Abstract
Homocysteine (Hcy) has been proposed to be a risk factor for cognitive dysfunction. We investigated the effects and the underlying mechanisms of action of propolis, which has antioxidant activity on Hcy-induced oxidative stress in vitro and in vivo. For the in vitro assays, neuroblastoma SH-SY5Y and glioblastoma U-251MG cells were cultured with Hcy and various concentrations of propolis. Cell death and reactive oxygen species production were significantly suppressed by propolis in dose-dependent manner, compared with Hcy alone. For the in vivo assays, mice were fed a propolis-containing diet and Hcy thiolactone in water. Cognitive function was evaluated using the Morris water maze test. Propolis suppressed cognitive dysfunction caused by hyperhomocysteinemia. Accumulation of aggregated protein in brain was accelerated in hyperhomocysteinemia, and the accumulation was suppressed by propolis. Hyperhomocysteinemia, however, did not enhance the oxidative stress in brain. In vitro amyloid formation assay showed that Hcy accelerated lysozyme aggregation and propolis inhibited the aggregation.
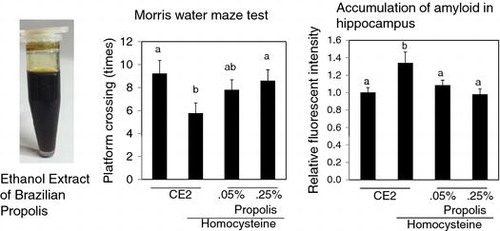
Graphic Abstract
Oral feeding of homocysteine thiolactone caused the cognitive dysfunction in mice. Brazilian Propolis ameliorated the cognitive dysfunction and suppressed the protein aggregation in hippocampus.
Homocysteine (Hcy) is a sulfur-containing amino acid derived from methionine. Its metabolism is at the intersection of two major pathways: remethylation and transsulfuration. The former requires vitamin B12 and folic acid, while the latter requires vitamin B6, as cofactors. Defects in these enzymatic pathways are found in a number of genetic diseases. Cofactor deficiencies lead to hyperhomocysteinemia.Citation1,2) Various studies suggest that hyperhomocysteinemia may be an independent risk factor for dementia.Citation3)
Alzheimer’s disease (AD) is a neurodegenerative disease that is pathologically characterized by neuritic plaques composed predominantly of amyloid-β peptides and neurofibrillary tangles formed by hyperphosphorylated forms of tau protein.Citation4) However, the pathogenesis of AD is unclear. Recent studies suggest that several harmful factors, including oxidative stress, may contribute to neurodegeneration in AD.Citation5–7) In addition, Aβ and copper ions form an Aβ-metal ion complex, which generates reactive oxygen species (ROS) via the Fenton reaction.Citation8) Although the mechanistic detail is not known, Hcy is a likely source of ROS through auto-oxidation.
We previously reported that although μM levels of Hcy do not affect the viability of SH-SY5Y neuroblastoma cells, Hcy can induce apoptosis of these cells in the presence of copper.Citation9) The apoptotic cell death is associated with ROS accumulation in these cells. In a more recent study, in the co-culture condition of SH-SY5Y and U-251MG glioblastoma, we showed that μM concentrations of Hcy induce SH-SY5Y apoptosis though ROS generation from nicotinamide adenine dinucleotide phosphate oxidase in U-251MG.Citation10)
Propolis is a resinous substance collected by honeybees from various plant sources. The composition of propolis depends on the plant sources and the region from which it is collected. Propolis has been reported to possess various biological activities, including antibacterial,Citation11) antiviral,Citation12) anti-inflammatory,Citation13) anticancer,Citation14,15) antifungal,Citation16) and antioxidative properties.Citation17,18) Artepillin C and other cinnamic acid derivatives have been identified in Brazilian propolis, which has been shown to botanically originate from Baccharis dracunculifolia by Kumazawa et al.Citation19) These compounds have potent neuroprotective effects.Citation20)
Although there are a number of in vitro neuronal cell culture studies of Hcy toxicity, in vivo studies are lacking. In this study, we investigated the effect of long-term Hcy thiolactone administration on cognitive function in mice, and we examined indices of oxidative injury in the brain. Furthermore, we studied whether ethanol extract of Brazilian propolis protects against cognitive dysfunction induced by Hcy. These results might contribute to resolve whether Brazilian propolis is beneficial for preventing human dementia.
Materials and methods
Cell culture
SH-SY5Y and U-251MG cells were purchased from the American Type Culture Collection (Manassas, VA) and Health Science Research Resources Bank (Osaka, Japan), respectively. These cell lines were co-cultured according to the procedure described previously.Citation10) Briefly, in co-culture conditions, U-251MG cells were seeded on 6-well cell culture inserts (Becton Dickinson, Franklin Lakes, NJ, USA), and SH-SY5Y cells were seeded on the 6-well cell culture insert companion plate. After 1 day of culture, the medium was replaced with medium containing DL-Hcy (Wako, Osaka, Japan) and various concentrations of propolis ethanol extract (propolis, 55% w/v in ethanol, Yamada Bee Farm Okayama, Japan). Cell viability was assessed by Alamar blue assay (Life Technology, Carlsbad, CA) on day 5. Three independent experiments were performed
Intracellular ROS detection
U-251MG cells were seeded onto a 96-well black plate. After 1 day in culture, the medium was replaced with 100 μL of medium containing 100 μM DL-Hcy and various concentrations of propolis. For detection of intracellular ROS after 3 days, 10 μL of 10 μM CM-DCF-DA (Life Technologies) was added directly to each well and incubated for 30 min. Cells were washed twice with cold PBS, and fluorescence intensity was measured immediately with a microplate reader (SH-9000; Corona Electric Co., Ibaraki, Japan). Three independent experiments were performed.
Animal experiment
DL-Hcy thiolactone hydrochloride (Sigma-Aldrich, Osaka, Japan) was dissolved in water (3.2 mg/mL). Propolis was mixed well with CE2 powder diet (CLEA Japan Inc., Osaka, Japan) and adjusted to 0.1 and 0.5% w/w. Therefore, the 0.05 and 0.25% (v/w) propolis ethanol extract-containing diets mentioned in this study refers to the 0.1 and 0.5% powdered propolis-containing diets, respectively.
Male C57BL/6 J mice were purchased from Kyudo (Saga, Japan) at 12 weeks of age. Prior to the intervention period, mice were adapted to a control diet and water for 1 week. At 13 weeks of age, mice were given preliminary examination (see Morris water maze test) and divided into four groups: control (normal diet and water), Hcy (normal diet and Hcy-containing water), Hcy-0.05P (0.05 % propolis diet and Hcy-containing water), and Hcy-0.25P (0.25 % propolis diet and Hcy-containing water) groups. Ten animals constituted each group. During intervention, mice were maintained on a 12 h:12 h light–dark cycle and given ad libitum access to diet and water. All procedures were compliant with the guidelines of the Kagoshima University Animal Ethics Committee (A10032).
After collecting blood samples, mice were sacrificed under anesthesia by bleeding. Immediately, brains were collected and their weights were measured. Three brains were fixed in 10% neutral-buffered formalin (Wako). Six or seven brains were frozen in liquid nitrogen and stored at −80 °C. Brains were homogenized to determine thiobarbituric acid reactive substance (TBARS) levels, protein carbonyl content, and superoxide dismutase (SOD) and catalase (CAT) activity. TBARS (Cayman Chemical Company, Ann Arbor, MI), protein carbonyl (Cayman Chemical Company), SOD (Cayman Chemical Company), CAT (Cayman Chemical Company), and protein concentration (Thermo Fisher Scientific Inc., Waltham, MA) assays were performed according to the manufacturer’s instructions provided with each kit. Absorbance was measured spectrophotometrically (Sunrise Thermo, Tecan, AG, Switzerland). Blood was collected for measurement of plasma Hcy concentration at 18, 27, and 40 weeks of age from tail. The quantitative analysis of Hcy concentration was according to previous reported method.Citation21)
Morris water maze test
Morris water maze test was performed to examine spatial localization in mice. A circular pool (100 cm in diameter) was filled with water (depth 17 cm, 25 °C) and divided into four quadrant zones: east, west, south, and north. A clear platform (10 cm in diameter and 16 cm in height) was hidden in the center of the north quadrant, submerged 1 cm below the water surface. Prior to the intervention period, all mice were given preliminary examination at 13 weeks of age. Mice were divided into four groups and given three trial sessions per day for five consecutive days to minimize behavioral variation. The average escape latency to finding the platform was determined for each group. A different starting position for each mouse was used in each trial. Mice were allowed to swim freely to find the hidden platform within 60 s and stay on the platform for 15 s. If a mouse failed to locate the platform within 60 s, it was placed on the platform for 15 s. At 29, 42, and 55 (last week of administration of propolis and Hcy) weeks, mice were given three trial sessions for 5 days in the same way. On the last day (day 5) of each session, probe trial testing was performed for a period of 120 s without the platform. We estimated the number of times the mouse crossed the virtual platform (crossing numbers).
Detection of amyloid
Amyloid in the brain section was detected using the ProteoStat Amyloid Plaque Detection Kit (Enzo Life Sciences Inc., Farmingdale, NY) according to the manufacturer’s instructions. Briefly, paraffin-embedded brain sections from each group were prepared (n = 3). The detection reagent interacts with the cross-β-sheet quaternary structure of amyloid fibrils on the slides and is readily excited by an argon ion laser source, with an emission maximum of 600 nm. The fluorescent intensity at the hippocampus was detected using a confocal laser microscope (EZ-C1, Nikon, Tokyo, Japan).
In vitro amyloid formation assay
The protein aggregation was measured according to the previous reported method.Citation22) In brief, 5 mg/mL lysozyme solution in 50 mM glycine-HCl pH 2.0 was prepared. Hcy (0 or 100 μM final concentration) and propolis (0, 0.27, or 1.38 μg/mL final concentration) were added in each lysozyme solution. Amyloid formation was undertaken at 56 °C. Thioflavin T (25 μM final concentration) was added to the incubated lysozyme solutions. The fluorescent intensity was measured (excitation at 440 nm, emission at 482 nm) using a Microplate reader (SH-9000).
Statistical analysis
The data were expressed as mean ± SD for in vitro experiments and mean ± SE for in vivo experiments. Data were analyzed by one-way ANOVA with post hoc Tukey’s HSD test using Statistical Package for the Social Sciences software (SPSS; International Business Machines Corporation, Armonk, NY). Differences with p values < 0.05 were considered significant. Each one mouse in the control group and the Hcy 0.5%-Pro group was excluded from all data analysis because they did not move in Morris water maze test.
Results
Cell viability
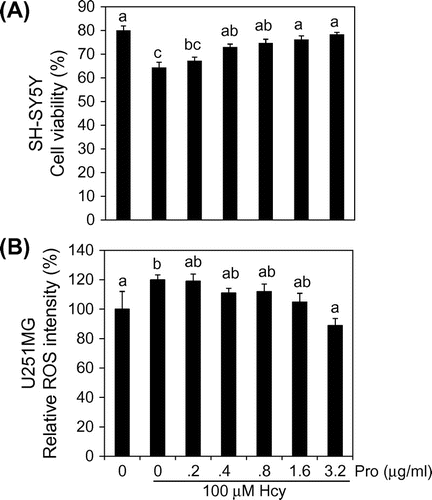
We evaluated the toxicity of propolis in SH-SY5Y and U-251MG cells. Each cell line was incubated with different concentrations of propolis for 1 day. In U-251MG cells,<70 μg/mL propolis did not influence cell viability, but complete cell death was observed with 140 μg/mL propolis. In comparison, propolis exhibited much greater cytotoxicity toward SH-SY5Y cells (<4.5 μg/mL did not affect cell viability). Thus, we chose propolis concentrations < 4.5 μg/mL for experiments.
Previously, we reported that 100 μM Hcy induces death of SH-SH5Y cells only when they are co-cultured with U-251MG cells.Citation10) We examined whether propolis could protect against Hcy cytotoxicity in co-cultures. SH-SY5Y and U-251MG cells were co-cultured for 5 days in each medium which is described in Fig. A. SH-SY5Y cell viabilities were 78.0 ± 4.9% and 66.1 ± 4.7% in normal and 100 μM Hcy-containing medium, respectively. Hcy significantly reduced cell viability. Propolis showed a protective effect against Hcy toxicity. Significant differences were observed with non-treated, 0.4, 0.8, 1.6, and 3.2 μg/mL propolis, compared with Hcy alone.
Fig. 1. Effects of propolis on cell viability in SH-SY5Y cells and relative ROS levels in U-251MG cells.
Notes: (A) U-251MG and SH-SY5Y cells were cultured with DL-Hcy and various concentrations of propolis-containing media, and SH-SY5Y cell viability was measured by counting at 5 days. (B) U-251MG cells were cultured in 100 μM DL-Hcy with various concentrations of propolis-containing medium. After 3 days, relative ROS level was assayed using CM-DCF-DA, and fluorescent intensity was measured. Results are expressed as mean ± SD (n = 3). Different characters indicate statistical significant differences (p < 0.05).
ROS detection
In SH-SY5Y and U-251MG co-cultures, Hcy-induced death of SH-SY5Y cells was accompanied with an accumulation of ROS in U-251MG cells.Citation10) We evaluated the effect of propolis on ROS production in U-251MG cells. ROS were detected using the DCF-DA fluorescent reagent. We represented the fluorescence intensity of cells in normal conditions as 100%. ROS levels significantly increased 1.2-fold when cells were treated with 100 μM Hcy for 3 days. Propolis dose-dependently suppressed the increase in ROS levels induced by Hcy. Significant differences were observed with 1.6 and 3.2 μg/mL propolis, compared with Hcy alone (Fig. B).
Plasma concentration of Hcy in Hcy administrated mice
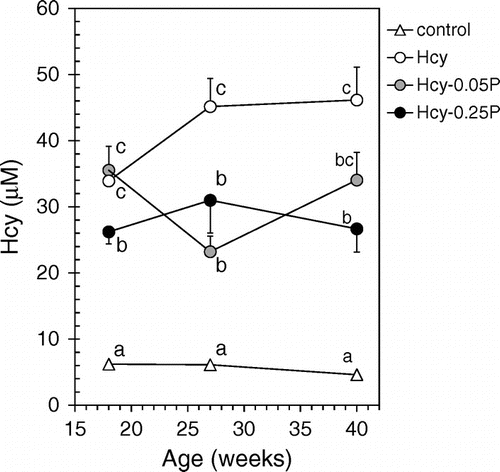
There was no significant difference in the weight of mice between the different groups during experimental period (Table ). Brain weight also was not different among the groups (data not shown). The plasma ALT and AST levels also did not show significant differences between the groups. Hcy concentrations in plasma of mice treated with Hcy (Hcy, Hcy-0.05P and Hcy-0.25P groups) were significantly higher than in the control group (Fig. and Table ). These mice displayed hyperhomocysteinemia. Plasma Hcy concentration was significantly decreased in the Hcy-0.25P groups, but there were no significant differences between Hcy-0.05P and Hcy-0.25P at 27 and 40 weeks of age.
Table 1. Weight and the levels of plasma Hcy, ALT and AST at the end of the experimental period.
Fig. 2. Plasma Hcy concentrations.
Notes: Mice were divided into four groups (control, Hcy, Hcy-0.05P, and Hcy-0.25P). Blood was collected from mice tail at 18, 27, and 40 weeks. Plasma Hcy concentrations were determined by HPLC. Results are expressed as mean ± SE (n = 9–10). Different characters indicate statistical significant differences in each week (p < 0.05). Hcy-0.05P, Hcy and 0.05% propolis; Hcy-0.25P, Hcy and 0.25% propolis.
Morris water maze test
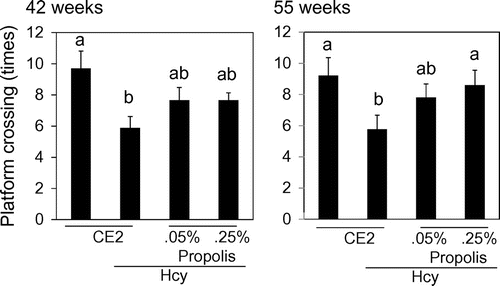
Mice were trained to recognize the area of platform for 5 days. On the last day, mice swum without platform, and we counted the number of times which mice crossed the area settled platform. There were no differences of the number of times among each group at 29 weeks. Cognitive loss was recognized in the Hcy group compared with the control group at 42 weeks and 55 weeks (Fig. ). Propolis-fed group ameliorate cognitive dysfunction caused by Hcy. There were no significant differences between the control group and the propolis-fed groups. In particular, at 55 weeks, Hcy-0.25P group was significantly high compared with Hcy group.
Fig. 3. Effects of propolis on cognitive function.
Notes: Mice were fed a control diet, Hcy, Hcy and 0.05% propolis (Hcy-0.05P), or Hcy and 0.25% propolis (Hcy-0.25P). Cognition was evaluated using the Morris water maze test (probe trial test) at 42 and 55 weeks. Results are expressed as mean ± SE (n = 9–10). Different characters indicate statistical significant differences (p < 0.05).
Indicators of oxidative stress and antioxidant enzyme levels
To evaluate the oxidative condition in the brain, we measured the TBARS level and protein carbonyl content, because they increase in oxidative condition.Citation23) TBARS levels in brain were not significantly different between the control and Hcy groups (Table ). Interestingly, however, TBARS levels in the Hcy-0.25P groups group were significantly increased compared with the Hcy group. In addition, protein carbonyl content in brain was also significantly increased in the Hcy-0.25P groups group compared with the Hcy group. The activities of the enzymes SOD and CAT in brain were not significantly different between the groups. TBARS levels in plasma were not significantly different among the groups (Table ).
Table 2. Levels of compounds related to oxidative stress and antioxidant enzyme activities in brain homogenates.
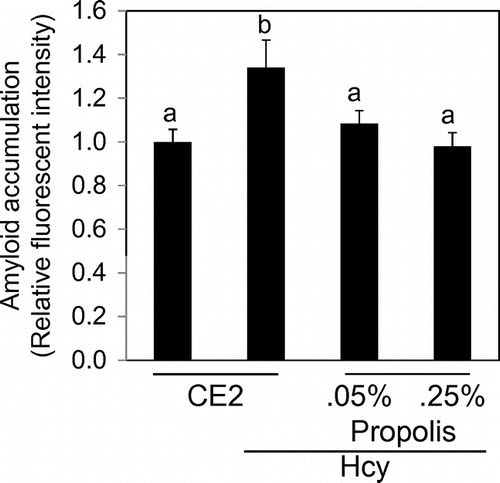
Accumulation of amyloid in brain
We detected amyloid with the protein aggregation detection dye on the section of the brain. Any obvious amyloid plaques could not be detected in all groups. We evaluated fluorescent intensity in the hippocampus region. As a result, the relative fluorescence intensity was high in the Hcy group compared to the control group (Fig. ). The intensity in propolis-fed groups was significantly lower than that of the Hcy group.
In vitro amyloids formation assay
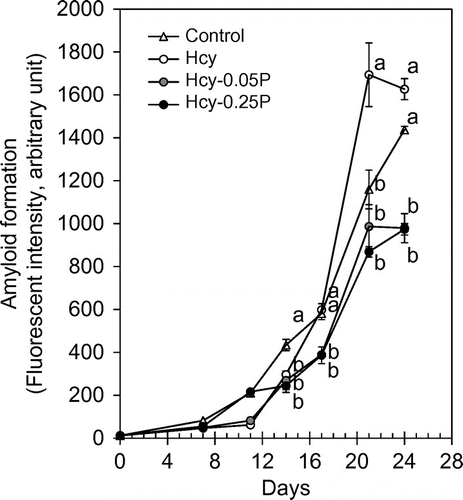
It has been established in vitro amyloid formation assay using lysozyme.Citation22) The aggregation of the lysozyme is undergone in the acidic solution. We evaluated whether Hcy accelerates the lysozyme aggregation and propolis suppress the aggregation. Figure shows that lysozyme aggregation was significantly accelerated in the 100 μM Hcy after 14 days. Both 0.85 and 1.7 μg/mL of propolis significantly suppressed the aggregation.
Fig. 5. In vitro amyloid formation assay.
Notes: Lysozyme (5 mg/mL) aggregation was evaluated using Thioflavin T. Hcy (100 μM) and propolis were added to the lysozyme solution. Results are expressed as mean ± SE (n = 3). Different characters indicate statistical significant differences (p < 0.05). Hcy-P, Hcy and propolis.
Discussion
In vitro studies showed that propolis have a protective effect against Hcy toxicity in SH-SY5Y cells in co-culture conditions. The cytotoxicity mediated by Hcy may involve ROS production by NADPH oxidase in U-251MG cells.Citation10) In this study, indeed, ROS accumulation was detected in Hcy-treated U-251MG cells. Propolis reduced the ROS accumulation in the cells. We suspect that these actions result from several antioxidant chemicals contained in the propolis.Citation18)
Hcy is an independent risk factor for cognitive dysfunction; however, there are only a few reports on Hcy-induced cognitive dysfunction in animal models.Citation24) In this experiment, we generated a mouse model of chronic hyperhomocysteinemia by oral administration of Hcy thiolactone. Short-term (<7 months) administration of Hcy thiolactone did not induce cognitive dysfunction. Administration exceeding 7 months successfully induced cognitive dysfunction. Body weight and tissue weights did not differ between the groups in this experiment.
Feeding propolis ameliorated hyperhomocysteinemia and cognitive dysfunction induced by Hcy. The mechanism how propolis decreased plasma Hcy concentration has remained unclear and there was no dose-dependency on Hcy-lowering effect of propolis. Cognitive function in the Hcy-0.25P group was higher than that in the Hcy-0.05P group. Therefore, amelioration of cognitive dysfunction may be related to not only Hcy-lowering effect but also other mechanisms. We suspect that the effect is related to antioxidative effect of propolis, however, TBARS and carbonyl protein values were not reduced by propolis, or rather these values were high in the Hcy-0.25P groups. Although a high level of ROS is detrimental for health in general,Citation25) Naviaux put forth the novel proposal that ROS are a response to disease, rather than the cause.Citation26) Indeed, antioxidant treatment in AD patients does not prevent the progression of the disease.Citation27) The relationship between oxidative balance in the brain and brain function needs to be clarified. So, it should be discussed carefully the extensive elevation of ROS by Hcy-0.25P and further studies are needed why propolis induce high ROS states in brain.
To clarify how Hcy induces cognitive dysfunction, we assessed amyloid protein accumulation. Hcy administration for 55 weeks did not induce amyloid plaque, but induced amyloid accumulation in the hippocampus. Propolis feeding appeared to decrease this accumulation. The accumulation of amyloid protein might be related to cognitive dysfunction. Derouiche et al. reported that proteasome activity was significantly deceased in heart but not in the aorta of rat hyperhomocysteinemia model.Citation28) The proteasome activity might also be decreased by Hcy in brain. In this study, we could not evaluate proteasome activity in the brain. To evaluate the direct effect of Hcy or propolis in amyloid formation, we examined the effects using in vitro amyloids formation assay. As a result, Hcy significantly accelerated amyloid formation. Hcy may bind cysteine residue in proteins resulting in unexpected protein foldings. Propolis inhibited the acceleration of amyloid formation caused by Hcy. Its mechanism remains unclear.
Hcy is considered an independent risk factor for dementia because it is a source of ROS. In this study, Hcy also induced ROS accumulation in vitro. However, we conclude that Hcy does not induce ROS accumulation in the brain. Propolis prevented ROS accumulation caused by Hcy in cells, but the effect could not suppress ROS production in the brain. Hcy impaired cognitive function, while propolis feeding was able to alleviate this behavioral decline. The cognitive dysfunction might to be related to plasma Hcy-lowering effect and the accumulation of amyloid proteins in the brain.
Author contribution
H. Kanouchi conceptualized and designed the study. Y. Miyazaki, Y. Sugimoto, and H. Kanouchi analyzed and interpreted the data. H. Kanouchi wrote the manuscript. Statistical analysis was done by Y. Miyazaki and H. Kanouchi. A. Fujita supervised the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This research was supported by the Yamada Research Grant.
References
- Schnyder G, Roffi M, Flammer Y, et al. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. J. Am. Med. Assoc. 2002;288:973–979.10.1001/jama.288.8.973
- Dimopoulos N, Piperi C, Salonicioti A, et al. Association of cognitive impairment with plasma levels of folate, vitamin B12 and homocysteine in the elderly. In Vivo. 2006;20:895–899.
- Seshadri S, Beiser A, Selhub J, et al. Plasma Homocysteine as a Risk Factor for Dementia and Alzheimer's Disease. N. Engl. J. Med. 2002;346:476–483.10.1056/NEJMoa011613
- Mudher A, Lovestone S. Alzheimer’s disease–do tauists and baptists finally shake hands? Trends Neurosci. 2002;25:22–26.10.1016/S0166-2236(00)02031-2
- Berr C. Oxidative stress and cognitive impairment in the elderly. J. Nutr. Health Aging. 2002;6:261–266.
- Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress1,2 1Guest Editors: mark A. Smith and George Perry 2This article is part of a series of reviews on “Causes and consequences of oxidative stress in Alzheimer’s disease.” The full list of papers may be found on the homepage of the journal. Free Radic. Biol. Med. 2002;32:1050–1060.10.1016/S0891-5849(02)00794-3
- Floyd RA, Hensley K. Oxidative stress in brain agingImplications for therapeutics of neurodegenerative diseases. Neurobiol. Aging. 2002;23:795–807.10.1016/S0197-4580(02)00019-2
- Huang HC, Chang P, Dai XL, et al. Protective Effects of Curcumin on Amyloid-β-induced neuronal oxidative damage. Neurochem. Res. 2012;37:1584–1597.10.1007/s11064-012-0754-9
- Hirashima Y, Seshimo S, Fujiki Y, et al. Homocysteine and copper induce cellular apoptosis via caspase activation and nuclear translocation of apoptosis-inducing factor in neuronal cell line SH-SY5Y. Neurosci. Res. 2010;67:300–306.10.1016/j.neures.2010.04.013
- Fujiki Y, Hirashima Y, Seshimo S, et al. Homocysteine induced SH-SY5Y apoptosis through activation of NADPH oxidase in U251MG cells. Neurosci. Res. 2012;72:9–15.10.1016/j.neures.2011.09.010
- Kujumgiev A, Tsvetkova I, Serkedjieva Y, et al. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999;64: 235–240.10.1016/S0378-8741(98)00131-7
- Amoros M, Lurton E, Boustie J, et al. Comparison of the anti-herpes simplex virus activities of propolis and 3-methylbut-2-enyl caffeate. J. Nat. Prod. 1994;64:235–240.
- Dobrowolski JW, Vohora SB, Sharma K, et al. Antibacterial, antifungal, antiamoebic, antiinflammatory and antipyretic studies on propolis bee products. J. Ethnopharmacol. 1991;35:77–82.10.1016/0378-8741(91)90135-Z
- Kimoto T, Aga M, Hino K, et al. Apoptosis of human leukemia cells induced by Artepillin C, an active ingredient of Brazilian propolis. Anticancer Res. 2001;21:221–228.
- Mitamura T, Matsuno T, Sakamoto S, et al. Effect of a new clerodane diterpenoid isolated from propolis on chemically induced skin tumors in mice. Anticancer Res. 1996;16:2669–2672.
- Murad JM, Calvi SA, Soares AM, et al. Effects of propolis from Brazil and Bulgaria on fungicidal activity of macrophages against Paracoccidioides brasiliensis. J. Ethnopharmacol. 2002;79:331–334.10.1016/S0378-8741(01)00404-4
- Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002;73:S1–S6.10.1016/S0367-326X(02)00185-5
- Kumazawa S, Hamasaka T, Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004;84:329–339.10.1016/S0308-8146(03)00216-4
- Kumazawa S, Yoneda M, Shibata I, et al. Direct evidence for the plant origin of Brazilian propolis by the observation of honeybee behavior and phytochemical analysis. Chem. Pharm. Bull. 2003;51:740–742.10.1248/cpb.51.740
- Nakajima Y, Shimazawa M, Mishima S, et al. Neuroprotective effects of Brazilian green propolis and its main constituents against oxygen-glucose deprivation Stress, with a gene-expression analysis. Phytother. Res. 2009;23:1431–1438.10.1002/ptr.v23:10
- Kakimoto T, Otsuka A, Kawaguchi H, et al. Plasma homocysteine concentrations in novel microminipigs. In Vivo. 2014;28:579–582.
- Sugimoto Y, Kamada Y, Tokunaga Y, et al. Aggregates with lysozyme and ovalbumin show features of amyloid-like fibrils. Biochem. Cell Biol. 2011;89:533–544.10.1139/o11-041
- Li Q, Zhao H, Zhao M, et al. Chronic green tea catechins administration prevents oxidative stress-related brain aging in C57BL/6 J mice. Brain Res. 2010;1353:28–35.
- Farkas M, Keskitalo S, Smith DE, et al. Hyperhomocysteinemia in Alzheimer’s disease: the hen and the egg? Alzheimers Dis. 2013;3: 1097–1104.
- Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007;47:143–183.10.1146/annurev.pharmtox.47.120505.105122
- Naviaux RK. Oxidative shielding or oxidative stress? J. Pharmacol. Exp. Therm. 2012;342:608–618.10.1124/jpet.112.192120
- Thal LJ, Grundman M, Berg J, et al. Idebenone treatment fails to slow cognitive decline in Alzheimer’s disease. Neurology. 2003;61:1498–1502.10.1212/01.WNL.0000096376.03678.C1
- Derouiche F, Bôle-Feysot C, Naïmi D, et al. Hyperhomocysteinemia-induced oxidative stress differentially alters proteasome composition and activities in heart and aorta. Biochem. Biophys. Res. Commun. 2014;452:740–745.10.1016/j.bbrc.2014.08.141