Abstract
Synthesized urushiol derivatives possessing different carbon atomic length in the alkyl side chain inhibited the growth of food spoilage and pathogenic microorganisms. Particularly, non-allergenic 3-pentylcatechol showed a broad antimicrobial spectrum on an agar plate. Most food spoilage and pathogenic microorganisms were sensitive to urushiol derivatives in the liquid culture. The morphologies of the microorganisms were changed after treatment of 3-pentylcatechol.
Urushiols are major components contained in the lacquer tree (Rhus verniciflua Stokes, Anacardiaceae), which has been used as food and medicinal herb for preventing stomach diseases, inflammatory diseases, and various cancers in Korea.Citation1,2) They consist of o-dihydroxybenzene (catechol) coupled with a saturated or unsaturated alkyl side chain of 15 or 17 carbons in the C-3 position of the benzene ring and are amphipathic compounds.Citation3) They exert various biological effects such as antioxidantCitation4) and anticancer Citation5) activities. In particular, natural urushiols have been reported to strongly inhibit the growth of food spoilage and pathogenic microorganisms.Citation2,6,7) In addition, several studies have implicated that urushiols inhibit the growth of Helicobacter pyroli in vitro and can eradicate H. pyroli from infected rat gastric tissue.Citation2,8) Natural phenolic lipids such as anacardic acid, cardanol, resorcinol, gingkolic acid, etc. have also been reported to show various biological effects including antioxidative, anticancer, and antimicrobial activities.Citation9) It has been indicated that the amphipathic and lipophilic properties of the phenolic lipids may key to their antimicrobial activity.Citation10,11) Therefore, the antimicrobial activity of natural urushiol might be due to its amphipathic and high lipophilic properties. However, the urushiols have the fatal defect of inducing strong allergic reactions such as skin redness, swelling, inflammation, and irritation on contact.Citation12,13)
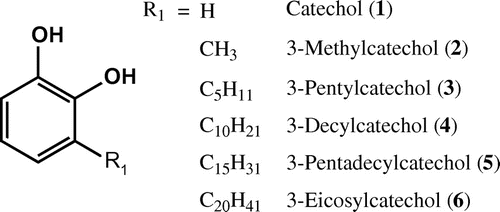
In the previous study, we chemically synthesized catechol (CTUD, 1–6)-type urushiol derivatives possessing different carbon atomic length in the alkyl side chain.Citation14) Some of the synthesized urushiol derivatives did not induce contact hypersensitivity, whereas 3-decylcatechol (4) and 3-pentyldecylcatechol (6) consisting of –C10H21 and –C15H31 in the alkyl side chains (Fig. ) caused an allergic reaction when the ears of rats were sensitized by the synthesized compounds every day for 20 days. In addition, the non-allergenic urushiol derivatives showed hydrophobic properties and very high affinities for phospholipid membranes. It was considered that the non-allergenic urushiol derivatives may have the potential to act as excellent antimicrobial agents. Therefore, we screened the antimicrobial activities of 12 synthesized urushiol derivatives with various carbon atomic lengths in the alkyl side chain against 5 food spoilage bacteria and 5 pathogenic microorganisms.
Antimicrobial activities of the urushiol derivatives (1–6) against food spoilage and pathogenic microorganisms were determined by an agar disk diffusion method.Citation15) Sterilized agar (80 mL) containing 0.2 mL of the cultured microorganisms was prepared with an equal thickness on petriplates (φ 15 cm petri dish, Corning, New York, USA). A paper disk treated with an urushiol derivative was placed on a solid plate. The diameter (mm) of the inhibition zone was measured after 16 h of incubation at the optimal growth temperature for each microorganism. H. pylori was incubated for 48 h at 37 °C in a 10% CO2 incubator (MCO175, Sanyo, Osaka, Japan). The inhibition zones of the CTUDs (1–6) against food spoilage and pathogenic microorganisms were measured, and their results are presented in Table . CTUDs (1–4) inhibited the growth of most microorganisms, although the activities on microorganisms varied depending on the alkyl side chain length (Table ). Compound 3 showed the highest antimicrobial activity among the CTUDs (1–6). Compound 3 was more effective against Escherichia coli and Candida albicans compared to the other tested microorganisms. Although compound 2 showed weaker antimicrobial activity than 3, its inhibitions were still more effective than benzoic acid (BA) at the same concentration of 5 μmol against all the tested microorganisms, except for Bacillus subtilis. However, compound 2 did show a stronger inhibitory effect than 3 against one microorganism, Vibrio vulnificus. Compound 4 inhibited the growth of four gram positive bacteria (Staphylococcus aureus, Staphylococcus epidermidis, B. subtilis, and Streptococcus mutans) and E. coli, but did not inhibit the growth of other microorganisms. In addition, compounds 5 and 6, which consisted of –C15H31 and –C20H41 in the alkyl side chain, did not inhibit the growth of all the tested microorganisms except for H. pyroli in agar plate. In the case of H. pyroli, compound 3 showed the strongest inhibitory activity, with even higher activity than those of nalidixic acid, erythromycin, tetracycline, and ampicillin, which have generally been used to eradicate H. pyroli.Citation16) The inhibition activities of 2, 4, and 5 against H. pyroli were similar to those of erythromycin and ampicillin. However, 1, which consisted of –H1 in the alkyl side chain, did not inhibit the growth of H. pyroli in agar plate.
Table 1. Inhibition of food spoilage and pathogenic microorganisms by catechol-type urushiol derivatives (1–6).
To measure MIC value in liquid culture, urushiol derivatives (1–6) and BA were prepared, respectively, at the final concentration of 4–500 μg/mL in sterilized broth (5 mL). Each culture of microorganisms was incubated for 16 h in a shaking incubator at optimal temperature. H. pylori was incubated for 18 h at 37 °C in a 10% CO2 incubator. The cultured broths (1 mL) were mixed with agar (14 mL) that was maintained at 50 °C, then poured on a plate. Agar plates were incubated under their optimal growth conditions for 16–18 h. The minimum concentration of the compound at which no colonies were detected on the agar plate was taken as the MIC value. Most food spoilage and pathogenic microorganisms were sensitive to the CTUDs (1–6), and their MIC values ranged from 0.03 to 2.68 μmol/mL. CTUDs (3–6) showed lower MIC values than BA in most cases (0.20–1.64 μmol/mL) (Table ). In contrast to the results obtained by the disk diffusion method, the CTUDs inhibited the growth of most tested microorganisms, although their MIC values were slightly different. Compounds 4–6, which consisted of the alkyl side chains with over 10 carbons, showed relatively higher antimicrobial activity than 1–3. This trend in results was similar for the antimicrobial activity of CTUDs against most tested microorganisms, except for C. albicans. In contrast, the lowest MIC value of CTUDs against C. albicans was observed in the case of CTUD (3). In the MIC results, CTUDs (1–6) exhibited relatively higher antimicrobial activity against H. pyroli among the tested microorganisms (Table ). The MIC values of 5 and 6, which consist of the alkyl side chain of –C15H31 and –C20H41, were 0.03 ± 0.0 μmol/mL, which were similar to those of nalidixic acid and erythromycin. The MIC value of 3, which possesses high inhibitory activity in the agar disk diffusion method, was relatively lower than those of 4–6, but higher than ampicillin.
Table 2. MIC of catechol-type urushiol derivatives (1–6) against food spoilage and pathogenic microorganisms.
The results of an agar disk diffusion assay showed 4–6 having alkyl side chains of over C10H21 in the catechol structure only slightly inhibited or did not at all inhibit the growth of microorganisms on the agar plate, while they showed high antimicrobial activities in the liquid culture (Table ). Several studies have indicated that the agar diffusion assay is not suitable for antimicrobial compounds, especially less polar compounds, because they have slower diffusion into agar medium, and therefore, their inhibitions are not frequently observed.Citation17,18) In our previous study, 4–6 showed relatively higher lipophilicities than other CTUDs (1–3).Citation14) Therefore, the lack of antimicrobial activity of 4–6 against the microorganisms tested in the agar disk diffusion assay might be due to their high lipophilicities.
In the results of MIC value in liquid culture, the antimicrobial activities of CTUDs (1–6) tended to increase with the increase in alkyl side chain length, although their activities varied somewhat among the tested microorganisms. This trend was very similar to that reported for the antimicrobial activities of natural and synthesized anacardic acid derivatives, which possess alkyl side chains of C8-C15 in salicylic acid.Citation19) They explained that alkyl chain length and partition coefficient (log P) value of anacardic acid derivatives were associated with the antimicrobial activity against S. aureus and S. mutans. In addition, several studies have implicated that lipophilic property is the key factor for determining antimicrobial activity.Citation9,20) Our previous study revealed that the lipophilicities of the CTUDs were not proportional to the length of the carbon atoms in the alkyl side chain, but simply increased with the increase in the length of the carbon atoms.Citation14) These observations suggested that the alkyl side chains act as an important factor for determining the antimicrobial activity of urushiol derivatives.
Of the non-allergenic CTUDs (1–3, 6), compound 3 showed a broad spectrum of antimicrobial activity both in solid and liquid cultures. Therefore, compound 3 was selected for analysis of morphological changes to the food spoilage and pathogenic microorganisms.Citation5) The tested microorganisms were treated with 3 (5 μmol/mL) for 18 h, fixed in 0.05 M cacodylate buffer (pH 7.2) containing 2% glutaraldehyde and 2% paraformaldehyde at 4 °C for 24 h. The samples were dehydrated with a graded series of ethanol and iso-amylacetate. After freeze-drying, the dried cells were coated with gold-palladium. The morphology of the cells was observed by an S-2400 Hitachi scanning electron microscopy (SEM, S-2400 Hitachi. Co. Ltd, Tokyo, Japan). The SEM images showed the morphologies with a loss of the normal form in comparison with the control, which exhibited a bright and smooth surface (supplementary data). In addition, the membrane surfaces of most tested microorganisms were disrupted, while some microorganisms including S. aureus, Salmonella enterica, V. vulnificus, and C. albicans formed clusters.
Several studies have reported that the efficiency of amphiphatic antimicrobial compounds is influenced by their affinity to the membranes of microorganisms.Citation9,10) Our previous reported that the affinity of CTUDs (1–6) to liposome membrane tended to increase with the increase of the carbon atomic length of the alkyl side chains.Citation14) In this study, we found that there was an apparent correlation between the antimicrobial activity and lipophilicity, although the activities observed varied slightly between the microorganisms tested. The results for morphological change of all of the tested microorganisms suggested that non-allergenic 3 may disorder the fluid bilayer of the microbial membranes. Further studies on the mechanism of action of the urushiol derivatives against microorganisms will be conducted.
In this study, we demonstrated that the CTUDs (1–6) effectively inhibited the growth of food spoilage and pathogenic microorganisms in solid and liquid cultures. Our previous study revealed that 4 and 5, which have 10 and 15 carbon atoms in the alkyl chain, caused serious contact hypersensitivity.Citation14) In contrast, the other CTUDs (1–3, 6) did not cause an allergenic reaction on contact and also had the ability to scavenge radicals generated in the liposome membrane and to chelate transition metal ions.Citation14) In particular, the non-allergenic urushiol derivatives, 3 and 6, which contain 5 and 20 carbon atoms in the alkyl chain, showed high growth inhibitory effect against the food spoilage and pathogenic microorganisms. Therefore, this study opens the possibilities for use of non-allergenic 3 and 6, which may become potential candidates for use as antimicrobial and antioxidative agents as food preservatives.
Author contribution
J.Y.C., K.Y.P, and J.H.M. designed research; J.Y.C. and K.Y.P. performed research; J.Y.C., K.Y.P., S.J.K., S.J.O., and J.H.M. analyzed data; J.Y.C. and J.H.M. wrote the manuscript.
Supplementary material
The supplementary material for this paper is available online at http://dx.doi./org/10.1080/09168451.2015.1061418.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology [grant number NRF-2013R1A1A2012410].
Supplementary_Data.doc
Download MS Word (165 KB)References
- Choi W, Jung H, Kim K, et al. Rhus verniciflua stokes against advanced cancer: A perspective from the Korean Integrative Cancer Center. J. Biomed. Biotechnol. 2012;2012:874276.
- Suk KT, Kim HS, Kim MY, et al. In vitro antibacterial and morphological effects of the urushiol component of the sap of the Korean lacquer tree (Rhus vernicifera Strokes) on Helicobacter pyroli. J. Korean Med. Sci. 2010;25:399–404.10.3346/jkms.2010.25.3.399
- Symes WF, Dawson CR. Poison Ivy “Urushiol”. J. Am. Chem. Soc. 1954;76:2959–2963.10.1021/ja01640a030
- Hong SH, Suk KT, Choi SH, et al. Anti-oxidant and natural killer cell activity of Korean red ginseng (Panax ginseng) and urushiol (Rhus vernicifera Stokes) on non-alcoholic fatty liver disease of rat. Food Chem. Toxicol. 2013;55:586–591.10.1016/j.fct.2013.01.022
- Choi JY, Park CS, Choi JO, Rhim HS, Chun HJ. Cytotoxic effect of urushiol on human ovarian cancer cells. J. Microbiol. Biotechnol. 2001;11:399–405.
- Kim JC, Ahn JK, Ko SY, Choi YH, Kim DH, Lee TY. Antimicrobial activities of urushiol and urushiol derivatives. Clean Technol. 2007;13:22–27. Korean.
- Kim D, Jeon SL, Seo J. The preparation and characterization of urushiol powders (YPUOH) based on urushiol. Prog. Org. Coat. 2013;76:1465–1470.10.1016/j.porgcoat.2013.05.034
- Suk KT, Baik SK, Kim HS, et al. Antibacterial effects of the urushiol component in the sap of the lacquer tree (Rhus verniciflua Stokes) on Helicobacter pylori. Helicobacter. 2011;16:434–443.10.1111/j.1523-5378.2011.00864.x
- Stasiuk M, Kozubek A. Biological activity of phenolic lipids. Cell. Mol. Life Sci. 2010;67:841–860.10.1007/s00018-009-0193-1
- Caturla N, Vera-Samper E, Villalaín J, Mateo R, Micol V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radical Biol. Med. 2003;34:648–662.10.1016/S0891-5849(02)01366-7
- Kubo I, Xiao P, Fujita K. Antifungal activity of octyl gallate: structural criteria and mode of action. Bioorg. Med. Chem. Lett. 2001;11:347–350.10.1016/S0960-894X(00)00656-9
- Ma XM, Lu R, Miyakoshi T. Recent advances in research on lacquer allergy. Allergol. Int. 2012;61:45–50.10.2332/allergolint.11-RA-0324
- Wakabayashi T, Hu DL, Tagawa Y, et al. IFN-gamma and TNF-alpha are involved in urushiol-induced contact hypersensitivity in mice. Immunol. Cell Biol. 2005;83:18–24.10.1111/icb.2005.83.issue-1
- Kim JY, Cho JY, Ma YK, Lee YG, Moon JH. Nonallergenic urushiol derivatives inhibit the oxidation of unilamellar vesicles and of rat plasma induced by various radical generators. Free Radical Biol. Med. 2014;71:379–389.10.1016/j.freeradbiomed.2014.03.041
- Zaika LZ. Spices and herbs. Their antimicrobial activity and its determination. J. Food Saf. 1998;9:97–118.
- Tabak M, Armon R, Neeman I. Cinnamon extracts’ inhibitory effect on Helicobacter pylori. J. Ethnopharmacol. 1999;67:269–277.10.1016/S0378-8741(99)00054-9
- Mann CM, Markham JL. A new method for determining the minimum inhibitory concentration of essential oils. J. Appl. Microbiol. 1998;84:538–544.10.1046/j.1365-2672.1998.00379.x
- Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radical Res. 2006;40:223–231.10.1080/10715760500473834
- Muroi H, Kubo I. Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillin-resistant Staphylococcus aureus. J. Appl. Bacteriol. 1996;80:387–394.10.1111/jam.1996.80.issue-4
- de Almeida CG, Diniz CG, Silva VL, Saraiva MF, Le Hyaric ML, de Almeida MV. Antibacterial activity of lipophilic fluoroquinolone derivatives. Med. Chem. 2009;5:419–421.10.2174/157340609789117859