Abstract
Huperzia serrata has been used as a Chinese folk medicine for many years. It contains huperzine A, which has a protective effect against memory deficits in animal models; however, it is unclear if H. serrata extract exerts any effects in Alzheimer’s disease (AD) models. We used H. serrata collected in Japan and determined its huperzine A content using HPLC. We determined its inhibitory effects on acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) activity. H. serrata extract (30 mg/kg/day) and donepezil (10 mg/kg/day) were orally administrated for 7 days. After repeated administration, we performed the Y-maze and passive avoidance tests. H. serrata extract contained 0.5% huperzine A; H. serrata extract inhibited AChE, but not BuChE. H. serrata extract ameliorated cognitive function in mice. These results indicate that Japanese H. serrata extract ameliorates cognitive function deficits by inhibiting AChE. Therefore, H. serrata extract may be valuable for the prevention or treatment of dementia in AD.
Repeated treatment with Huperzia serrata extract (30 mg/kg/day) for six or seven days ameliorated the memory impairment induced by scopolamine in the two behavioral tests.
Introduction
Alzheimer’s disease (AD) is one of the most notable forms of dementia, and is characterized by progressive deficits in memory and cognitive function.Citation1) Eventually, the ability to perform simple daily tasks is lost. A recent report estimated that by 2050, the number of patients with AD worldwide would increase fourfold to 36 million patients.Citation2) AD pathology is characterized by accumulation of β-amyloid protein (Aβ), which forms senile plaques. Aβ affects neuronal cells and induces phosphorylation of the tau protein,Citation3) subsequently causing neurofibrillary changes and neuronal death. As AD progresses, cholinergic neurons are lost in the basal forebrain and activity of choline acetyltransferase (ChAT), an acetylcholine synthesizing enzyme, is reduced.Citation4,5) Furthermore, ChAT activity is associated with acetylcholinesterase (AChE) activity and cognitive disorders.Citation6) AChE inhibitors, such as donepezil and galantamine, were developed to treat AD symptoms.Citation7,8)
Scopolamine, a muscarinic anticholinergic drug, suppresses central cholinergic neurons and impairs learning and memory.Citation9) Thus, it is widely used to generate dementia models by impairing learning and memory.Citation10,11)
Huperzia serrata, known as Chinese club moss, is used in traditional Chinese herbal medicine to treat contusions, schizophrenia, strains, and swelling.Citation12) Huperzine A, a novel Lycopodium alkaloid, can be isolated from H. serrata.Citation13) Other plants, such as Huperzia selago and Lycopodium selago, are known to contain huperzine A.Citation14) The molecular weight of huperzine A is 242.32, and its half-life in blood is approximately 5 h.Citation15) Huperzine A, reversibly and selectively inhibits AChE, crosses the blood–brain barrier (BBB) and prevents acetylcholine (ACh) breakdown. Huperzine A may ameliorate the memory deficits in rodents and monkeys via AChE inhibition.Citation16,17)
In the present study, we investigated the effects of Japanese H. serrata extract on inhibition of AChE activity and memory. Specifically, mice with scopolamine-induced cognitive impairment were subjected to Y-maze and passive avoidance tests.
Materials and methods
Animals
All experiments were performed using six-week-old male ICR mice (Japan SLC, Hamamatsu, Japan). The mice were housed at 24 ± 2 °C under a 12/12 h light/dark cycle (lights on 8:00 am) and had ad libitum access to food and water. All procedures related to animal care and treatment conformed to the animal care guidelines issued by the Gifu Pharmaceutical University Animal Experiment Committee.
H. serrata extract preparation and HPLC analysis
H. serrata was collected from Yamagata city in the Gifu Prefecture. Ground whole of H. serrata (298 g) was dried and extracted using ethanol (15 h at room temperature); and subsequent evaporation produced a dry solid as a standard extraction (59.67 g). A portion of the standard extraction was dissolved in 1% (v/v) HCl in H2O and then extracted using ethyl acetate (EtOAc). The aqueous layer was removed, Na2CO3 was added to make it basic (pH 9.0), and the basic solution was extracted with CHCl3. The soluble CHCl3 portion was evaporated to obtain an alkaloidal enriched fraction (AEF) (2.9 g).
HPLC instrument consisted of a Shimadzu (Kyoto, Japan) 6A pump, Shimadzu CTO-10Avp column oven, Shimadzu SPD-10A UV–vis spectrophotometry detector, and a SPD-M10Avp UV–vis diode array detector. Separation was accomplished over a Capcell Pak C18 column (MG II, 5 μm, 4.6 mm i.d.×250 mm; SHISEIDO, Tokyo, Japan). The elution conditions were as follows: flow rate, 1.0 mL/min; column temperature 40 °C; injection volume, 10 μL; and detection, UV absorption (308 nm). The solvent system used was isocratic methanol/water/0.1% (v/v) trifluoroaceticacid (20:80).
Stock solutions of a huperzine A [(−)—huperzine A, purity > 97%, LKT Laboratories Inc., St. Paul, MN, USA] standard were prepared at a concentration of 1.0 mg/mL. A three-point calibration curve was prepared using diluted standard huperzine A at concentration ranging from 1.0 to 8.0 μg/mL. Parts of the standard extraction and AEF were dissolved in methanol and passed through a 0.45-μm Millipore poly syringe filter into a measuring flask, and were then prepared at a concentration of 10 mg/mL. The H. serrata extract used in subsequent was the AEF.
Measurement of AChE activity
Mice were sacrificed, and the whole brains were removed. The brains were added into 1% Tris-HCl buffer (4× the brain weight; pH 7.4) and homogenized. Homogenates were centrifuged at 3500 rpm for 10 min at 4 °C. The supernatant was used as AChE enzyme source and stored at −80 °C use. AChE activity was measured using an Amplite™ Colorimetric AChE Assay Kit (AAT Bioquest®, Sunnyvale, CA, USA). H. serrata extract, huperzine A (LKT Laboratories Inc., St. Paul, MN, USA), and donepezil (Wako, Osaka, Japan) were dissolved in 1% dimethyl sulfoxide (DMSO). The test samples were incubated for 30 min after being mixed with a 50-fold diluted AChE enzyme source and each drug (9:1). Assays were performed according to the manufacturer’s protocol. Absorption was measured at 405 and 412 nm using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Measurement of butyrylcholinesterase (BuChE) activity
Mice were anesthetized with pentobarbital; blood was collected from the postcaval vein. Blood samples were centrifuged at 3500 rpm for 10 min at 4 °C. The supernatant was used as a BuChE enzyme source and stored at −80 °C until use. BuChE activity was measured using cholinesterase Wako (Wako, Osaka, Japan). Each drug was dissolved in 1% DMSO. Test samples were incubated for 30 min after being mixed with a fivefold diluted BuChE enzyme source and drug (9:1). Assays were performed according to the manufacturer’s protocol, with slight modifications. The test sample, the coloration test solution, and the substrate solution were added at 2.5, 200, and 50 μL, respectively. Absorption was measured at 405 nm using a microplate reader.
Y-maze test
H. serrata extract (30 mg/kg/day) and donepezil (10 mg/kg/day) were suspended in 0.5% carboxymethyl cellulose (CMC), and administered orally once a day. On day six of drug treatment, the Y-maze test was performed to evaluate short-term memory. The Y-maze apparatus used in this experiment consisted of three gray arms (40 cm in length, 10 cm in width, and 12 cm in height). One hour prior to the behavioral test, mice were treated with H. serrata extract or donepezil. Thirty minutes after H. serrata extract or donepezil treatment and 30 min before the behavioral test, cognitive impairment was induced by administering scopolamine (3 mg/kg, i.p.; Sigma Aldrich, St. Louis, MO, USA). Saline (10 mL/kg) was administered to the control group instead of scopolamine. At the beginning of the behavioral test, mice were placed in one fixed arm, and the number of arm alternation was observed. An alternation was defined as entrance into each of the three arms in rotation. Mouse behavior was recorded using a video camera, and alternations were calculated according to the following mathematical formula: Alternation (%) = ([The number of alternations]/[Total arm invasion − 2]) × 100. The Y-maze arms were cleaned with 70% ethanol after every test to remove odors and excrement.
Passive avoidance test
H. serrata extract (30 mg/kg/day) and donepezil (10 mg/kg/day) were each suspended in 0.5% CMC, and administrated orally once per day. On days 5–7, the passive avoidance test was performed to evaluate memory. The experimental apparatus comprised a well-lit chamber (15.5 cm long, 9.6 cm wide, and 18 cm high) and a dark chamber (32 cm long, 32 cm wide, and 27 cm high). The two chambers were separated by a sliding guillotine door. On day five, mice were habituated to the apparatus. Mice were placed in the well-lit chamber, and 30 seconds later the door was opened. When the hind legs of the mouse entered the dark chamber, the door closed, and 30 s later the mice was removed from the chamber. The training trial was performed 24 h after the habituation. In this trial, mice received a foot shock (0.16 mA, 2 s) upon entering the dark chamber. A retention trial was performed 24 h after the training trial. Latency was calculated as the time it took for a mouse to enter the dark chamber after the door opened. Latency was measured up to 600 s using a stopwatch. Mice were treated with H. serrata extract or donepezil 1 h before each trial. Scopolamine (3 mg/kg, i.p.) was administered 30 min after H. serrata extract or donepezil treatment and 30 min before the behavioral test to induce cognitive impairment. The apparatus was cleaned with 70% ethanol after every test to remove odors and excrements.
Statistical analysis
Data are represented as the mean ± standard error of the mean (SEM). The results were analyzed using Student’s t-tests or Dunnett’s test. Statistical significance was considered at a value of p < 0.05.
Results
H. serrata extract contained huperzine A
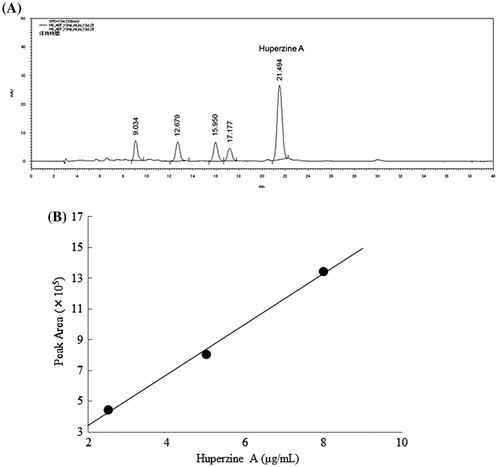
HPLC analysis of the AEF showed that the highest peak was detected at 21.494 min. The component of the peak was huperzine A (Fig. (A)). Stock solutions of huperzine A standard were prepared at a concentration of 1.0 mg/mL. A three-point calibration curve was prepared using diluted standard huperzine A at concentration ranging from 1.0 to 8.0 μg/mL (Fig. (B)). A portion of the AEF was dissolved in methanol and passed through a 0.45 mm Millipore poly syringe filter into a measuring flask, and then it was prepared at a concentration of 10 mg/mL (n = 3). The HPLC analysis revealed that huperzine A was the major alkaloid in the AEF. Huperzine A content was 0.5 ± 0.003% in the AEF.
H. serrata extract inhibited AChE
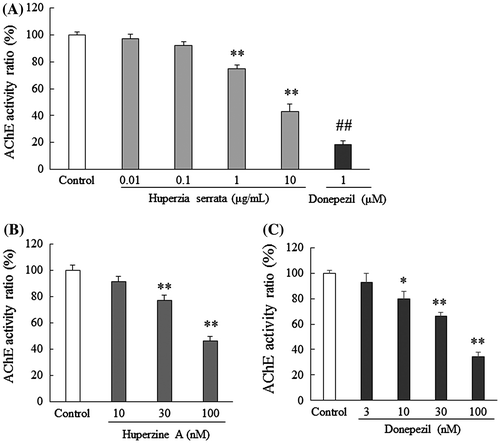
Next, we investigated the effects of H. serrata extract on AChE. H. serrata extract inhibited AChE activity in a concentration-dependent manner, and its effects were significant at concentrations of 1–10 μg/mL (p < 0.01, Fig. (A)). Both huperzine A, which was contained within the H. serrata extract, and donepezil inhibited AChE. Huperzine A (30 and 100 nM; p < 0.01) and donepezil significantly inhibited AChE (10, 30, and 100 nM; Fig. (B) and (C)). The 50% inhibitory concentration (IC50) of the extract of H. serrata, huperzine A and donepezil were 5.96 μg/mL, 87.17, and 55.56 nM, respectively.
Fig. 2. Both H. serrata extract and huperzine A inhibit AChE activity.
Notes: Each graph shows AChE activity after treatment with (A) H. serrata extract, (B) huperzine A, or (C) donepezil. The AChE inhibitory activity observed in the control was considered to be 100%. H. serrata extract (10 μg/mL) corresponds to 0.206 μM of huperzine A. Values are expressed as the mean ± SEM (n = 3 to 5). ##p < 0.01 vs. control (Student’s t-test). **p < 0.01, *p < 0.05 vs. control (Dunnett’s test).
H. serrata extract had no effect on BuChE
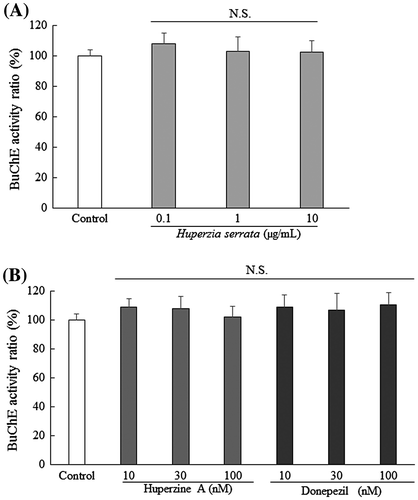
To examine the selectivity H. serrata extract on AChE, we determined whether it inhibited BuChE. The same drug concentrations were used as in the AChE experiment. H. serrata extract had no effect on BuChE at any concentration (Fig. (A)). Similarly, neither huperzine A nor donepezil inhibited BuChE (Fig. (B)). These results suggest that H. serrata extract, huperzine A, and donepezil are of high selectivity for AChE.
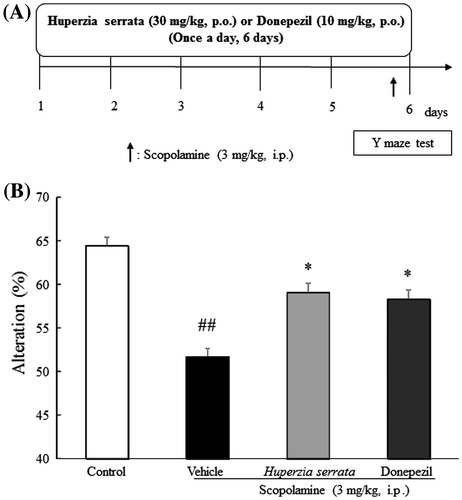
Effects of H. serrata extract on mouse behavior in the Y-maze test
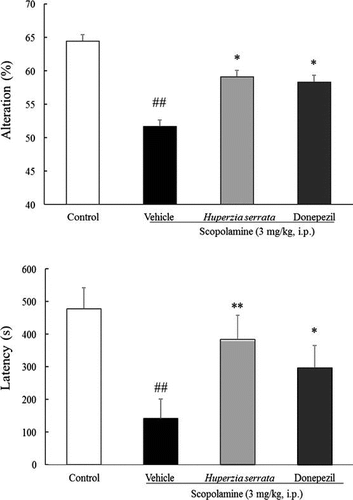
Next, to investigate the effects of H. serrata extract on cognitive function, we employed the Y-maze test in a scopolamine-induced cognitive impairment model. Mice were tested in the Y-maze after six days of drug treatment (Fig. (A)). Scopolamine treatment significantly decreased the number of alterations compared with the control group (p < 0.01). H. serrata extract (30 mg/kg, p.o.) and donepezil (10 mg/kg, p.o.) significantly ameliorated cognitive impairment induced by scopolamine (p < 0.05; Fig. (B)). These results suggest that repeated H. serrata extract administration ameliorates scopolamine-induced memory impairment.
Fig. 4. Effects of H. Serrata extract on short-term memory in a scopolamine-induced cognitive impairment mice model using the Y-maze test.
Notes: (A) An outline of the behavioral test is shown. H. serrata extract (30 mg/kg/day) or donepezil (10 mg/kg/day) was administered orally once a day for 6 days. On day 6, the Y-maze test was performed. (B) Effects of H. serrata on short-term memory in a scopolamine-induced cognitive impairment mouse model. Donepezil is the positive control. Values are expressed as the mean ± SEM (n = 9 or 10). ##p < 0.01 vs. control mice (Student’s t-test). *p < 0.05 vs. vehicle mice (Student’s t-test).
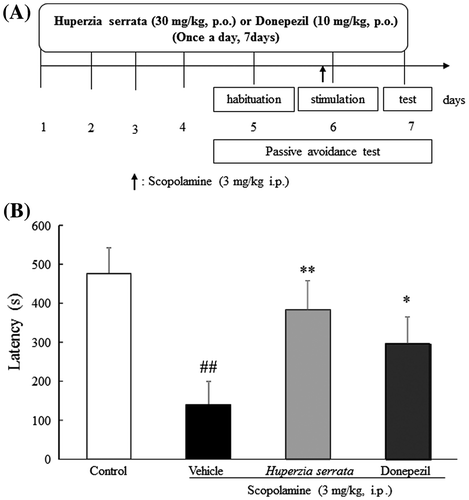
Effects of H. serrata extract on the passive avoidance test
We performed the passive avoidance test 5–7 days after drug treatment (Fig. (A)). On day seven, scopolamine treatment significantly decreased the latency to enter the dark box compared to control (p < 0.01). H. serrata extract (30 mg/kg, p.o.) or donepezil (10 mg/kg, p.o.) significantly ameliorated the latency to enter the dark box (p < 0.01, p < 0.05, respectively, Fig. (B)). These results suggest that repeated H. serrata extract treatment improves memory.
Fig. 5. Effects of H. Serrata extract on memory in a scopolamine-induced cognitive impairment mouse model using the passive avoidance test.
Notes: (A) An outline of the behavioral test is shown. H. serrata extract (30 mg/kg/day) or donepezil (10 mg/kg/day) was administered orally once a day for 7 days. On days 5–7, the passive avoidance test was performed. (B) Effects of H. serrata on learning and memory in a scopolamine-induced cognitive impairment mouse model. Donepezil is the positive control. Values are expressed as the mean ± SEM (n = 9 or 10). ##p < 0.01 vs. control mice (Student’s t-test). **p < 0.01, *p < 0.05 vs. vehicle mice (Student’s t-test).
Discussion
Content of huperzine A in H. serrata extract was 0.5% used in this study. The content of huperzine A in other plants, such as H. selago and L. selago, are known to contain huperzine A were 0.044–0.159%.Citation14,18) Moreover, in H. serrata it has been reported that the content of huperzine A was less than 0.02%.Citation19) These results suggest that H. serrata collected by in Yamagata city, Gifu prefecture is plant resource containing abundant huperzine A.
In the present study, we examined the effects of Japanese H. serrata extract on AChE. Donepezil and huperzine A reportedly inhibit AChE in a concentration-dependent manner.Citation20,21) H. serrata extract also inhibited AChE in a concentration-dependent manner. These results suggest that the effects H. serrata extract on AChE are partly associated with the effect of huperzine A. Huperzine A exists as two stereoisomers; (+)—huperzine A and (−)—huperzine A. (−)—Huperzine A more potently inhibits than (+)—huperzine A.Citation22) These results suggest that huperzine A, contained within H. serrata extract, is associated with AChE inhibition.
BuChE, a cholinesterase, degrades ACh as well AChE. The present study revealed that neither huperzine A nor donepezil inhibited BuChE under the same concentrations that inhibited AChE. Huperzine A inhibits BuChE at much greater concentrations than those that inhibit AChE.Citation17,20) Therefore, huperzine A selectively inhibited AChE. H. serrata extract did not inhibit BuChE at the same concentrations of AChE. These results suggest that H. serrata extract inhibits AChE are highly selective.
A mouse model of scopolamine-induced cognitive impairment is widely used as an animal model of that mimics the memory deficits observed in patients with AD. Alternation behavior in the Y-maze test represents short-term and working memory.Citation23,24) The passive avoidance test is used to evaluate long-term and learning-associated memory.Citation25) Scopolamine administration-induced memory impairment, as evidenced by decreased alternation number and latency in these behavioral tests. Repeated treatment with H. serrata extract (30 mg/kg/day, p.o.) or donepezil (10 mg/kg/day, p.o.) ameliorated the memory impairment induced by scopolamine in both behavioral tests. These results suggest that H. serrata extract improved the scopolamine-induced cognitive impairment in mice by activating the cholinergic system via AChE inhibition. Furthermore, in the two behavioral tests, H. serrata extract (30 mg/kg/day, p.o.) and donepezil (10 mg/kg/day, p.o.) were similarly effective. H. serrata extract at 30 mg/kg corresponds to 1.4 mg/kg huperzine A. Therefore, in the present study huperzine A is more potent than donepezil. However, the AChE inhibitory effect of donepezil was more potent than that of huperzine A. Huperzine A reportedly increases norepinephrine and dopamine levels and antagonizes glutamic acid activity.Citation26,27) These findings indicate that other constituents present in H. serrata extract and other effects of huperzine A may contribute to improving cognitive impairment. In previous reports, both of huperzine A and donepezil increased in the level of ACh in the rat cerebral cortex. Moreover, a lower dose (0.5 μmol/kg) of huperzine A and a higher dose (4 μmol/kg) of donepezil affected similarly.Citation28) Additionally, huperzine A could cross the BBB, higher oral bioavailability, and longer AChE inhibition duration than donepezil,Citation14) suggesting that H. serrata extract may be also associated with the activity in vivo.
We also investigated locomotor activity using the open field test (Fig. S1). Anticholinergic drugs such as scopolamine increase locomotor activity in the open field test and cause hyperactivity.Citation29–31) In the present study, while scopolamine treatment significantly increased locomotor activity in mice; neither H. serrata extract (30 mg/kg, p.o.) nor donepezil (10 mg/kg, p.o.) showed an effect. These results suggest that treatment with H. serrata extract or donepezil improves memory without influencing locomotor behavior in mice.
In conclusion, Japanese H. serrata extract and its constituent, huperzine A, were effective in improving cognitive function, including short-term and long-term memory. The mechanism involved in memory improvement induced by H. serrata extract might be associated with regulation of the cholinergic system via AChE inhibition. These findings indicate that H. serrata extract is useful for prophylaxis of memory impairment-related diseases.
Author contribution
H.H. made experimental plans of the manuscript and organized all of the studies. O.T. and Y.Y. and I.M. conceived and performed the experiments. A.N. and O.M performed experiment and analyzed the data on Fig. . T.K. and S.M. and T.T. and H.H. participated in its design and coordination and helped to draft the manuscript. All authors discussed results and commented on the manuscript.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2015.1052773.
Supplemental Materials
Download Zip (36.3 KB)Acknowledgments
We would like to thank Mr. Isao Sakurai at Kawamura Hospital and Mr. Muneaki Yokoyama at Lequio Pharma Co. for their cooperation, support, and advice regarding this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Watson WJ, Seiden HS. Alzheimer’s disease: a current review. Can. Fam. Physician. 1984;30:595–599.
- Gomez-Ramirez J, Wu J. Network-based biomarkers in Alzheimer’s disease: review and future directions. Front. Aging Neurosci. 2014;6:12.
- Takashima A, Noguchi K, Sato K, et al. Tau protein kinase I is essential for amyloid beta-protein-induced neurotoxicity. Proc. Natl. Acad. Sci. USA. 1993;90:7789–7793.10.1073/pnas.90.16.7789
- Francis PT, Palmer AM, Snape M, et al. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J. Neurol. Neurosurg. Psychiatry. 1999;66:137–147.10.1136/jnnp.66.2.137
- Kar S, Slowikowski SP, Westaway D, et al. Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer’s disease. J. Psychiatry Neurosci. 2004;29:427–441.
- Gil-Bea FJ, García-Alloza M, Domínguez J, et al. Evaluation of cholinergic markers in Alzheimer’s disease and in a model of cholinergic deficit. Neurosci. Lett. 2005;375:37–41.10.1016/j.neulet.2004.10.062
- Sugimoto H, Yamanish Y, Iimura Y, et al. Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr. Med. Chem. 2000;7:303–339.10.2174/0929867003375191
- Lilienfeld S. Galantamine—a novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer’s disease. CNS Drug Rev. 2002;8:159–176.
- Anagnostaras SG, Maren S, Fanselow MS. Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol. Learn. Mem. 1995;64:191–194.10.1006/nlme.1995.0001
- Yang JH, Han SJ, Ryu JH, et al. Ginsenoside Rh2 ameliorates scopolamine-induced learning deficit in mice. Biol. Pharm. Bull. 2009;32:1710–1715.10.1248/bpb.32.1710
- Uchida S, Endo S, Akita K, et al. The cyanine dye NK-4 improves scopolamine-induced memory impairments in mice. Biol. Pharm. Bull. 2012;35:1831–1835.10.1248/bpb.b12-00370
- Guo B, Xu L, Wei Y, et al. [Research advances of Huperzia serrata (Thunb.) Trev]. Zhongguo Zhong Yao Za Zhi. 2009;34:2018–2023.
- Zhang HY, Zheng CY, Yan H, et al. Potential therapeutic targets of huperzine A for Alzheimer’s disease and vascular dementia. Chem. Biol. Interact. 2008;175:396–402.10.1016/j.cbi.2008.04.049
- Ma X, Tan C, Zhu D, et al. Huperzine A from Huperzia species—an ethnopharmacolgical review. J. Ethnopharmacol. 2007;113:15–34.10.1016/j.jep.2007.05.030
- Suh WH, Suslick KS, Suh YH. Therapeutic agents for Alzheimer’s disease. Curr. Med. Chem. 2005;5:259–269.
- Tang XC, Han YF. Pharmacological profile of huperzine A, a novel acetylcholinesterase inhibitor from Chinese herb. CNS Drug Rev. 1999;5:281–300.
- Ye JW, Cai JX, Wang LM, et al. Improving effects of huperzine A on spatial working memory in aged monkeys and young adult monkeys with experimental cognitive impairment. J. Pharmacol. Exp. Ther. 1999;288:814–819.
- Szypuła WJ, Kiss AK, Pietrosiuk A, et al. Determination of huperzine a in Huperzia selago plants from wild population and obtained in in vitro culture by high-performance liquid chromatography using a chaotropic mobile phase. Acta Chromatographica. 2011;23:339–352.10.1556/AChrom.23.2011.2.11
- Ferreira A, Rodrigues M, Fortuna A, et al. Huperzine A from Huperzia serrata: a review of its sources, chemistry, pharmacology and toxicology. Phytochem. Rev. 2014. DOI 10.1007/s11101-014-9384-y.
- Bai DL, Tang XC, He XC. Huperzine A, a potential therapeutic agent for treatment of Alzheimer’s disease. Curr. Med. Chem. 2000;7:355–374.10.2174/0929867003375281
- Kasa P, Papp H, Kasa P Jr, et al. Donepezil dose-dependently inhibits acetylcholinesterase activity in various areas and in the presynaptic cholinergic and the postsynaptic cholinoceptive enzyme-positive structures in the human and rat brain. Neuroscience. 2000;101:89–100.10.1016/S0306-4522(00)00335-3
- Saxena A, Qian N, Kovach IM, et al. Identification of amino acid residues involved in the binding of huperzine A to cholinesterases. Protein Sci. 1994;3:1770–1778.10.1002/pro.v3:10
- Mandillo S, Tucci V, Hölter SM, et al. Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol. Genomics. 2008;34:243–255.10.1152/physiolgenomics.90207.2008
- Kumar H, Kim BW, Song SY, et al. Cognitive enhancing effects of alpha asarone in amnesic mice by influencing cholinergic and antioxidant defense mechanisms. Biosci. Biotechnol. Biochem. 2012;76:1518–1522.10.1271/bbb.120247
- Oike Y, Hata A, Mamiya T, et al. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum. Mol. Genet. 1999;8:387–396.10.1093/hmg/8.3.387
- Zhu XD, Giacobini E. Second generation cholinesterase inhibitors: Effect of (L)-huperzine-A on cortical biogenic amines. J. Neurosci. Res. 1995;41:828–835.10.1002/(ISSN)1097-4547
- Gordon RK, Nigam SV, Weitz JA, et al. The NMDA receptor ion channel: a site for binding of huperzine A. J. Appl. Toxicol. 2001;21:S47–S51.10.1002/(ISSN)1099-1263
- Wang R, Yan H, Tang XC. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol. Sin. 2006;27:1–26.10.1111/aphs.2006.27.issue-1
- Shannon HE, Peters SC. A comparison of the effects of cholinergic and dopaminergic agents on scopolamine-induced hyperactivity in mice. J. Pharmacol. Exp. Ther. 1990;255:549–553.
- Ukai M, Kobayashi T, Kameyama T. Characterization of the effects of scopolamine on the habituation of exploratory activity: differential effects of oxotremorine and physostigmine. Gen. Pharmacol. 1994;25:433–438.10.1016/0306-3623(94)90193-7
- Hornick A, Schwaiger S, Rollinger JM, et al. Extracts and constituents of Leontopodium alpinum enhance cholinergic transmission: Brain ACh increasing and memory improving properties. Biochem. Pharmacol. 2008;76:236–248.10.1016/j.bcp.2008.04.015