Abstract
Bacterial bio-production during the stationary phase is expected to lead to a high target yield because the cells do not consume the substrate for growth. Bacillus subtilis is widely used for bio-production, but little is known about the metabolism during the stationary phase. In this study, we focused on the dipicolinic acid (DPA) production by B. subtilis and investigated the metabolism. We found that DPA production competes with acetoin synthesis and that acetoin synthesis genes (alsSD) deletion increases DPA productivity by 1.4-fold. The mutant showed interesting features where the glucose uptake was inhibited, whereas the cell density increased by approximately 50%, resulting in similar volumetric glucose consumption to that of the parental strain. The metabolic profiles revealed accumulation of pyruvate, acetyl-CoA, and the TCA cycle intermediates in the alsSD mutant. Our results indicate that alsSD-deleted B. subtilis has potential as an effective host for stationary-phase production of compounds synthesized from these intermediates.
Graphical abstract
Dipicolinic acid production in B. subtilis during the stationary phase. The deletion of alsSD enhanced the dipicolinic acid productivity by 1.4-fold.
Bacillus subtilis is a gram-positive bacterium that is widely applied for bio-production, such as that of various secreted proteins,Citation1) riboflavin,Citation2) isobutanol,Citation3) 2,3-butanediol,Citation4) and acetoin.Citation5) Bioproduction during the stationary phase, so-called non-growth-associated production, is expected to increase the yield of target compounds compared with that during the growth phase because the cells can produce the target material without consuming the substrate for cell growth during the stationary phase.Citation6) However, little is known about the stationary-phase metabolism in B. subtilis.
B. subtilis produces acetoin to maintain intracellular pHCitation7) and for external carbon storage.Citation8) One mole of acetoin is produced from 2 mol of pyruvate via acetolactate by alpha-acetolactate synthase and acetolactate decarboxylase, which are encoded by alsS and alsD, respectively. Although acetoin is used as a food additive, in cosmetics, and as a precursor for the synthesis of pharmaceutical products, it is an undesirable by-product during the production of other metabolites. In this study, we first examined the effect of the blocking of acetoin biosynthesis on cellular metabolism during the stationary phase in an alsSD deletion strain of B. subtilis.
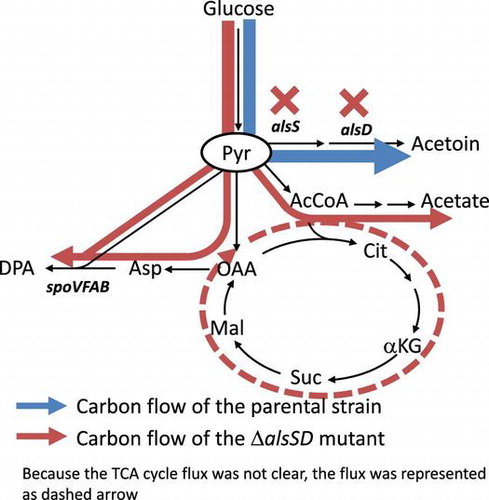
Dipicolinic acid (DPA) is a major constituent of the spore core in Bacillus species and is involved in germinationCitation9) and heat resistance of spores.Citation10) Moreover, DPA is expected to be useful for a wide variety of applications, including for use in antimicrobial agents, antioxidants, chelating reagents, and polymer precursors.Citation11) In B. subtilis, DPA is synthesized via the lysine biosynthetic pathway from pyruvate in glycolysis and from oxaloacetate in the TCA cycle as precursors (Fig. ). Recently, Takahashi et al. developed a DPA-producing strain by replacing the promoter of the spoVFAB operon, which encodes DPA synthase, with spoVG promoter.Citation11) The sporulation gene, spoVG is highly expressed during the stationary phase.Citation12,13) In this study, we focused on DPA as a target chemical for stationary-phase bioproduction. We examined the DPA production in this recombinant strain during the stationary phase and the effect of alsSD deletion on DPA production to evaluate the potential of B. subtilis as a host microorganism for stationary bioproduction. Cost of medium is also an important concern for practical bioproduction. Therefore, in this study, the DPA production was conducted by used of inexpensive synthetic medium containing glucose as a sole carbon source, instead of rich medium supplemented with yeast extract and glutamate in the previous study.Citation11)
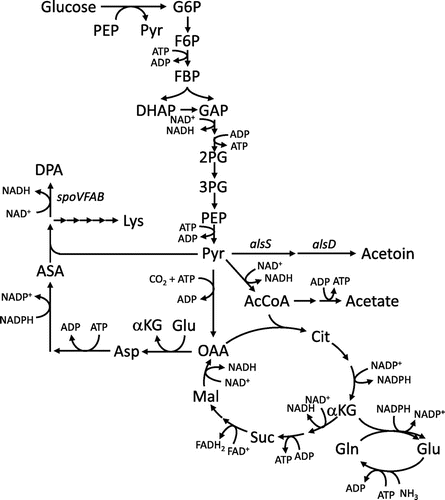
Fig. 1. Central carbon metabolic pathways with DPA biosynthetic pathways in B. subtilis.
Notes: G6P, glucose-6-phosphate; F6P, fructose-6-phosphate; FBP, fructose-1,6-bisphosphate; DHAP, dihydroxyacetone phosphate; 2PG, 2-phosphoglycerate; 3PG, 3-phosphoglycerate; PEP, phosphoenolpyruvate; Pyr, pyruvate; AcCoA, acetyl-CoA; Cit, citrate; αKG, α-ketoglutarate; Suc, succinate; Mal, malate; OAA, oxaloacetate; Glu, glutamate; Asp, aspartate; ASA, aspartate semialdehyde; Lys, lysine.
Materials and methods
Strain construction
The strains used in this study are listed in Table . The genome in wild-type B. subtilis strain 168 contains 2 prophages (SPβ and PBSX) and 7 prophage-like regions (pro1 to pro7), which place cells at risk of lysis by prophage induction upon host cell damage, and genes encoding the synthesis of plipastatin and bacillaene (pps and pks, respectively), which may reduce viability during long-term culture. Thus, a prophage- and antibiotic-free strain, OA105, was used in this study. The alsSD deletion mutant OA105-ΔalsSD was constructed by a marker-free disruption method.Citation14) The disruptions in OA105 and OA105-ΔalsSD were confirmed by PCR and DNA sequencing. The construction of OA105 and OA105-ΔalsSD is detailed in the Supplementay material.
Table 1. Strains used in this study.
To construct the DPA-producing strains, the native spoVFA promoter was replaced with the spoVG promoter in OA105 and OA105-alsSD strains according to the methods described by Takahashi et al.Citation11) The primer sequences for PCR are listed in Table . A chloramphenicol resistance cassette was amplified from the template pDLT3 plasmid using primers CmF2 and CmR2.Citation15) The spoVG promoter sequence was amplified using the primers spoVGpro-F and spoVGpro-R, and upstream and downstream sequences of the spoVFA promoter region were amplified using the primer sets spoVFA-F1 and spoVFA-R1, and spoVFA-F2 and spoVFA-R2, respectively. These fragments were connected by overlap extension PCR. The OA105 and OA105-ΔalsSD strains were transformed with the ligated product, and the transformants obtained were designated OA105-DPA and OA105ΔalsSD-DPA, respectively. The fragment insertions were checked by PCR, and the inserted regions were confirmed by DNA sequencing. KOD plus Neo (Toyobo) was used as the polymerase for all PCRs.
Table 2. Primer list for DPA-producing strain construction.
Culture conditions
The strains were cultured in M(MOPS) synthetic medium consisting of 1.5 g L−1 K2HPO4, 6 g L−1 NH4Cl, 0.35 g L−1 MgSO4·7H2O, 0.05 g L−1 MnSO4·5H2O, 10.5 g L−1 3-morpholinopropanesulfonic acid, 13.5 mg L−1 FeCl3·6H2O, 7.3 mg L−1 CaCl2·2H2O, 3.6 mg L−1 ZnSO4·7H2O, 0.65 mg L−1 CuSO4·5H2O, 0.6 mg L−1 CoCl2·6H2O, and 0.6 mg L−1 Na2MoO4·2H2O.Citation16) First, 2 μL of glycerol stock was inoculated into 5 mL of synthetic medium supplemented with 20 g L−1 glucose, 0.5 g L−1 Bacto yeast extract (Difco Laboratories), and 0.2 g L−1 casamino acids (Difco Laboratories) in a test tube and cultured at 37 °C. For the preculture, this starter culture was used to inoculate 30 mL of the synthetic medium containing 10 g L−1 glucose, and the culture was incubated at 37 °C with rotation at 200 rpm in a 500-mL baffled Erlenmeyer flask until the optical density at 600 nm (OD) reached approximately 2. For the main culture, this preculture was inoculated to achieve an OD of 0.1 in 100 mL of the synthetic medium with 100 g L−1 glucose and the culture incubated under the same conditions as the preculture. Note that because the DPA-producing strains could not grow with 100 g L−1 glucose, the cells were initially cultured with 50 g L−1 glucose. Substrate solution containing 500 g L−1 glucose and 60 g L−1 NH4Cl was added at 12 h at 1/10 of the culture volume. The decrease in the pH levels in the culture was prevented by adding 40 g L−1 CaCO3. All culture experiments were conducted in triplicate.
Analysis of OD, metabolites, and glucose levels in the culture broth
The OD was measured using a UVmini-1240 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) and was converted to the dry cell concentration using experimentally measured conversion coefficients of 0.35 g L−1 OD−1.Citation16)
The glucose concentration was measured using Bioanalyzer BF-5 enzymatic electrode sensors (Oji Scientific Instruments, Hyogo, Japan). The concentrations of acetoin, acetate, isobutyrate, isovalerate, lactate, and succinate in the culture supernatants were measured using high-performance liquid chromatography (HPLC) (Shimadzu Corporation) with TSKgel OApak-A and TSKgel OApak-P columns (Tosoh, Tokyo, Japan) and a UV light detector (210 nm), as described previously.Citation16) Because the peak of acetate overlapped with that of DPA, the acetate concentration was measured enzymatically using an F-kit (Roche Diagnostics, Mannheim, Germany) for analysis of the samples from DPA-producing strains.
DPA was quantified by HPLC with a high-performance carbohydrate column (Waters, Tokyo, Japan) and a UV light detector (270 nm). The eluting solution was 50% acetonitrile containing 10 mM EDTA (pH 3.4), and the flow rate was set to 1 mL min−1.
Measurement of intracellular metabolites
An appropriate volume of culture, calculated from the 20/OD (mL), was taken at 20 h corresponding to the stationary phase. The cells were trapped using a filter with 0.5-μm pores (Advantec, CA, USA) and washed twice with 5 mL of Milli-Q water. The following sample pretreatment procedures, including quenching and metabolite extraction, have been described elsewhere.Citation17) The intracellular metabolite levels were measured by capillary electrophoresis (CE) time-of-flight mass spectrometry (TOF-MS). The analysis was performed by an Agilent 7100 CE system equipped with an Agilent 6224 TOF-MS system, and analytical conditions for anionic metabolites have been described elsewhere.Citation17)
Results
Effect of blocking acetoin production during the stationary phase in B. subtilis
To investigate metabolism at the stationary phase in B. subtilis, the parental strain OA105 was cultured aerobically in M(MOPS) synthetic media with glucose as the sole carbon source. The time courses of cell growth and the concentrations of glucose and excreted metabolites, including acetoin and acetate, in the culture medium are shown in Fig. . Here, we defined the stationary phase as when the growth rate was significantly reduced compared to that during the exponential growth, but the carbon source still remained in the culture. The cell growth mostly stopped at 18 h, and the stationary phase lasted until 33 h when the OD of the culture decreased as a result of cell lysis after glucose depletion.
Fig. 2. Batch cultures of B. subtilis OA105 and OA105-ΔalsSD strains.
Notes: (A) OD600, (B) glucose concentration, (C) acetoin concentration and (D) acetate concentration; open circles, OA105; closed circles OA105-ΔalsSD. All cultures were performed in triplicate, and the average ± standard deviation is shown.
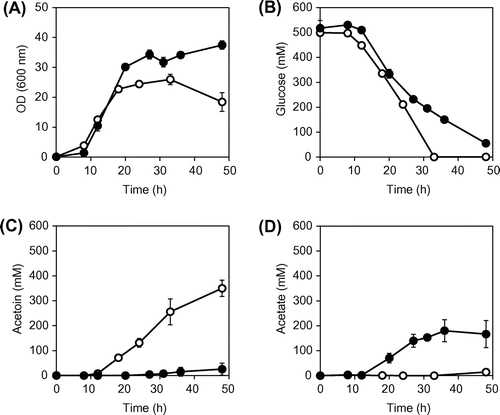
The specific rates of glucose consumption and metabolite production during the stationary phase from 18 to 33 h are summarized in Table . Acetoin was a primary product of the wild-type strain during the stationary phase, the yield during stationary phase was 0.56 ± 0.14% (mol mol−1), and its maximum concentration was 350 ± 32 mM at 48 h (Fig. (C)). Acetate was not detected during the stationary phase. These results indicate that acetoin appears to be a main by-product during the stationary-phase bioproduction, and thus, we expected that deletion of the acetoin synthesis genes (alsSD) would enhance the yield of desirable products. Therefore, we examined effect of alsSD deletion on metabolism during the stationary phase.
Table 3. Comparison of the specific rates between wild-type and ΔalsSD strains during the stationary phase.
As expected, in the alsSD deletion mutant, acetoin synthesis was decreased to less than 5% of that in the parental strain. Rather, the mutant showed increased acetate production, as the concentration at 48 h reached 166 ± 54 mM and the yield during the stationary phase was 0.59 ± 0.10% (mol mol−1). The cell concentration was 1.6-fold higher than that of the parental strain, leading to enhancement of the production rate in a culture volume favorable for bioproduction. Interestingly, the specific glucose consumption rate decreased significantly to 38.5% of that of the parental strain (Table ), possibly because of inhibition of the phosphotransferase system for glucose uptake as a result of pyruvate accumulation, as described later.
DPA production from glucose in B. subtilis
As shown above, B. subtilis efficiently converts glucose to acetoin during the stationary phase, suggesting that B. subtilis cells would be appropriate for production of compounds from pyruvate, which is a precursor of acetoin. DPA that is useful as a biopolymer precursor is synthesized from pyruvate and oxaloacetate in the central carbon metabolism (Fig. ). Therefore, DPA was chosen as a target compound in this study. The DPA synthetic reactions from the central carbon metabolism were summarized as follows.
pyruvate + oxaloacetate + l-glutamate + ATP + NADPH + NAD+ → DPA + α-ketoglutarate + ADP + NADP+ + NADH + Pi + 2H2O + H+
In B. subtilis, glutamate is synthesized via the glutamine synthetase (l-glutamate + ATP + NH3 → l-glutamine + ADP + Pi + H+) and glutamate synthase (l-glutamine + α-ketoglutarate + NADPH + H+ → 2 l-glutamate + NADP+) cycle, and oxaloacetate is formed via pyruvate carboxylase from pyruvate and HCO3−. Therefore, the DPA synthetic reactions were summarized as
2Pyruvate + HCO3− + 3ATP + 2NADPH + NAD+ + NH4→ DPA + 3ADP + 2NADP+ + NADH + 3Pi + 3H2O + H+
Considering glycolysis, the DPA synthetic reaction from glucose is expressed as
Glucose + HCO3− + ATP + 2NADPH + 3NAD+ + NH4→ DPA + ADP + 2NADP+ + 3NADH + Pi + 5H2O + 3H+
Because DPA synthesis is associated with NADH generation, the aerobic condition is assumed to be preferable for DPA production.
To produce DPA during the stationary phase, the promoter of the DPA synthase operon, spoVFA, in the wild-type strain (OA105) was replaced with the spoVG promoter, which is induced with entry into stationary phase.Citation11–13) The resultant strain OA105-DPA was cultured in M(MOPS) medium as described above, but it did not grow in the presence of 100 g L−1 glucose (data not shown), although it did grow when the cells were initially cultured in M(MOPS) with 50 g L−1 glucose. To achieve a final input glucose concentration of 100 g L−1, additional glucose at 50 g L−1 was supplied after entry into stationary phase (12 h). In this condition, cell growth mostly ceased at 18 h, and the stationary phase continued until approximately 40 h (Fig. ). The specific rates of glucose consumption and metabolite productions during the stationary phase from 20 to 32 h are summarized in Table . As expected, DPA was produced during the stationary phase (0.03 ± 0.00 mol mol−1), and acetoin was produced as the primary by-product (0.58 ± 0.02 mol mol−1) as observed in the wild-type strain OA105. The specific glucose consumption rate was 2.19 ± 0.14 mmol g−1 h−1, which corresponds to 82.3% of that of the OA105 strain.
Fig. 3. Batch cultures of B. subtilis OA105-DPA and OA105ΔalsSD-DPA strains.
Notes: (A) OD, (B) glucose concentration, (C) acetoin concentration and (D) acetate concentration (E) DPA concentration; Open circles, OA105-DPA; closed circles OA105ΔalsSD-DPA. All cultures were performed in triplicate, and average ± standard deviation is shown.
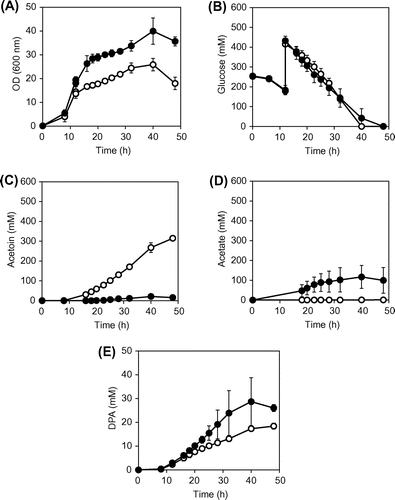
Table 4. Comparison of the specific rates of DPA production between wild-type and ΔalsSD strains during the stationary phase.
Effect of alsSD deletions on DPA production during the stationary phase
To improve DPA productivity, the alsSD deletion was introduced into the OA105-DPA strain to inactivate the acetoin biosynthetic pathway. The culture profiles are shown in Fig. . As expected, the DPA concentration of OA105ΔalsSD-DPA strain at 48 h increased by 1.4-fold (from 18.4 ± 1.3 to 26.0 ± 1.4 mM). The specific rates of glucose consumption and metabolite productions during the stationary phase are summarized in Table . As observed in the OA105ΔalsSD strain, the specific glucose consumption rate was decreased in OA105ΔalsSD-DPA strain, to 55.3% of that in OA105-DPA strain; however, the level of the specific glucose consumption rate was higher than that of the OA105ΔalsSD strain (38.3%), suggesting that DPA production stimulates glucose uptake, although the mechanism is still not clear. It is possible that DPA may inhibit enzyme activities on the central carbon metabolism. It is also possible that, because the DPA is known as chelating agents,Citation11) DPA production may trap intercellular metallic ions that are necessary for efficient glucose uptake. The maximum cell density increased in OA105ΔalsSD-DPA strain by approximately 50% as observed in the OA105ΔalsSD strain, and thus, the volumetric glucose consumption in OA105ΔalsSD-DPA strain was almost identical to that of the OA105-DPA strain because of the counteracting effects of decreased glucose uptake and increased cell concentration at the stationary phase (Fig. ).
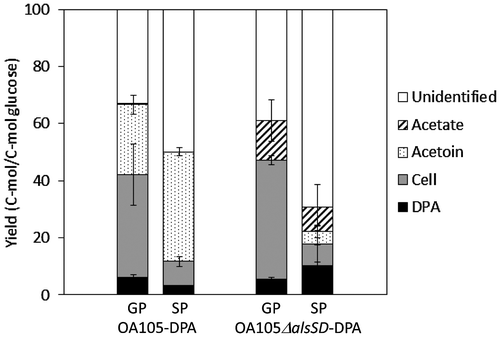
The carbon recoveries of the OA105-DPA and OA105ΔalsSD-DPA strains in the growth phase and stationary phase are shown in Fig. . During the growth phase, approximately 40% of the carbon from glucose was converted to cellular components. The DPA yield was highest in the OA105ΔalsSD-DPA strain during the stationary phase. Because this mutant produced acetate (8% of uptake carbons as glucose) during stationary phase, additional blockage of acetate synthesis may further enhance the DPA yield. Furthermore, the total carbon recovery was only 35%, implying an increase of unknown by-products. Identification of the unknown by-products and rewiring of the carbon flow to the target pathway is likely necessary for further improvement of the DPA yield.
Intracellular metabolic states in DPA-producing strains in the stationary phase
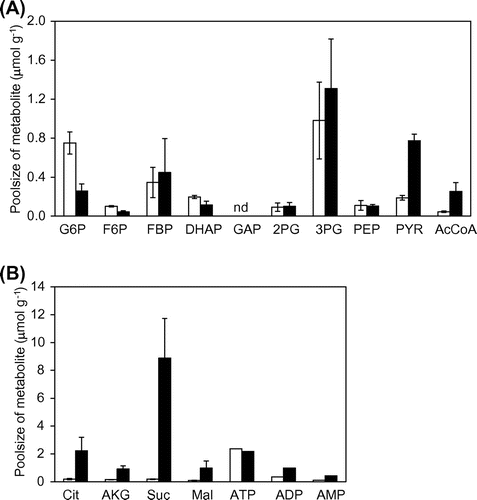
To investigate the effect of the alsSD deletion on metabolism in DPA-producing strains, metabolic profiles of the OA105-DPA and OA105ΔalsSD-DPA strains at 20 h, corresponding to the stationary phase, were compared (Fig. ). The concentrations of pyruvate and acetyl-CoA were increased in the alsSD deletion strain, whereas accumulation of glycolytic intermediates upstream of pyruvate was not observed. The increase of pyruvate and acetyl-CoA is thought to be direct effect of lack of the acetoin biosynthetic pathway. The decrease in glucose-6-phosphate concentration accompanying the alsSD deletion may be related to the slow specific glucose consumption rate in OA105ΔalsSD-DPA. Furthermore, significant accumulation was also observed for TCA cycle intermediates such as citrate, α-ketoglutarate, succinate, and malate. In particular, the accumulation of succinate was prominent, i.e. 30-fold higher than that of the OA105-DPA strain. Further improvement of DPA productivity can thus be expected if the accumulated TCA cycle intermediates are utilized for the DPA synthetic pathway.
Fig. 5. Concentrations of intracellular metabolite in the TCA cycle at the stationary phase in DPA-producing B. subtilis.
Notes: Intracellular pool sizes of metabolites in glycolysis (A) and the TCA cycle (B) are shown. Open and closed bars represent the intracellular pool sizes in OA105-DPA and OA105ΔalsSD-DPA, respectively. The average ± standard deviation of triplicate cultures is shown. nd. denotes not detected.
Discussion
In the present study, we investigated the metabolic system of B. subtilis during the stationary phase and demonstrated its application for DPA production. In the parental strain, acetoin was a primary product during the stationary phase, and blockage of acetoin synthesis resulted in acetate production. The production of acetate, which was not observed in the parental strain during the stationary phase, by the ΔalsSD mutant likely occurred because the mutant reluctantly produced acetate to overcome the accumulation of intermediates resulting from the blockage of acetoin synthesis. The enzymes of acetate synthetic pathways thus appear to be directly or indirectly induced by the accumulated intermediates.
DPA production at 48 h was increased 1.4-fold by alsSD deletion. One of the issues in bioproduction in the stationary phase has been slow glucose uptake. The specific glucose consumption rate in the stationary phase for the parental strain was approximately half of that in the growth phase.Citation16) Although the specific glucose consumption rate was significantly decreased by alsSD deletion, this decrease was alleviated by the production of DPA. Because the accumulated metabolites in the cells with the alsSD deletions were utilized in the DPA synthetic pathway, the inhibition of glucose uptake was ameliorated. Furthermore, the maximum cell concentration during the stationary phase increased by 50% in cells with the alsSD deletion with or without DPA production. It was reported that the cell growth of B. subtilis was seriously inhibited by acetoin in culture broth.Citation18) Therefore, the decrease of acetoin production may contribute to the increase of the maximum OD in the ΔalsSD mutants. Otherwise, the accumulations of the precursor metabolites such as pyruvate, acetyl-CoA, oxaloacetate, and α-ketoglutarate by alsSD deletion may lead to increase cell growth during the stationary phase. The increase in cell concentration is preferable for enhancing the conversion rate from the substrate to the target compound per culture volume.
The DPA-producing strains did not grow in the presence of a high concentration of glucose (100 g L−1). Because the strains were able to grow at a lower concentration of glucose (50 g L−1), the osmotic stress caused by the high glucose concentration may have caused this growth defect. However, the reason why DPA production decreased the resistance to the high glucose concentration remains unclear. The mutant with tolerance to high glucose concentration should be developed to make practical use of this strain. Furthermore, despite the deletion of alsSD, acetoin was produced at low levels in both OA105-ΔalsSD and OA105ΔalsSD-DPA strains. B. subtilis possesses an isozyme of acetolactate synthase, encoded by ilvBH, which converts pyruvate to acetolactate.Citation19) The conversion from acetolactate to acetoin is also catalyzed by acetoin dehydrogenase encoded by acoAB. Thus, the remaining acetoin production in the ΔalsSD mutants may have occurred through such alternative reactions.
Measurement of intracellular metabolites revealed significant accumulation of TCA cycle intermediates such as citrate, α-ketoglutarate, succinate, and malate in the OA105ΔalsSD-DPA strain (Fig. ). Increased accumulation of the TCA cycle intermediates is expected to be caused by the elevated accumulation of pyruvate and acetyl-CoA, instead of acetoin production, in the ΔalsSD mutant In the case of Escherichia coli, α-ketoglutarate inhibits enzyme I activity in the phosphotransferase system for glucose uptake.Citation20) The metabolism of B. subtilis also may have such a feedback regulatory system for glucose uptake by TCA cycle intermediates.
The impact of alsSD deletion on the stationary phase metabolism in B. subtilis is not limited to DPA production, and it may be applicable to the productions of many compounds from pyruvate, acetyl-CoA, and the TCA cycle intermediates. B. subtilis cells have potential as an effective cell factory for the stationary phase production of such compounds.
Author contribution
YT, TH, KO, NO, and HS designed research. SI, CO, TM, SL, KM, and YK constructed the mutants. YT, TM, SL, KM, and YK evaluated the metabolism of the mutants. YT, TH, SI, CO, and HS wrote the article.
Supplementary material
The supplementary material for this article is available online at http://dx.doi.10.1080/09168451.2015.1060843
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental_141222.pdf
Download PDF (311.5 KB)References
- Westers L, Westers H, Quax WJ. Bacillus subtilis as cell factory for pharmaceutical proteins: a biotechnological approach to optimize the host organism. Biochim. Biophys. Acta. 2004;1694:299–310.10.1016/j.bbamcr.2004.02.011
- Perkins JB, Sloma A, Hermann T, et al. Genetic engineering of Bacillus subtilis for the commercial production of riboflavin. J. Ind. Microbiol. Biotechnol. 1999;22:8–18.10.1038/sj.jim.2900587
- Jia X, Li S, Xie S, Wen J. Engineering a metabolic pathway for isobutanol biosynthesis in Bacillus subtilis. Appl. Biochem. Biotechnol. 2012;168:1–9.10.1007/s12010-011-9268-1
- Biswas R, Yamaoka M, Nakayama H, et al. Enhanced production of 2,3-butanediol by engineered Bacillus subtilis. Appl. Microbiol. Biotechnol. 2012;94:651–658.10.1007/s00253-011-3774-5
- Wang M, Fu J, Zhang X, Chen T. Metabolic engineering of Bacillus subtilis for enhanced production of acetoin. Biotechnol. Lett. 2012;34,1877–1885.
- Tashiro Y, Shinto H, Hayashi M, Baba S, Kobayashi G, Sonomoto K. Novel high-efficient butanol production from butyrate by non-growing Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) with methyl viologen. J. Biosci. Bioeng. 2007;104:238–240.10.1263/jbb.104.238
- Tsau JL, Guffanti AA, Montville TJ. Conversion of pyruvate to acetoin helps to maintain pH homeostasis in Lactobacillus plantarum. Appl. Environ. Microbiol. 1992;58:891–894.
- Yoshida KI, Fujita Y, Ehrlich SD. An operon for a putative ATP-binding cassette transport system involved in acetoin utilization of Bacillus subtilis. J. Bacteriol. 2000;182:5454–5461.10.1128/JB.182.19.5454-5461.2000
- Paidhungat M, Ragkousi K, Setlow P. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 2001;183:4886–4893.10.1128/JB.183.16.4886-4893.2001
- Lindsay JA, Murrell WG. Solution spectroscopy of dipicolinic acid interaction with nucleic acids: role in spore heat resistance. Curr. Microbiol. 1986;13:255–259.10.1007/BF01568649
- Takahashi F, Sumitomo N, Hagihara H, Ozaki K. Increased dipicolinic acid production with an enhanced spoVF operon in Bacillus subtilis and medium optimization. Biosci. Biotechnol. Biochem. 2015;79:505–511.10.1080/09168451.2014.978261
- Eymann C, Mittenhuber G, Hecker M. The stringent response, σ H-dependent gene expression and sporulation in Bacillus subtilis. Mol. Gen. Genet. 2001;264:913–923.10.1007/s004380000381
- Zuber P, Losick R. Use of a lacZ fusion to study the role of the spoO genes of Bacillus subtilis in developmental regulation. Cell. 1983;35:275–283.10.1016/0092-8674(83)90230-1
- Morimoto T, Ara K, Ozaki K, Ogasawara N. A simple method for introducing marker-free deletions in the Bacillus subtilis genome. Methods Mol. Biol. 2011;765:345–358.
- Morimoto T, Loh PC, Hirai T, et al. Six GTP-binding proteins of the Era/Obg family are essential for cell growth in Bacillus subtilis. Microbiology. 2002;148:3539–3552.
- Toya Y, Hirasawa T, Morimoto T, et al. 13C-metabolic flux analysis in heterologous cellulase production by Bacillus subtilis genome-reduced strain. J. Biotechnol. 2014;179:42–49.10.1016/j.jbiotec.2014.03.025
- Ito Y, Hirasawa T, Shimizu H. Metabolic engineering of Saccharomyces cerevisiae to improve succinic acid production based on metabolic profiling. Biosci. Biotechnol. Biochem. 2014;78:151–159.10.1080/09168451.2014.877816
- Zhu F, Cai J, Wu X, et al. The main byproducts and metabolic flux profiling of γ-PGA-producing strain B. subtilis ZJU-7 under different pH values. J. Biotechnol. 2013;164:67–74.10.1016/j.jbiotec.2012.12.009
- Shivers RP, Dineen SS, Sonenshein AL. Positive regulation of Bacillus subtilis ackA by CodY and CcpA: establishing a potential hierarchy in carbon flow. Mol. Microbiol. 2006;62:811–822.10.1111/mmi.2006.62.issue-3
- Doucette CD, Schwab DJ, Wingreen NS, Rabinowitz JD. α-ketoglutarate coordinates carbon and nitrogen utilization via enzyme I inhibition. Nat. Chem. Biol. 2011;7:894–901.10.1038/nchembio.685