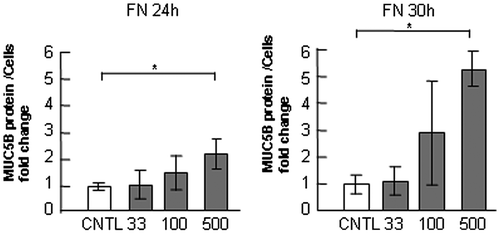
Fig. 5. Evaluation of MUC5B protein in NCI-H292 cells on laminin.
Notes: NCI-H292 cells (2 × 104 cells/well) were cultured in 96-well plates precoated with PBS (CNTL), or with 33, 100, 500 μg/mL of laminin (LM). The cells were cultured for 24 or 30 h and their culture media were sampled. The samples were analyzed using the mucin protein assay to detect the levels of MUC5B protein in culture media. Fold changes were based on CNTL level of MUC5B in culture media (mean ± SD, n = 5, one-way ANOVA). The representative results of three independent experiments are shown.
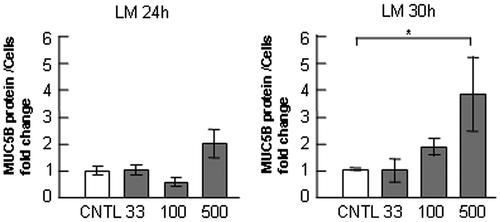
Fig. 6. Effects of integrin β1 inhibition on the regulation of MUC5B and MUC5AC production in NCI-H292 cells on ECM components.
Notes: NCI-H292 cells (2 × 104 cells/well) were cultured in 96-well plates precoated with PBS (CNTL), or with 500 μg/mL of type-IV collagen (C4), fibronectin (FN), or laminin (LM). The cells were cultured with an integrin β1 inhibitor (200 μM: integrin inh: +) or with the same concentration of DMSO (−). After culturing for 30 h, the culture media and cells were sampled and analyzed using the mucin protein assay to detect the levels of MUC5B and MUC5AC protein. The sampled culture media was used for MUC5B detection and the sampled cells were used for MUC5AC detection. Fold changes were based on a control, which was cultured with DMSO (mean ± SD, n = 5, one-way ANOVA). Fold changes were normalized to cell numbers. Asterisks indicate statistical probability, *p < 0.05 (ANOVA). The representative results of three independent experiments are shown.
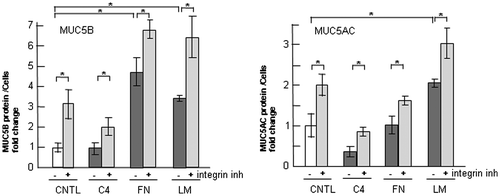
Fig. 7. Effects of p38 inhibition on the regulation of MUC5B production in NCI-H292 cells on ECM components.
Notes: (A) NCI-H292 cells (2 × 104 cells/well) were cultured in 96-well plates pretreated with PBS. The cells were cultured with a p38 inhibitor (10 μM: p38 inh: +) or with the same concentration of DMSO (−) for 30 h and the cells were sampled. The samples were analyzed using western blot analysis to detect the levels of phosphorylated and activated form of p38 (p-p38), total p38, and β-actin. (B) NCI-H292 cells (2 × 104 cells/well) were cultured in a 96-well plate precoated with PBS (CNTL), 500 μg/mL of type-IV collagen (C4), fibronectin (FN), or laminin (LM). The cells were cultured with a p38 inhibitor (10 μM: +) or with the same concentration of DMSO (−) for 30 h and their culture media were sampled. The samples were analyzed using the mucin protein assay to detect the levels of MUC5B protein. Fold changes were based on a control, which was cultured with DMSO (mean ± SD, n = 5, one-way ANOVA). (C) NCI-H292 cells (2 × 104 cells/well) were cultured in a 96-well plate precoated with PBS (CNTL), 500 μg/mL of type-IV collagen (C4), fibronectin (FN), or laminin (LM). The cells were cultured with a p38 inhibitor (10 μM: +) or with the same concentration of DMSO (−) for 30 h and the cells were sampled. The samples were analyzed using the mucin protein assay to detect the levels of MUC5AC protein. Fold changes were based on a control, which was cultured with DMSO (mean ± SD, n = 5, one-way ANOVA). Fold changes were normalized to cell numbers. Asterisks indicate statistical probability, *p < 0.05 (ANOVA). The representative results of three independent experiments are shown.
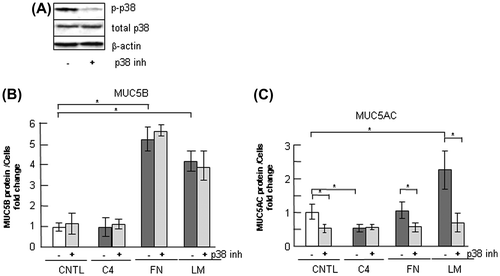
Fig. 8. Effects of ERK inhibition on the regulation of MUC5B production in NCI-H292 cells.
Notes: (A) NCI-H292 cells (2 × 104 cells/well) were cultured in 96-well plates pretreated with PBS. The cells were cultured with an ERK inhibitor (10 μM: ERK inh: +) or with the same concentration of DMSO (−) for 30 h and the cells were sampled. The samples were analyzed using western blot analysis to detect the levels of phosphorylated and activated form of ERK (p-ERK), total ERK, and β-actin. (B) NCI-H292 cells (2 × 104 cells/well) were cultured in a 96-well plate precoated with PBS (CNTL), 500 μg/mL of type-IV collagen (C4), fibronectin (FN), or laminin (LM). The cells were cultured with a ERK inhibitor (10 μM: +) or with the same concentration of DMSO (−) for 30 h and their culture media were sampled. The samples were analyzed using the mucin protein assay to detect the levels of MUC5B protein. Fold changes were based on a control, which was cultured with DMSO (mean ± SD, n = 5, one-way ANOVA). (C) NCI-H292 cells (2 × 104 cells/well) were cultured in a 96-well plate precoated with PBS (CNTL), 500 μg/mL of type-IV collagen (C4), fibronectin (FN), or laminin (LM). The cells were cultured with an ERK inhibitor (10 μM: +) or with the same concentration of DMSO (−) for 30 h and the cells were sampled. The samples were analyzed using the mucin protein assay to detect the levels of MUC5AC protein. Fold changes were based on a control, which was cultured with DMSO (mean ± SD, n = 5, one-way ANOVA). Fold changes were normalized to cell numbers. Asterisks indicate statistical probability, *p < 0.05 (ANOVA). The representative results of four independent experiments are shown.
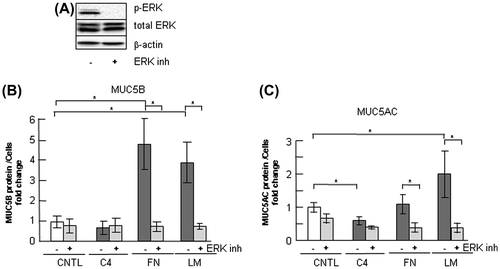
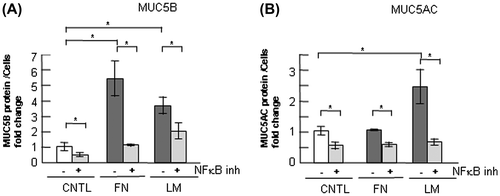
Fig. 9. Effects of NF-κB inhibition on the regulation of MUC5B and MUC5AC production in NCI-H292 cells cultured on ECM components.
Notes: (A) NCI-H292 cells (2 × 104 cells/well) were cultured in a 96-well plate precoated with PBS (CNTL), 500 μg/mL of fibronectin (FN), or laminin (LM). The cells were cultured with a NF-κB inhibitor (2.5 μM: +) or with the same concentration of DMSO (−). After culturing for 30 h, the cells were sampled and analyzed using the mucin protein assay to detect the levels of MUC5B protein. Fold changes were based on a control, which was treated with PBS (mean ± SD, n = 5, one-way ANOVA). (B) NCI-H292 cells (2 × 104 cells/well) were cultured in a 96-well plate precoated with PBS (CNTL), 500 μg/mL of fibronectin (FN), or laminin (LM). The cells were cultured with a NF-κB inhibitor (2.5 μM: +) or with the same concentration of DMSO (−). After culturing for 30 h, the cells were sampled and analyzed by the mucin protein assay to detect the levels of MUC5AC protein. Fold changes were based on a control, which was treated with PBS (mean ± SD, n = 5, one-way ANOVA). Fold changes were normalized to cell numbers. Asterisks indicate statistical probability, *p < 0.05 (ANOVA). The representative results of three independent experiments are shown.
MUC5B production was upregulated by inhibition of integrin β1
In NCI-H292 cells, MUC5AC production has been suggested to be downregulated by the integrin β1 subunit. Inhibition of integrin β1 with an inhibitory antibody induces the upregulation of MUC5AC production.Citation13) We examined the function of integrin β1 in the regulation of secreted MUC5B in the cells cultured on type-IV collagen, fibronectin, or laminin. We treated cells on ECM components with TC-I 15, an integrin β1 inhibitor, and examined MUC5B and MUC5AC production. TC-I 15 inhibited the interaction of cells with type-IV collagen in a dose-dependent manner, and inhibited the interaction most effectively at the concentration of 200 μM without cell toxicity (data not shown). MUC5B and MUC5AC production was upregulated following treatment with TC-I 15 on ECM components (Fig. ). These effects suggest that integrin β1 downregulates MUC5B and MUC5AC production on ECM components.
MUC5B production was downregulated by inhibition of ERK but not by inhibition of p38
p38 is a representative transducer in the integrin intervening pathway. We tested whether p38 MAPK acts in the regulation of MUC5B production. The p38 MAPK was activated by MKK3/6, and was autophosphorylated and activated by the interaction of TAB1 (transforming growth factor-beta-activated protein kinase 1 binding protein 1).Citation26) A p38 inhibitor, PH-797804, suppressed p38 activity (Fig. (A)). The secreted MUC5B was unchanged following treatment with the p38 inhibitor in cells cultured on type-IV collagen, fibronectin, or laminin, whereas MUC5AC production was downregulated on fibronectin or on laminin (Fig. (B) and (C)).
ERK is a key regulator of the integrin pathway and MUC5AC production has been shown to be strongly regulated by ERK activation.Citation27) Treatment with a ERK pathway inhibitor, U0126, suppressed ERK activity (Fig. (A)). U0126 treatment induced downregulation of MUC5B production on fibronectin and laminin (Fig. (B)). MUC5AC production was downregulated on fibronectin or on laminin (Fig. (C)). These results indicate that integrin β1 and ERK but not p38 are involved in the regulation of MUC5B production and suggest that ERK upregulates MUC5B production on fibronectin and laminin.
MUC5B production was regulated, in part, by the transcription factor NF-κB
In terms of the regulation of MUC5AC production, the transcription factor NF-κB plays a primary role in MUC5AC gene expression. In a previous study, we found that regulation of the expression of MUC5AC by the ECM is mainly mediated by NF-κB.Citation25) We examined the function of NF-κB in the regulation of increased MUC5B in cells on fibronectin or on laminin but not on type-IV collagen. Inhibition of NF-κB resulted in recovery of MUC5B overproduction on fibronectin or on laminin (Fig. (A)). MUC5AC production was downregulated to the level of the control on fibronectin or on laminin by inhibition of NF-κB (Fig. (B)). These results suggest that regulation of MUC5B production by the ECM is mediated by the transcription factor NF-κB.
Discussion
MCC is an important self-clearing mechanism of bronchi. Inhaled irritants are trapped in the viscous mucus in the airway surface, and the mucus is excluded by the movement of cilia. A previous study on mice demonstrated that MUC5B mucin deficiency is associated with MCC dysfunction and causes difficulty in biodefense of the airway.Citation10) However, overproduction of MUC5B or MUC5AC mucin causes narrowing of the airway. A primary symptom of patients with COPD and asthma is hypersecretion of airway mucus with hyperplasia and it causes airflow limitation. One method of treating COPD and asthma is airway clearance. Therefore, adequate regulation of MUC5B and MUC5AC levels is required to maintain a healthy condition.
The current study is the first to recognize that MUC5B production can be regulated by signals from ECM components. Our results demonstrated that great majority of MUC5B was secreted in the medium and that production of both MUC5B and MUC5AC was upregulated in NCI-H292 cells cultured on laminin (Figs. and ). In the airway of OVA treated asthmatic mouse, increase in laminin was found.Citation28) Our results suggest that an increase in laminin in the airway may lead to a risk of hypersecretion of mucus and narrowing of the airway.
Type-IV collagen and fibronectin showed different effects on the regulation of MUC5B and MUC5AC. Type-IV collagen halved MUC5AC production but did not change MUC5B production (Figs. and ). Fibronectin induced a 5-fold increase in MUC5B production but did not affect MUC5AC production (Figs. and ). These results suggest that MUC5AC and MUC5B production was differently regulated by fibronectin and type-IV collagen.
Our results suggest that integrin β1 downregulates and ERK upregulates MUC5B production and suggest that the signal from ECM components such as fibronectin and laminin is transmitted via the integrin β1 and ERK intervening pathway but not via the p38 pathway in the regulation of MUC5B production (Figs. ). Inhibition of transcription factor NF-κB suppressed MUC5B production (Fig. ). This result suggests that upregulation of MUC5B on fibronectin or laminin occurs via NF-κB.
Hypersecretion of MUC5AC and MUC5B induces airflow limitation; however, MUC5B is indispensable for biodefense by movement of cilia. In view of mediation of COPD and asthma by ECM components, downregulation of MUC5AC overproduction but maintaining a steady level of MUC5B production is effective. Our result suggests that type-IV collagen may be useful for the reduction of MUC5AC production with a steady level of MUC5B production in the airway of patients with COPD or asthma. In some patients, it has been reported that MUC5B production decreases at airway surfaces.Citation29) Fibronectin may be useful for the recovery of decreased MUC5B production in patients with unchanged MUC5AC production. Our results suggest that nebulization of type-IV collagen and fibronectin together would be a useful tool for the treatment of asthmatic airway by downregulation of MUC5AC production and a steady level of MUC5B production in the airway.
Author contribution
Yuho Ito, Jun Iwashita, and Jun Murata conceived the experiments. Yuho Ito, Arisa Kudoh, and Chika Kuramata performed the experiments. All authors analyzed the data, discussed the results, and commented on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research (C) from Japan Society for the Promotion of Science [grant number 23570238]; a Grant-in-Aid for Feasible Study stage: A-STEP from Japan Science and Technology Agency [grant number AS251Z02371Q].
Acknowledgments
The authors thank Dr Hajime Muraguchi for his helpful advices.
Notes
Abbreviations: ECM, extracellular matrix; COPD, chronic obstructive pulmonary disease; ERK, extracellular-signal-regulated kinases.
References
- Fahy JV, Dickey BF. Airway mucus function and dysfunction. N. Engl. J. Med. 2010;363:2233–2247.10.1056/NEJMra0910061
- Button B, Cai LH, Ehre C, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941.10.1126/science.1223012
- Aikawa T, Shimura S, Sasaki H, et al. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101:916–921.10.1378/chest.101.4.916
- Vestbo J. Epidemiological studies in mucus hypersecretion. Novartis Found. Symp. 2002;248:3–12; discussion 12–19, 277–282.
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 2006;86:245–278.10.1152/physrev.00010.2005
- Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest. 2002;122:320S–326S.10.1378/chest.122.6_suppl.320S
- Lai HY, Rogers DF. Mucus hypersecretion in asthma: intracellular signalling pathways as targets for pharmacotherapy. Curr. Opin. Allergy Clin. Immunol. 2010;10:67–76.10.1097/ACI.0b013e328334643a
- Davies JR, Svitacheva N, Lannefors L, et al. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochem. J. 1999;344:321–330.10.1042/0264-6021:3440321
- Chen Y, Zhao YH, Di YP, et al. Characterization of human mucin 5B gene expression in airway epithelium and the genomic clone of the amino-terminal and 5′-flanking region. Am. J. Respir. Cell Mol. Biol. 2001;25:542–553.10.1165/ajrcmb.25.5.4298
- Roy MG, Livraghi-Butrico A, Fletcher AA, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416.
- Song KS, Lee WJ, Chung KC, et al. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J. Biol. Chem. 2003;278:23243–23250.10.1074/jbc.M300096200
- Iwashita J, Ose K, Ito H, et al. Inhibition of E-cadherin dependent cell–cell contact promotes MUC5AC mucin production through the activation of epidermal growth factor receptors. Biosci. Biotechnol. Biochem. 2011;75:688–693.10.1271/bbb.100830
- Iwashita J, Yamamoto T, Sasaki Y, et al. MUC5AC production is downregulated in NCI-H292 lung cancer cells cultured on type-IV collagen. Mol. Cell. Biochem. 2010;337:65–75.10.1007/s11010-009-0286-z
- Ingber DE, Dike L, Hansen L, et al. Cellular tensegrity: exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int. Rev. Cytol. 1994;150:173–224.
- Johnson PR. Role of human airway smooth muscle in altered extracellular matrix production in asthma. Clin. Exp. Pharmacol. Physiol. 2001;28:233–236.10.1046/j.1440-1681.2001.03426.x
- Johnson PR, Black JL, Carlin S, et al. The production of extracellular matrix proteins by human passively sensitized airway smooth-muscle cells in culture: the effect of beclomethasone. Am. J. Respir. Crit. Care Med. 2000;162:2145–2151.10.1164/ajrccm.162.6.9909111
- Johnson PR, Burgess JK, Underwood PA, et al. Extracellular matrix proteins modulate asthmatic airway smooth muscle cell proliferation via an autocrine mechanism. J. Allergy Clin. Immunol. 2004;113:690–696.10.1016/j.jaci.2003.12.312
- Sasaki T, Fassler R, Hohenester E. Laminin: the crux of basement membrane assembly. J. Cell Biol. 2004;164:959–963.10.1083/jcb.200401058
- Heino J, Kapyla J. Cellular receptors of extracellular matrix molecules. Curr. Pharm. Des. 2009;15:1309–1317.10.2174/138161209787846720
- De Arcangelis A, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 2000;16:389–395.10.1016/S0168-9525(00)02074-6
- McCall-Culbreath KD, Zutter MM. Collagen receptor integrins: rising to the challenge. Curr. Drug Targets. 2008;9:139–149.
- Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Curr. Opin. Cell Biol. 1995;7:736–747.10.1016/0955-0674(95)80117-0
- Yee KL, Weaver VM, Hammer DA. Integrin-mediated signalling through the MAP-kinase pathway. IET Syst. Biol. 2008;2:8–15.10.1049/iet-syb:20060058
- Aikawa R, Nagai T, Kudoh S, et al. Integrins play a critical role in mechanical stress-induced p38 MAPK activation. Hypertension. 2002;39:233–238.10.1161/hy0202.102699
- Iwashita J, Ito Y, Yokoo M, et al. Akt induces down regulation of MUC5AC production in NCI-H292 human airway epithelial cells cultured on extracellular matrix. Biosci. Biotechnol. Biochem. 2014;78:212–221.10.1080/09168451.2014.877829
- Ge B, Gram H, Di Padova F, et al. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–1294.10.1126/science.1067289
- Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann. Med. 2006;38:116–125.10.1080/07853890600585795
- Christie PE, Jonas M, Tsai CH, et al. Increase in laminin expression in allergic airway remodelling and decrease by dexamethasone. Eur. Respir. J. 2004;24:107–115.10.1183/09031936.04.00013303
- Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 2009;180:388–395.10.1164/rccm.200903-0392OC
