Abstract
Most currently available vaccines rely on the induction of long-lasting protective humoral immune responses by memory B cells and plasma cells. Antibody responses against most antigens require interactions between antigen-specific B cells and CD4+ T cells. Follicular helper T cells (TFH cells) are specialized subset of T cells that provide help to B cells and are essential for germinal center formation, affinity maturation, and the development of high-affinity antibodies. TFH-cell differentiation is a multistage process involving B-cell lymphoma 6 and other transcription factors, cytokines, and costimulation through inducible costimulator (ICOS) and several other molecules. This article reviews recent advances in our understanding of TFH cell biology, including their differentiation, transcriptional regulation, and function.
Graphic Abstract
Development of follicular helper T cells is controlled by TCR, cytokine, and ICOS-mediated signals, and downstream transcription factors.
Cognate interactions between antigen (Ag)-specific B cells, CD4+ T cells, and dendritic cells (DCs) in response to foreign Ags lead to the formation of germinal centers (GCs). GCs are specialized structures in B-cell follicles of secondary lymphoid organs. In GCs, somatic hypermutation of immunoglobulin variable genes is induced in B cells and selection of high-affinity B cells takes placeCitation1), followed by the generation of long-lived memory B cells or plasma cells. This process ensures the development of long-lived humoral immunity after infection or vaccination with T cell-dependent Ag.
It has been known that CD4+ T cells are required for the formation of productive GC responsesCitation1–3), as well as for generating Ag-specific memory B cells and plasma cells. However, the exact nature of the CD4+ T cells that provides help to B cells remained enigmatic. More than two decades ago, an in vitro study by Coffman and Mosman first discovered the heterogeneity of effector T cells, which were then named as T-helper 1 (TH1) or TH2 cellsCitation4). It showed that TH2 cells were the major TH subset engaged in helping B cells by secreting interleukin 4 (IL-4), IL-5, IL-10, and IL-13. TH1 cells also contributed to the regulation of antibody response by producing interferon-γ (IFN-γ) and inducing B-cell class switching toward IgG2a. The TH1/TH2 dichotomy has dominated the field of immune regulation until recently. However, it has been unclear whether those cells really represent the cells which engage in B cell help in lymphoid organs.
In recent years, T follicular helper (TFH) cells have been recognized as the key cell type required for the formation of GCs and the generation of long-lived humoral memoryCitation5). Similar to other CD4+ T-cell lineages (TH1, TH2, TH17, and regulatory T cells), the generation of TFH cells requires signaling pathways activated downstream of cytokines and cell surface molecules, and the subsequent activation of transcription factors. Here, I discuss recent advances in understanding the requirements for the generation and acquisition of effector function by TFH cells.
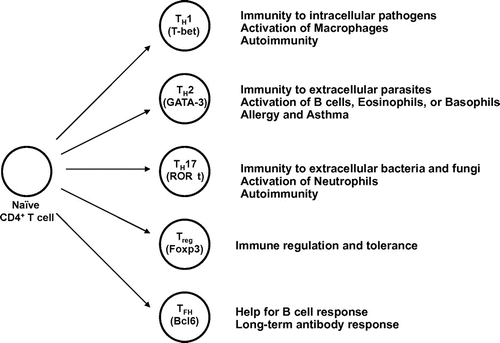
I. T follicular helper cells
The presence of CD4+ T cells expressing CXCR5, a chemokine receptor required for the migration into B-cell follicles, was first reported in 1999Citation6). CXCR5 was up-regulated on antigen-specific CD4+ T cells in the lymph nodes in Ag-immunized mice. Subsequently in the early 2000, the presence and function of human CXCR5+CD4+ T cells have been revealed. CD4+ T cells in human tonsils were found to express CXCR5. Importantly, such CXCR5+CD4+ T cells were potent to induce antibody production by B cells in vitro, relative to CXCR5− counterparts. On the basis of their localization and functions, tonsillar CXCR5+ CD4+ T cells were designated as TFH cellsCitation7–9). A superior capacity of CXCR5+ TFH cells to induce B-cell activation was confirmed in mouse systemCitation10). TFH cells show distinct cytokine production and gene expression pattern from TH1, TH2, and TH17 cellsCitation11–13). TFH cells utilize TNF family molecule CD40L and the cytokine IL-21 and IL-4 to help B cellsCitation14–17). In 2009, the transcription factor Bcl6 was discovered to be an essential factor for TFH-cell generation in vivo in miceCitation18–20) and since then TFH cells have been recognized as a TH subset distinct from TH1, TH2, and TH17 cells (Fig. ).
II. Development of TFH cells
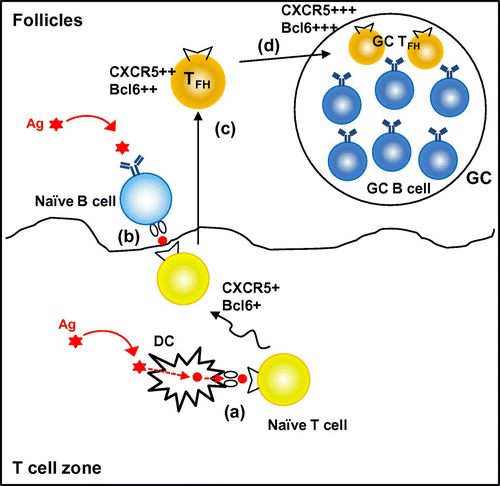
TFH-cell differentiation has been proposed to be a multistage process involving serial interaction of CD4+ T cells, first with DCs and then with antigen-presenting B cells (Fig. ). The presentation of antigen by DCs is required to initiate the TFH-cell differentiation programCitation21). Bcl6 induction occurs quite early during the DC priming stage and inducible costimulator ICOS is a key upstream molecule for induction of Bcl6 and thus instruction of TFH-cell differentiationCitation22). Initial Bcl6 induction and subsequent CXCR5 expression occur in T-cell zone; the activated CD4+ T cells then relocate to the T–B border, where early TFH cells interact with B cells to maintain Bcl6 expression for full differentiation and relocation into GCs as mature TFH cellsCitation18,21–24). The origin of TFH cells may not be restricted to naïve CD4+ T cells. Some evidences have suggested that other TH subsets including TH1, TH2, TH17, and regulatory T cells have a potential to acquire TFH-cell phenotype in GCs, although there is still controversy regarding this issueCitation5). Importantly, GC-TFH cells show heterogeneity in cytokine expression patterns under different immunization protocols and by different types of infectious agents. They are capable of expressing not only TFH signature cytokine IL-21, but also IL-4 or IFN-γCitation25,26).
Fig. 2. Multiple steps involved in TFH-cell development.
Notes: TFH-cell development requires serial interaction with DCs and antigen-specific B cells. Upon priming with dendritic cells, some of the activated T cells up-regulate a chemokine receptor, CXCR5, and transcription factors, including Bcl6 (a). The activated CD4+ T cells migrate toward the border of T-cell zone and B-cell follicle, where the T cells interact with antigen-specific B cells (b). This interaction with B cells further up-regulates CXCR5 and Bcl6, while down-regulates CCR7, which allows the T cells relocate to B-cell follicle (TFH) (c). Some of TFH cells participate in germinal center (GC) reaction (GC-TFH) (d).
Differentiation of CD4+ T cell into distinct effector TH subset is mediated in large part by exposure to various cytokines. For example, Th1 cells develop in the presence of IL-12 and IFN-γ, whereas differentiation of TH2 cells requires IL-4Citation27). TFH-cell differentiation is not exceptional. IL-6 appears to be the earliest cytokine signal involved in initiation of TFH-cell differentiation in vivo. In the absence of IL-6, early Bcl6+CXCR5+ TFH differentiation is severely impairedCitation28). Nonetheless, in vitro culture of murine CD4+ T cells with IL-6 failed to generate the T cells with complete TFH phenotypes. In vitro, IL-6 is an indeed potent inducer of IL-21Citation19,29). Although IL-6 was initially reported to drive CXCR5 and Bcl6 expression in vitroCitation19), neither Bcl6 nor CXCR5 was induced by IL-6 in vitro in two other studiesCitation29,30). These studies suggest that cytokines other than IL-6 may be involved in complete TFH differentiation. Indeed, a recent paper reports that type I IFNs (IFN-α/IFN-β) are potent cytokines to induce TFH-like cells in vitroCitation31). Type I IFNs promote sustained Bcl6 expression and induces CXCR5+PD1+ cells. Although type I IFNs fail to induce IL-21 by activated CD4 + T cells, addition of IL-6 compensates IL-21 production. Hence, type I IFNs + IL-6 appears to be the most successful conditions for murine TFH-cell differentiation in vitro, with the robust induction of CXCR5, PD1, Bcl6, and IL-21. In contrast, IL-2 interferes with TFH-cell development. It has been demonstrated that IL-2 administration impaired the differentiation of TFH cells, thus resulting in a reduction of GCs and long-lived antibody responsesCitation32). IL-2 signaling directly inhibits TFH-cell response, since IL-2Rα-deficient CD4+ T cells are more prone to become TFH cells in vivoCitation32).
In a past few years, significant progress has been made in understanding of human TFH-cell development. It appears that the development of TFH cells might differ between mice and humans. In humans, TGF-β acts with IL-12 or IL-23 to promote the expression of multiple TFH signature molecules, including CXCR5, IL-21, and Bcl6Citation33). This is in sharp contrast to murine CD4+ T cells, in which TGF-β inhibits the expression of TFH molecules such as IL-21, ICOS, and Bcl6Citation19,34,35), implying a difference in TFH differentiation mechanisms between species. In vitro human TFH cells generated in the presence of TGF-β and IL-12 or IL-23 had enhanced B cell help activity, indicating that TGF-β signaling is important for human TFH differentiation and function.
III. Transcriptional regulation of TFH-cell development
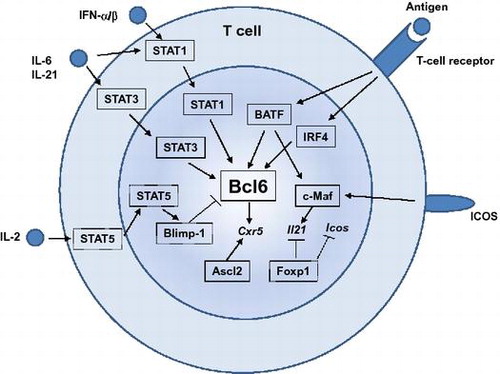
Bcl6 is highly expressed in murine and human TFH cells and is essential for TFH differentiation. In the absence of Bcl6, TFH cells are unable to form and subsequently GCs are not presentCitation18–20). Like other lineage-defining factors for other TH subsets, such as T-bet, GATA3, RORγt, or Foxp3, overexpression of Bcl6 not only enforces TFH-cell differentiation but also attenuates differentiation to other TH subsetsCitation18–20). In addition to Bcl6, several transcription factors are demonstrated to be required for TFH development. These include basic leucine zipper transcription factor (Batf), interferon regulatory factor 4 (IRF4), Maf, achaete-scute homologue 2 (Ascl2), Foxp1, and signal transducers and activators of transcription (STATs). Action of these transcription factors in TFH development is summarized in Fig. .
Fig. 3. Transcription factors that regulate TFH-cell development.
Notes: Many transcription factors are involved in TFH-cell development. Transcription factors shown in boxes with straight lines are the positive regulators, whereas those in boxes with dashed lines are the negative regulators for TFH development. Among them, Bcl6 plays a central role during TFH-cell development. STAT1, STAT3, BATF, and IRF4 positively regulate Bcl6 induction, whereas a STAT5-Blimp1 axis inhibits Bcl6 induction. Ascl2 is involved in CXCR5 expression. c-Maf is required for optimal IL-21 expression. Foxp1 negatively regulates IL-21 or ICOS expression.
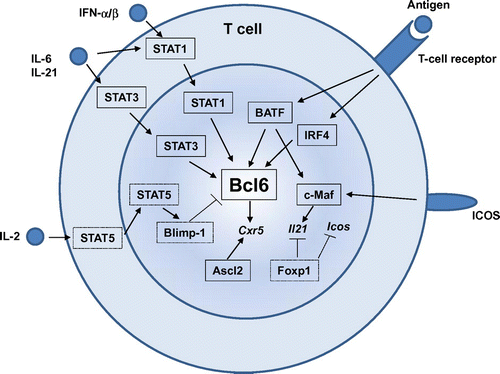
Batf and IRF4 are both essential for TFH-cell developmentCitation36,37) but they are required for multiple different CD4+ T-cell programs, including TH17Citation38,39), TH2Citation40), and TH9Citation41,42). Batf and IRF4 are induced in non-polarized T cells receiving stimulation and are found to be bound to the target genes independently of polarizing conditionsCitation43). Thus, it can be considered that these factors act as “pioneering factors” that enable expression and function of downstream cell-fate-determining factorsCitation44), including Bcl6.
c-Maf is highly expressed in TFH cells and c-Maf-deficient chimeric mice exhibited defected TFH cellsCitation45). TFH-cell impairment in the absence of c-Maf is due to defective induction or maintenance of IL-4 and IL-21 in an ICOS-dependent mannerCitation45). A recent study has demonstrated that Bcl6 and c-Maf cooperate to instruct human TFH-cell differentiation and that c-Maf induces IL-21 and CXCR5 expressionCitation46).
A basic helix-loop-helix (bHLH) transcription factor, Ascl2, is highly expressed in TFH cellsCitation30). Overexpression of Ascl2 specifically up-regulates CXCR5, but not Bcl6 or other TFH signatures in T cell in vitro, as well as accelerating T-cell migration to the B-cell follicles and TFH-cell development in vivoCitation30). Acute deletion of Ascl2 results in impaired TFH-cell development and GC responsesCitation30). Ascl2 directly regulates TFH-related genes including CXCR5 or CXCR4, whereas it inhibits expression of TH1 and TH17 signature genesCitation30).
The transcription factor Foxp1 is expressed in resting naïve CD4+ T cells and is required for quiescence and homing of naïve CD4+ T cellsCitation47). Naïve CD4+ T cells deficient in Foxp1 preferentially differentiate into TFH cells, which results in substantial enhancement of GC and antibody responsesCitation48). Foxp1 directly and negatively regulates IL-21. Foxp1 also dampens expression of ICOS and its downstream signaling at early stages of T-cell activation. Thus, Foxp1 is a critical negative regulator of TFH-cell differentiation.
In response to cytokine stimulation, STATs are also deeply involved in TFH differentiation Function of STATs during TFH differentiation is somewhat complex and overlapping. STAT3-deficient CD4+ T cells have a profound defect in TFH-cell differentiationCitation49,50). It has been shown that STAT3 is involved in IL-21 expression by CD4+ T cellsCitation35) and in down-regulation of IL-2Rα to limit TH1 cell differentiationCitation28). STAT1 is required for IL-6-mediated Bcl6 induction for early TFH differentiationCitation28). STAT1 is also activated by type I IFN and activated STAT1 directly regulates key TFH genes, including Bcl6 and CXCR5Citation31). Interestingly, IL-12-STAT4 axis positively regulates the differentiation of TFH cells in mice and human, although STAT4 appears to be more important in human TFH differentiationCitation51). In contrast, as mentioned above, IL-2 signals through STAT5 to limit TFH-cell differentiationCitation32,52,53). STAT5 induces the transcriptional repressor Blimp-1Citation52), which serves to repress Bcl6 and TFH-cell differentiationCitation18). Thus, the IL-2/STAT5/Blimp1 axis limits TFH-cell generation by suppressing Bcl6.
IV. Function of TFH cell
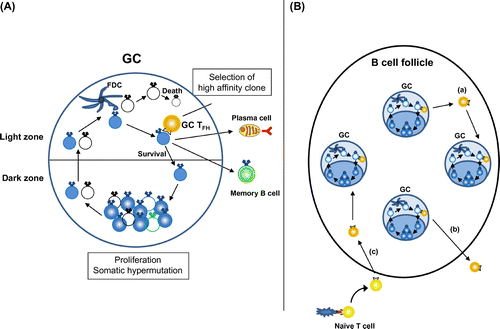
TFH cells are essentially required for the induction of GC. However, TFH cells play important roles even after GCs are established (Fig. ). Once TFH cells are generated, some of them further up-regulate CXCR5 and Bcl6 and migrate from follicles to GC (GC-TFH cells). As mentioned at the beginning, GC is the primary site of affinity maturation of B cells and recent evidences have shown that GC-TFH cells are essential for achieving the goal of GC responses, which is to generate and select GC B cells with higher affinity for the antigensCitation1). GC is divided into two anatomically distinct regions: the light zone (LZ) and the dark zone (DZ). GC B cells undergo proliferation and somatic hypermutation in the DZ and then migrate to the LZ. In the LZ, GC B cells capture antigen through surface immunoglobulin and present it. Selection of high-affinity GC B cells take place in LZ as a result of the interaction with cognate GC-TFH cells. GC-TFH cells discern the high-affinity GC B cells according to the level of presented antigenic peptide on their MHC class II and help high-affinity GC B cell clones to expand, hypermutate, or differentiate into long-lived plasma cells or memory B cells (Fig. ). These selection events are controlled by TFH-cell-derived signals, including ICOS, CD40L, and cytokines IL-4 and IL-Citation5). Recent intravital imaging revealed that GC-TFH cells continually scan the surface of many GC B cells in the LZ and form short-lived contacts which results in the selection of high-affinity B-cell clonesCitation54,55). Interestingly, GC-TFH cells are not confined to GC. Unlike GC B cells, which are clonally restricted, TFH cells distributed among all GCs and continually emigrated into the follicle and entered a different GCCitation55) (Fig. ). Moreover, newly activated TFH cells invade preexisting GCs, where they contribute to B-cell selection. Conceivably, the dynamic exchange of TFH cells between GCs ensures diversified and robust support for B-cell clonal expansion and affinity maturation.
Fig. 4. Function of TFH cells in GCs.
Notes: (A) GC-TFH cells localize in the light zone of GCs and scan the amount of antigenic peptide presented on GC B cells. GC B cells with high-affinity BCRs present high levels of antigenic peptide and get survival signals from GC-TFH cells. Selected high-affinity B cells differentiate into plasma cells or memory B cells, or migrate back to the dark zone of GC to undertake further round of proliferation and somatic hypermutation. (B) GC-TFH cells are not confined to GC. GC-TFH cells can emigrate to the follicles and enter different GCs (a) or relocate to the T-cell zone (b). Further, newly generated TFH cells can participate in preexisting GCs (c).
V. Memory TFH
Although the existence of TFH-cell memory was initially controversial, accumulating evidences now clearly show that TFH cells can give rise to resting memory T cells (Memory TFH cells) in both mice and humanCitation24,56–60). Memory TFH cells can be long-lived in the absence of the antigensCitation60). Upon leaving a GC, the TFH cells acquire a less activated and less polarized phenotype: Memory TFH cells down-regulate most of the canonical TFH markers, including PD1 and Bcl6, but up-regulate CCR7, IL-7Rα, and CD62LCitation57,58,60,61). Notably, memory TFH cells maintain low, but significant levels of CXCR5 expressionCitation57,58,62), which makes memory TFH cells distinguishable from other memory T cells. Memory TFH cells have the capacity to recirculate in bloodCitation59,63). Circulating TFH memory cells are a heterogeneous pool of cells having distinct functions which is broadly categorized according to the expression of several chemokine receptors. This issue is discussed in recent reviewsCitation64).
Memory TFH cells preferentially become TFH cells and GC-TFH cells upon reactivationCitation24,58,60). Thus, it appears that TFH memory cells are committed populations that are poised for the lineage-specific reexpression of effector molecules upon recall. How this could be achieved is currently unknown. But it has been demonstrated that distinct DNA methylation pattern of Gzmb locus between memory TFH and memory TH1 cellsCitation58), suggesting that epigenetic modification of lineage-specific gene locus reinforces the TFH lineage commitment. Functionally, memory TFH cells are potent in helping B-cell activationCitation65). A recent study demonstrated that memory TFH cells are required for efficient memory B-cell responsesCitation62). In the absence of memory TFH cells, activation of memory B cells to differentiate into plasma cells is compromised. Importantly, antigen-specific memory B cells, but not DCs, function as antigen-presenting cells for memory TFH cellsCitation62), suggesting that cognate interaction between memory TFH cells and memory B cells elicit efficient activation of memory antibody responses.
VI. Conclusion
Since the identification of TFH cells just over a decade ago, much has been discovered about the function of TFH cells and the molecules required for their differentiation. TFH cells are essential for the generation of affinity-matured antibodies; therefore, they have an obvious role not only in protective immunity against pathogen but also in the induction of a range of diseases, particularly autoimmune diseases. The progress in the understanding of TFH biology will lead to improved vaccine designs and better management of autoimmune diseases or allergy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes
This review was written in response to the author’s receipt of the Japan Society for Bioscience, Biotechnology, and Agrochemistry Award for the Encouragement of Young Scientist in 2011.
References
- Victora GD, Nussenzweig MC. Germinal centers. Annu. Rev. Immunol. 2012;30:429–457.10.1146/annurev-immunol-020711-075032
- Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202.10.1016/j.immuni.2007.07.009
- MacLennan IC. Germinal centers. Annu Rev. Immunol. 1994;12:117–139.10.1146/annurev.iy.12.040194.001001
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173.10.1146/annurev.iy.07.040189.001045
- Crotty S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011;29:621–663.10.1146/annurev-immunol-031210-101400
- Ansel KM, McHeyzer-Williams LJ, Ngo VN, et al. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 1999;190:1123–1134.10.1084/jem.190.8.1123
- Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000;192:1545–1552.10.1084/jem.192.11.1545
- Kim CH, Rott LS, Clark-Lewis I, et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J. Exp. Med. 2001;193:1373–1381.10.1084/jem.193.12.1373
- Schaerli P, Willimann K, Lang AB, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000;192:1553–1562.10.1084/jem.192.11.1553
- Campbell DJ, Kim CH, Butcher EC. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nat. Immunol. 2001;2:876–881.10.1038/ni0901-876
- Chtanova T, Tangye SG, Newton R, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 2004;173:68–78.10.4049/jimmunol.173.1.68
- Rasheed AU, Rahn HP, Sallusto F, et al. Follicular B helper T cell activity is confined to CXCR5hiICOShi CD4 T cells and is independent of CD57 expression. Eur. J. Immunol. 2006;36:1892–1903.10.1002/(ISSN)1521-4141
- Vinuesa CG, Cook MC, Angelucci C, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458.10.1038/nature03555
- Bentebibel SE, Schmitt N, Banchereau J, et al. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc. Nat. Acad. Sci. USA. 2011;108:E488–E497.10.1073/pnas.1100898108
- Bryant VL, Ma CS, Avery DT, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J. Immunol. 2007;179:8180–8190.10.4049/jimmunol.179.12.8180
- Casamayor-Palleja M, Khan M, MacLennan IC. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J. Exp. Med. 1995;181:1293–1301.10.1084/jem.181.4.1293
- Han S, Hathcock K, Zheng B, et al. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 1995;155:556–567.
- Johnston RJ, Poholek AC, DiToro D, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010.10.1126/science.1175870
- Nurieva RI, Chung Y, Martinez GJ, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005.10.1126/science.1176676
- Yu D, Rao S, Tsai LM, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468.10.1016/j.immuni.2009.07.002
- Goenka R, Barnett LG, Silver JS, et al. Cutting edge: dendritic cell-restricted antigen presentation initiates the follicular helper T cell program but cannot complete ultimate effector differentiation. J. Immunol. 2011;187:1091–1095.10.4049/jimmunol.1100853
- Choi YS, Kageyama R, Eto D, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946.10.1016/j.immuni.2011.03.023
- Deenick EK, Chan A, Ma CS, et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253.10.1016/j.immuni.2010.07.015
- Liu X, Yan X, Zhong B, et al. Bcl6 expression specifies the T follicular helper cell program in vivo. J. Exp. Med. 2012;209:1841–1852, S1841-1824.10.1084/jem.20120219
- Lüthje K, Kallies A, Shimohakamada Y, et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat. Immunol. 2012;13:491–498.10.1038/ni.2261
- Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 2009;10:385–393.10.1038/ni.1715
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793.10.1038/383787a0
- Choi YS, Eto D, Yang JA, et al. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J. Immunol. 2013;190:3049–3053.10.4049/jimmunol.1203032
- Eto D, Lao C, DiToro D, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE. 2011;6:e17739.10.1371/journal.pone.0017739
- Liu X, Chen X, Zhong B, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014;507:513–518.10.1038/nature12910
- Nakayamada S, Poholek AC, Lu KT, et al. Type I IFN induces binding of STAT1 to Bcl6: divergent roles of STAT family transcription factors in the T follicular helper cell genetic program. J. Immunol. 2014;192:2156–2166.10.4049/jimmunol.1300675
- Ballesteros-Tato A, Leon B, Graf BA, et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856.10.1016/j.immuni.2012.02.012
- Schmitt N, Liu Y, Bentebibel SE, et al. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat. Immunol. 2014;15:856–865.10.1038/ni.2947
- Nurieva RI, Chung Y, Hwang D, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149.10.1016/j.immuni.2008.05.009
- Suto A, Kashiwakuma D, Kagami S, et al. Development and characterization of IL-21-producing CD4+ T cells. J. Exp. Med. 2008;205:1369–1379.10.1084/jem.20072057
- Bollig N, Brustle A, Kellner K, et al. Transcription factor IRF4 determines germinal center formation through follicular T-helper cell differentiation. Proc. Nat. Acad. Sci. USA. 2012;109:8664–8669.10.1073/pnas.1205834109
- Ise W, Kohyama M, Schraml BU, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat. Immunol. 2011;12:536–543.10.1038/ni.2037
- Brüstle A, Heink S, Huber M, et al. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 2007;8:958–966.10.1038/ni1500
- Schraml BU, Hildner K, Ise W, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409.
- Lohoff M, Mittrucker HW, Prechtl S, et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc. Nat. Acad. Sci. USA. 2002;99:11808–11812.10.1073/pnas.182425099
- Jabeen R, Goswami R, Awe O, et al. Th9 cell development requires a BATF-regulated transcriptional network. J. Clin. Invest. 2013;123:4641–4653.10.1172/JCI69489
- Staudt V, Bothur E, Klein M, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202.10.1016/j.immuni.2010.07.014
- Ciofani M, Madar A, Galan C, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303.10.1016/j.cell.2012.09.016
- Vahedi G, A CP, Hand T, et al. Helper T-cell identity and evolution of differential transcriptomes and epigenomes. Immunol. Rev. 2013;252:24–40.10.1111/imr.12037
- Bauquet AT, Jin H, Paterson AM, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 2009;10:167–175.10.1038/ni.1690
- Kroenke MA, Eto D, Locci M, et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J. Immunol. 2012;188:3734–3744.10.4049/jimmunol.1103246
- Feng X, Wang H, Takata H, et al. Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells. Nat Immunol. 2011;12:544–550.10.1038/ni.2034
- Wang H, Geng J, Wen X, et al. The transcription factor Foxp1 is a critical negative regulator of the differentiation of follicular helper T cells. Nat. Immunol. 2014;15:667–675.10.1038/ni.2890
- Ma CS, Avery DT, Chan A, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119:3997–4008.10.1182/blood-2011-11-392985
- Ray JP, Marshall HD, Laidlaw BJ, et al. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40:367–377.10.1016/j.immuni.2014.02.005
- Schmitt N, Morita R, Bourdery L, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169.10.1016/j.immuni.2009.04.016
- Johnston RJ, Choi YS, Diamond JA, et al. STAT5 is a potent negative regulator of TFH cell differentiation. J. Exp. Med. 2012;209:243–250.10.1084/jem.20111174
- Nurieva RI, Podd A, Chen Y, et al. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. J. Biol. Chem. 2012;287:11234–11239.10.1074/jbc.M111.324046
- Gitlin AD, Shulman Z, Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509:637–640.10.1038/nature13300
- Shulman Z, Gitlin AD, Targ S, et al. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677.10.1126/science.1241680
- Bentebibel SE, Lopez S, Obermoser G, et al. (2013) Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci. Transl. Med. 2013;5:176ra132.
- Choi YS, Yang JA, Yusuf I, et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J. Immunol. 2013;190:4014–4026.10.4049/jimmunol.1202963
- Hale JS, Youngblood B, Latner DR, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–817.10.1016/j.immuni.2013.02.020
- Locci M, Havenar-Daughton C, Landais E, et al. Human circulating PD-1+CXCR3-CXCR5+ memory tfh cells are highly functional and correlate with broadly neutralizing hiv antibody responses. Immunity. 2013;39:758–769.10.1016/j.immuni.2013.08.031
- Weber JP, Fuhrmann F, Hutloff A. T-follicular helper cells survive as long-term memory cells. Eur. J. Immunol. 2012;42:1981–1988.10.1002/eji.v42.8
- Kitano M, Moriyama S, Ando Y, et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972.10.1016/j.immuni.2011.03.025
- Ise W, Inoue T, McLachlan JB, et al. Memory B cells contribute to rapid Bcl6 expression by memory follicular helper T cells. Proc. Nat. Acad. Sci. USA. 2014;111:11792–11797.10.1073/pnas.1404671111
- He J, Tsai LM, Leong YA, et al. Circulating precursor CCR7lo PD-1hi CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781.10.1016/j.immuni.2013.09.007
- Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35:436–442.10.1016/j.it.2014.06.002
- MacLeod MK, David A, McKee AS, et al. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J. Immunol. 2011;186:2889–2896.10.4049/jimmunol.1002955