Abstract
Collagen tripeptide (CTP) is a collagen-derived compound containing a high concentration of tripeptides with a Gly-X-Y sequence. In this study, the concentrations and metabolites of CTP were monitored in rat plasma after its administration. We performed a quantitative analysis using high-performance liquid chromatography tandem mass spectrometry according to the isotopic dilution method with stable isotopes. We confirmed that the tripeptides Gly-Pro-Hyp, Gly-Pro-Ala, and Gly-Ala-Hyp were transported into the plasma. Dipeptides, which are generated by degradation of the N- or C-terminus of the tripeptides Gly-Pro-Hyp, Gly-Pro-Ala, and Gly-Ala-Hyp, were also present in plasma. The plasma kinetics for peroral and intraperitoneal administration was similar. In addition, tripeptides and dipeptides were detected in no-administration rat blood. The pharmacokinetics were monitored in rats perorally administered with Gly-[3H]Pro-Hyp. Furthermore, CTP was incorporated into tissues including skin, bone, and joint tissue. Thus, administering collagen as tripeptides enables efficient absorption of tripeptides and dipeptides.
Graphical abstract
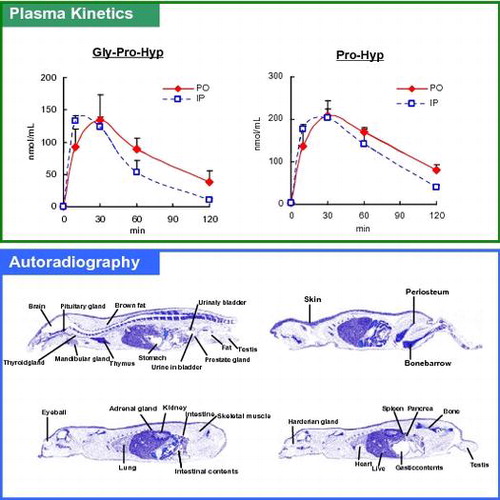
Plasma kinetics of peptides in the two routes of administration, and autoradiographs after peroral administration of Gly-[3H]Pro-Hyp.
Collagen is a major protein that constitutes approximately 30% of all proteins in vivo. It is found primarily in skin, bone, cartilage, and tendons where it plays a critical role in both structure and function. Collagen hydrolysates, which are widely used in cosmetics and food products, are produced by hydrolysis of gelatine extracted from animal or fish collagen. Several studies have indicated that perorally administered gelatine or collagen hydrolysates have beneficial effects, such as improving joint pain,Citation1) increasing bone density,Citation2,3) decreasing blood pressure,Citation4) increasing the moisture content of stratum corneum,Citation5,6) and modulating the circulatory system.Citation7)
Collagen has a characteristic acases, only amino acid sequence in which glycine is present at every third position (Gly-X-Y). Hence, the Gly-X-Y triplet is the minimum unit of collagen. Among these sequences, Gly-Pro-Hyp is the most abundant, followed by Gly-Pro-Ala and Gly-Ala-Hyp.Citation8) Iwai et al. reported that dipeptides and tripeptides were present in the blood after ingesting collagen hydrolysates, thus confirming collagen hydrolysates transported into the blood stream without complete degradation to amino acids.Citation9) Furthermore, short-chain peptides originating from collagen have physiological activity. The Gly-Pro-Hyp tripeptide interacts with plateletsCitation10,11) and the central nervous systemCitation12), whereas the Pro-Hyp dipeptide affects cellular proliferation and hyaluronic acid synthesisCitation13) and helps in protecting joint cartilage.Citation14) Peroral administration of isotopically labeled [14C]Pro-Hyp in rats, followed by whole-body autoradiography, confirmed that the isotopically labeled peptide reached the tissues after its administration.Citation15) Thus, the physiological activity of short-chain peptides is believed to participate in metabolism and have beneficial effects in vivo. Therefore, efficient intake methods for the functional components of collagen are in demand.
Because they were randomly processed by endopeptidase in a molecular-weight-reduction treatment, the length or sequence of peptide fragments in collagen hydrolysates was inconsistent. Therefore, we developed a method that uses a specific protease to hydrolyze collagen into collagen tripeptide (CTP) fragments that contain highly pure Gly-X-Y tripeptides. These CTP are produced in several sequences, including Gly-Pro-Hyp, Gly-Pro-Ala, and Gly-Ala-Hyp. CTP can help in healing boneCitation16,17) and has several beneficial effects on the skin, including protection against photoaging caused by UV-B irradiationCitation18) and anti-wrinkle and anti-pruritus effects,Citation19) and enhancing hyaluronic acid synthesis.Citation19) In addition, a hydrolysate of chicken collagen peptides rich in Gly-Pro-Hyp can affect osteoporosis.Citation20) These reports indicate that collagen-derived tripeptides contain functional peptides and are efficacious when administered perorally.
The peptide transporter PEPT1, which is located on the brush border membrane (BBM) of the small intestine, contributes to short-chain peptide (i.e. CTP) transport into the blood. PEPT1 recognizes dipeptides and tripeptides as direct substrates and transports them across intestinal lumen into the blood circulation.Citation21) An experiment that used artificially constructed BBM vesicles to examine Gly-Pro-Hyp intake found only Pro-Hyp inside the vesicles,Citation22) and the result might mean that Gly-Pro-Hyp could not be transported across BBM and into the blood stream. However, Watanabe-Kamiyama et al. performed an experiment in which they perorally administered isotopically labeled Gly-Pro-Hyp to rats and analyzed the radioactive tracer in blood plasma by thin layer chromatography (TLC). They confirmed that the tripeptide was present in blood plasma in its intact form.Citation20) However, they also detected proline, dipeptides, and other unidentified components in the plasma, suggesting only a partial degradation of the tripeptides. Therefore, the transport efficiency of the intact form remains uncertain.
There are few reports concerning the degradation of collagen-derived tripeptides. In addition, it is generally known that peptide hydrolysis occurs in cells and in the blood. Accordingly, factors other than enzymatic degradation in the digestive tract and membrane digestion should be considered to understand the efficiency of CTP transport into the blood. Quantitative examination of the extent to which intact CTP is transported into the blood, as well as its metabolite peptides, is of great importance for understanding in vivo absorption of functional peptides.
We measured metabolite levels in rat blood plasma after administering CTP perorally or intraperitoneally. Previous studies have examined enzymatic degradation of CTP through the digestive tract and its degradation by peptide transporters. This study was designed to compare the plasma kinetics of CTP and its metabolites with and without digestion. We analyzed eight peptides, including the major tripeptide components (Gly-Pro-Hyp, Gly-Pro-Ala, and Gly-Ala-Hyp), the potential dipeptide components (Pro-Hyp, Gly-Pro, Pro-Ala, and Ala-Hyp), and a dipeptide component that cannot be generated from CTP (Hyp-Gly). Furthermore, previous reports on collagen metabolites in plasma were limited to peroral administration and only identified components present at high levels.Citation9,23) In this study, we established a high-performance liquid chromatography tandem mass spectrometry (HPLC–MS/MS) system based on a stable isotope dilution that enabled analyses at lower concentrations.
Materials and methods
CTP preparation
CTP-100 used in this study was from Jellice Co., Ltd. Briefly, CTP was prepared from porcine skin collagen digested using a collagenase-type protease (Protease N, Nagase Chemtex Corporation, Osaka, Japan). The digest was deionized using an ion-exchange resin (DIAION type SK, Mitsubishi Chemical, Tokyo, Japan), passed through a 0.2-μm filter, and was then subjected to ion-exchange chromatography using Toyopearl DEAE-650 (TOSOH Corp., Japan). The tripeptide fraction was isolated using reverse-phase high-pressure liquid chromatography (HPLC). The mean molecular weight of CTP is approximately 300. The purity of CTP was analyzed using HPLC with a Superdex peptide gel filtration column (GE Healthcare UK Ltd., England) and quantitated by integrating the absorbance at 214 nm of the major peak. The composition of tripeptide components is expressed as the area ratio (%). The tripeptide content was ≥ 90% after this purification step, and its major components include peptides such as Gly-Pro-Hyp (34%), Gly-Pro-Ala (15%), and Gly-Ala-Hyp (4%). Moreover, CTP includes other tripeptides (about 40%) such as Gly-Pro-Ser, Gly-Pro-Lys, Gly-Pro-Pro, Gly-Pro-Arg, Gly-Pro-Hyl, Gly-Pro-Gln, Gly-Ala-Ala, Gly-Ala-Arg, Gly-Ala-Asp, Gly-Ala-Lys, Gly-Ala-Ser, Gly-Ser-Hyp, Gly-Ser-Ala, Gly-Lys-Asp, and Gly-Glu-Gln, and each tripeptide was less than 3%. On the other hand, CTP also contained Gly-Pro (5%) as a dipeptide, but it did not contain almost the other dipeptides such as Pro-Hyp (0.5%), Ala-Hyp (0.3%), and Hyp-Gly (0.05%.), and the peptide composed of four or more amino acids.
Chemicals
Saline was purchased from Otsuka Pharmaceutical Co., Ltd. Gly-Pro-Hyp, Gly-Pro-Ala, Gly-Ala-Hyp, Pro-Hyp, Gly-Pro, Pro-Ala, Ala-Hyp, and Hyp-Gly were purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). Isotopically labeled tripeptides ([13C2]Gly-Pro-Hyp, [13C2]Gly-Pro-Ala, and [13C2]Gly-Ala-Hyp) were synthesized by Peptide Institute Inc. (Osaka, Japan). Isotopically labeled dipeptides ([13C15N]Pro-Hyp, Gly-[13C15N]Pro, [13C15N]Pro-Ala,[13C3,15N]Ala-Hyp, and Hyp-[13C2,15N]Gly) were synthesized by Scrum Inc. (Tokyo, Japan). HPLC–MS/MS graded water, acetonitrile (ACN), formic acid, trifluoroacetic acid (TFA), and trichloroacetic acid (TCA) were purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan).
Pharmacokinetic studies
This experiment adhered to the Guidelines for Animal Experimentation of Kanagawa Dental University. Nine-week-old Wister rats were purchased from Clea Japan, Inc. (Tokyo, Japan) and housed in a cage room maintained at a constant temperature of 23 ± 2 °C, humidity of 55 ± 2%, and 12-h light–dark cycle. Solid food and water were freely available. After 3 days of preliminary rearing, the rats were divided into three groups to minimize weight variation among individuals. The groups were administered CTP perorally (n = 5), intraperitoneally (n = 5), or were not treated (no-administration, n = 3). The peroral administration group was fed CTP dissolved in saline (446 mg/kg body weight) using gastric sonde. Blood was collected under anesthesia through a small excision at the tip of the tail (tail snip) at 0, 10, 30, 60, and 120 min after CTP administration. For the intraperitoneal group, CTP was dissolved in saline (446 mg/kg body weight) and injected intraperitoneally using a syringe. Blood was collected from the caudal vein at 0, 10, 30, 60, and 120 min after CTP administration. For the no-administration group, blood was collected from the tip of the tail. Blood samples were centrifuged to obtain plasma, which was stored at −80 °C for further analysis.
Preparation of standard samples
The standards were dissolved with 0.1% TFA. The final concentrations of standard solutions (STD 1–4) were the following: Gly-Pro-Hyp: 7, 35, 175, and 351 pmol/mL; Gly-Pro-Ala: 8, 41, 205, and 410 pmol/mL; Gly-Ala-Hyp: 8, 39, 193, and 386 pmol/mL; Pro-Hyp: 9, 44, 219, and 438 pmol/mL, Gly-Pro: 12, 58, 290, and 581 pmol/mL; Pro-Ala: 11, 54, 269, and 537 pmol/mL; Ala-Hyp: 10, 49, 247, and 495 pmol/mL; and Hyp-Gly: 11, 53, 266, and 531 pmol/mL. Peptides labeled with stable isotopes were added to each STD 1–4 as internal standards. The final concentration of each internal standard was as follows: Gly-Pro-Hyp, 36 pmol/mL; Gly-Pro-Ala, 43 pmol/mL; Gly-Ala-Hyp, 40 pmol/mL; Pro-Hyp, 60 pmol/mL; Gly-Pro, 51 pmol/mL; Pro-Ala, 63 pmol/mL; Ala-Hyp, 51 pmol/mL; and Hyp-Gly, 61 pmol/mL. The purity of corresponding dipeptides and tripeptides in each internal standard was over 90% whenestimated with HPLC.
Preparation of blood samples
Internal standards were added to blood plasma (diluted if necessary) with a final concentration equivalent to that of STD. Furthermore, the spiked plasma samples were deproteinized using 4% (v/v) TCA and centrifuged at 14,000 rpm for 15 min at 4 °C. 10 μL of the supernatant was directly injected into the column and analyzed by an HPLC–MS/MS system described below.
HPLC–MS/MS (MRM) analysis
An HPLC–MS/MS system including ultra-fast liquid chromatography (UFLC) (Shimazu, Kyoto, Japan) and 4000 QTRAP (AB Sciex, Way-Carlsbad, USA) was used to analyze all samples. The supernatant sample (10 μL) was injected and separated by a Sunniest RP-AQUA column (2.1 × 150 mm, 5 μm; ChromaNIK Technologies, Osaka, Japan) and then detected by the multiple reaction monitoring (MRM) mode.
The HPLC gradient elution was conducted with eluents A [0.1% (v/v) formic acid/water] and B [0.1% (v/v) formic acid/acetonitrile]. Eluent B was linearly increased from 0 to 10% (0.0–0.5 min) and then to 30% (0.5–4.0 min). The flow rate was 0.2 mL/min, and the column temperature was 30 °C.
The MS analytical conditions used were the following: positive ion electrospray (ESI) mode at a capillary voltage of 4.5 kV, desolvation voltage of 25 V, curtain gas (nitrogen) at 12 L/h, 65 psi, and 600 °C. The MRM acquisition mode was used at a nitrogen gas pressure of 65 psi and collision voltage of 30 V. All data were acquired and processed using the Analyst software, version 1.4.1 (AB Sciex, Way-Carlsbad, USA).
Whole-body autoradiography
This experiment was conducted according to the Guidelines for Animal Experimentation of Sekisui Medical Co., Ltd. Five-week-old male Crj:CD (SD) IGS rats were purchased from Charles River Laboratories Japan, Inc., Yokohama, Japan. They were conditioned for 1 week in a room maintained with a temperature of 23 ± 2 °C, a relative humidity of 55 ± 10%, and light for 12 h per day from 6:00 to 18:00. The rats were allowed free access to food and water (AIN-93G, Oriental Yeast Co., Ltd., Tokyo, Japan).
Tritium labeled Gly-[3H]Pro-Hyp (0.35 mmol/kg, 5 mL/kg, 74 MBq/kg) was administered via a gastric tube to rats that had fasted for 16 h. The animals were sacrificed 24 h after administration by an overdose of inhaled ether. After euthanasia, the rat carcasses were deep-frozen in dry ice–acetone. After rapidly removing the fur, nasal cavity, and anus of each animal, the animal was closed with 5% (w/v) sodium carboxymethyl cellulose and deep-frozen in dry ice–acetone. The frozen samples were then mounted onto a Cryo-microtome (Cryo-microcut, Lica, Germany) after the legs and tail had been removed. The resulting sections were exposed on an X-ray film (Hyperfilm, Amersham Pharmacia Biotech) for 12 days at 4 °C.
Results
Analysis of standard samples
Limit of quantification (LOQ) for each peptide was the following: Gly-Pro-Hyp, 0.175 pmol/mL; Gly-Pro-Ala, 0.410 pmol/mL; Gly-Ala-Hyp, 1.157 pmol/mL; Pro-Hyp, 4.381 pmol/mL; Gly-Pro, 1.162 pmol/mL; Pro-Ala, 0.054 pmol/mL; Ala-Hyp, 0.989 pmol/mL; and Hyp-Gly, 0.159 pmol/mL. These values indicated a sensitive quantitation at low concentrations.
Detection of peptides in plasma in no-administration rats
All peptides expect for Gly-Ala-Hyp were detectable in blood plasma for the no-administration group, but Gly-Ala-Hyp could be barely detected. Components present in a particularly high abundance were Gly-Pro-Hyp (0.484 ± 0.239 nmol/mL), Pro-Hyp (3.034 ± 0.315 nmol/mL), and Gly-Pro (0.214 ± 0.056 nmol/mL). The other detected peptides were Gly-Pro-Ala (0.026 ± 0.003 nmol/mL), Ala-Hyp (0.024 ± 0.005 nmol/mL), Pro-Ala (0.050 ± 0.014 nmol/mL), and Hyp-Gly (0.034 ± 0.007 nmol/mL).
Concentration of peptides in plasma after CTP administration
Fig. shows the plasma levels of tripeptides and dipeptides after peroral or intraperitoneal administration of CTP. And the Tmax (min), Cmax (nmol/mL), and AUC(min nmol/mL) of each peptide are shown in Table . Compared with the no-administration group, significant increases were observed in the plasma concentrations of all tripeptides after peroral and intraperitoneal administration, indicating the detected tripeptides originated from the administered CTP. The concentration of Gly-Pro-Hyp drastically increased as early as 10 min after administration and reached the maximum concentration (Cmax) within 30 min. Cmax of Gly-Pro-Hyp exceeded 130 nmol/mL, which was the highest among all tripeptides. In addition, the AUC for Gly-Pro-Hyp was the highest among the tripeptides. Although the plasma concentrations of Gly-Pro-Ala and Gly-Ala-Hyp increased as early as 10 min after administration, their subsequent increases were moderate and their time to reach Cmax (Tmax) was longer than that for Gly-Pro-Hyp, which had a relatively low Cmax and AUC. There were no differences in concentrations, Cmax, or AUC between perorally and intraperitoneally administered tripeptides. However, Tmax tended to be earlier in these tripeptides with intraperitoneal administration than with peroral administration.
Fig. 1. Plasma levels of tripeptides and dipeptides after proral or intraperitoneal administration of CTP.
Notes: (A) Gly-Pro-Hyp, (B) Gly-Pro-Ala, (C) Gly-Ala-Hyp, (D) Pro-Hyp, (E) Gly-Pro, (F) Pro-Ala, (G) Ala-Hyp, and (H) Hyp-Gly. Values presented as the mean ± standard error, n = 5 subjects. Symbols: p.o.(◆), i.p.(□).
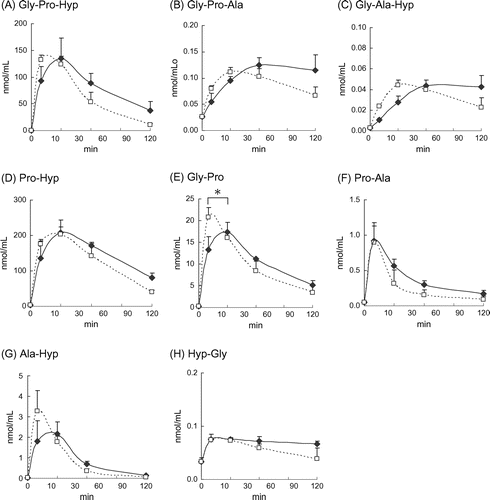
Table 1. AUC, Cmax, and Tmax of tripeptides and dipeptides in plasma after peoral administration of CTP.
Enzymatic hydrolysis of amino acid residues from the N- or C-terminus of tripeptides generates dipeptides. Therefore, blood-concentration changes for Pro-Hyp, Pro-Ala, Ala-Hyp, and Gly-Pro were indicative of degradation. Significant increase in Pro-Hyp, Pro-Ala, Ala-Hyp, and Gly-Pro concentrations occurred with peroral and intraperitoneal administration when compared with those in the no-administration group.
The Pro-Hyp concentration rapidly increased 10 min after administration with a quantity that was significantly higher than that of other dipeptides. Pro-Hyp Cmax exceeded 200 nmol/mL, which was higher than its precursor Gly-Pro-Hyp. Gly-Pro had the second highest concentration after Pro-Hyp in dipeptides, but its Cmax and AUC values were ≤0.1 times those of Pro-Hyp. Furthermore, the Cmax and AUC values of Gly-Pro were six times or more lower than those of Gly-Pro-Hyp, one of its potential precursors. These Gly-Pro values, however, were higher than those of Gly-Pro-Ala and Pro-Ala, and therefore, it was considered that Gly-Pro-Ala was another precursor candidate. The Cmax and AUC values of Pro-Ala were higher than those of its precursor Gly-Pro-Ala. Similarly, the Cmax and AUC values of Ala-Hyp were higher than those of its precursor Gly-Ala-Hyp.
Cmax, Tmax, and AUC of Pro-Hyp and Pro-Ala were similar between the two administration routes. Gly-Pro showed a significantly earlier Tmax with intraperitoneal administration; however, no differences were observed for Cmax and AUC. There were no significant difference, but Tmax of Ala-Hyp tended to be earlier with intraperitoneal administration than with peroral administration. Cmax and AUC of Ala-Hyp were similar between the two administration routes. For Hyp-Gly, which is a sequence not likely to be generated from the tripeptides, a slight change in concentration was observed with both peroral and intraperitoneal administration; however, the change was negligible compared with its initial concentration.
Whole-body autoradiogram after Gly-Pro-Hyp administration
To monitor the in vivo biodistribution of the tripeptides most abundant in blood, Gly-Pro-Hyp was labeled with tritium to prepare Gly-[3H]Pro-Hyp. Gly-[3H]Pro-Hyp was perorally administered to rats, and whole-body autoradiography was used to investigate its uptake and distribution in the body. Administration of 0.35 mmol/kg (74 MBq/kg) Gly-[3H]Pro-Hyp to fasted male rats resulted in a high level of radioactivity in the periosteum, followed by the bone, bone marrow, periosteum, thymus, and skin (Fig. ).
Discussion
In this study, we examined the absorption and in vivo metabolism of collagen-derived tripeptides administered to rats. Previous studies mainly analyzed plasma metabolites after administering collagen hydrolysates of larger macromolecules.Citation9,23,24) In such cases, only specific sequences of digestion-resistant short-chain peptides were present in blood plasma after successfully traversing several digestion processes, such as enzymatic digestion in the intestinal lumens and membrane digestion at BBM.Citation25) On the other hand, CTP is a short chain substrate for PEPT1 and should be absorbed in a nearly intact form. Therefore, specific sequences may be transported into the blood stream, and tripeptides and dipeptides may be efficiently absorbed into the body. By degrading collagen polypeptide chains into fragments of three amino acid residues, we successfully obtained tripeptides of the minimum unit of a conserved collagen sequence (CTP). Further, since the health effect was revealed by ingestion of CTP in previous studies, we examined for the absorption and blood kinetics of the tripeptides and the dipeptides, which are their metabolites, when administered CTP with the N-terminal amino acid of glycine.
HPLC–MS/MS was used to perform a quantitative analysis with an isotopic dilution method using stable isotopes. One study analyzed peptides derived from collagen in the blood in the external standard method.Citation23) However, to the best our knowledge, this analysis is the first to use the isotope dilution method. If the retention for the ODS column is weak, the retention time and peak shapes are likely to change this group of peptides. In such cases, the identification and quantification by isotope dilution method is effective. This is particularly effective in analyses with low concentrations. Dipeptides and tripeptides were detected when the plasma from no-administered rats was examined. The tripeptides in CTP are enzymatically0 prepared and have been degraded using collagen as a raw material. These tripeptides were detected in the blood of no-administration rat, which was a new finding in this study. These tripeptides are considered to result from in vivo collagen metabolism, suggesting that these tripeptides are endogenous peptides and may play physiological roles in vivo. For example, tripeptides such as Gly-Pro-Hyp have physiological activity.Citation10–12) Tripeptides that exist in low blood concentrations may be natural homeostatic factors that maintain the structure and function of the bones, skin, joints, and blood vessels, which contain collagen. Future research is expected to focus on this issue.
After peroral administration of CTP, temporal changes in the concentrations of dipeptides and tripeptides were observed that substantially exceeded the initial concentrations. This clearly suggests that CTP is absorbed in blood. Cmax of Gly-Pro-Hyp, which is most abundant in CTP, was the highest among all the tripeptides, exceeding 130 nmol/mL. Meanwhile, Cmax of Pro-Hyp exceeded 200 nmol/mL, suggesting a partial degradation of Gly-Pro-Hyp into Pro-Hyp. Watanabe-Kamiyama et al., using TLC, demonstrated that Gly-Pro-Hyp was present in its intact form in plasma of rats after peroral administration.Citation20) In this study, we demonstrated that the intact tripeptide was present in high blood concentrations with peroral administration of CTP. Furthermore, our findings revealed increases in blood concentration occurred as early as 10 min after administration. This indicates that the absorption of these tripeptides from the small intestines to the blood is rapid, and the contact time with enzymes in BBM or cytosol is limited. Using an artificially constructed BBM vesicle from the small intestine of porcine, Aito-Inoue et al. demonstrated that only Pro-Hyp was absorbed into blood because Gly-Pro-Hyp is degraded into glycine and Pro-Hyp in BBM.Citation22) However, in their experiment, Gly-Pro-Hyp was mixed with BBM for 0.5–8 h, followed by centrifugation and other procedures that created an extended time delay before measurement, which may have been attributed to the difference from the administration studies. In addition, Watanabe-Kamiyama et al. indicated that the use of porcine small intestine in Inoue’s experiment might yield different results compared with those from rat studies.Citation20) Because this study also used rats, a similar explanation might be applicable.
When the plasma concentrations of the three tripeptides were compared after peroral administration, AUC and Cmax of Gly-Pro-Ala and Gly-Ala-Hyp were lower than those of Gly-Pro-Hyp. This difference did not reflect the content ratio of the product. These tripeptides may be hardly absorbed into the blood or be susceptible to hydrolysis to dipeptides or amino acids. The reason is because the blood concentrations of Pro-Ala and Ala-Hyp, which are the metabolites of Gly-Pro-Ala and Gly-Ala-Hyp, respectively, were higher than those of their parent tripeptides.
Gly-Pro is produced by C-terminal hydrolysis of Gly-Pro-Hyp or Gly-Pro-Ala and is unlikely to undergo enzymatic degradation by peptidases.Citation26) Although this sequence is often repeated in collagen, it has never been examined for collagen metabolites because it contains no hydroxyproline. In our analysis, the Cmax and AUC values of Gly-Pro were the second highest among those of all the dipeptides studied and were only less than those of Pro-Hyp. The origin of Gly-Pro in plasma between the two possible parent tripeptides is not certain. However, because the Gly-Pro concentration is low in plasma compared with that of Pro-Hyp, it can be predicted that Pro-Hyp is more easily produced from Gly-Pro-Hyp by simply removing the N-terminal amino acid, whereas Gly-Pro occurs as a result of removing the C-terminal amino acid.
We further examined the difference in absorption and concentration–time changes of metabolites between the two routes of administration. We found that the concentration–time changes were similar between peroral and intraperitoneal administration for most metabolites. Only Tmax of Gly-Pro showed a significantly earlier value with intraperitoneal administration.
Until recently, the mechanism by which tripeptides are degraded into dipeptides after intraperitoneal administration, and the exact site or organ of degradation, remains uncertain. Considering that the degradation and absorption quantities for intraperitoneal administration were equivalent to those for peroral administration, a degradation system may be present between the abdominal cavity and the site of absorption into blood. In addition, degradation from tripeptides to dipeptides rarely occurs during membrane digestion in the small intestine. Rather, after rapid absorption into the blood, degradation occurs at a site common to both routes of administration (such as the blood and liver). Further study is needed to elucidate the answer to this question; however, monitoring plasma kinetics in the portal vain may partially reveal the mechanism.
We monitored the in vivo kinetics of tripeptides in the blood to determine how the rapidly absorbed tripeptides that transported into the blood stream were later distributed in the target organs or tissues.
We investigated the biodistribution of orally administered Gly-[3H]Pro-Hyp, which was a main component in CTP, in rats to determine the uptake levels of tripeptides into the organs or tissues. After a single administration, radio activity was observed in the connective tissues including bone and skin within 24 h. The beneficial effects on the similar tissues from CTP administration have been confirmed, suggesting an association between the site of transport and the physiological activity of functional peptides. Based on the kinetics of the metabolites in plasma, Gly-Pro-Hyp was likely to be partially degraded into dipeptides. There is also a possibility that amino acids generated from the degradation migrated. Because we aimed to investigate the in vivo kinetics of labeled tripeptide Gly-[3H]Pro-Hyp and its degradation metabolites, this study does not differentiate between such cases; however, a previous study confirmed the difference in transport compared with Pro when the transport to each organ was compared after Gly-Pro-Hyp or Pro administration.Citation20) In addition, the differences in transport were indicated in a study that compared the in vivo kinetics of Pro-Hyp and Pro following peroral administration.Citation15) Based on these studies, it is likely that at least a part of Gly-Pro-Hyp has been degraded and metabolized to dipeptides; however, it is considered that not all decomposed into a single amino acid.
This study demonstrated the absorption and transport of collagen-derived tripeptides into the blood stream in their intact forms. That is, CTP was absorbed into the blood rapidly and thereafter also transported into the tissues rapidly because the mean molecular weight of CTP is much smaller than that of conventional collagen peptide and the digestion process of CTP is omitted in digestive tract. In addition, it was shown that a partial degradation generates dipeptides, but the degradation rate is a future task. Furthermore, it was indicated that the plasma kinetics for peroral and intraperitoneal administration was similar. This cause needs investigation. Some of the dipeptides have beneficial physiological activity;Citation13,14,27,28) thus, it was implied that administering collagen as tripeptides enables efficient absorption of functional peptides.
Author contribution
S.Y. and Y.S designed the study; S.Y., F.H., K.D., T.O. and T.F. performed experiments; S.Y. and K.D. analyzed data and wrote the manuscript; and Y.S. provided project management.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Moskowitz RW. Role of collagen hydrolysate in bone and joint disease. Semin. Arthritis Rheum. 2000;30:87–99.10.1053/sarh.2000.9622
- Wu J, Fujioka M, Sugimoto K, Mu G, Ishimi Y. Assessment of effectiveness of oral administration of collagen peptide on bone metabolism in growing and mature rats. J. Bone. Miner. Metab. 2004;22:547–553.10.1007/s00774-004-0522-2
- Nomura Y, Oohashi K, Watanabe M, Kasugai S. Increase in bone mineral density through oral administration of shark gelatin to ovariectomized rats. Nutrition. 2005;21:1120–1126.10.1016/j.nut.2005.03.007
- Saiga-Egusa A, Iwai K, Hayakawa T, Takahata Y, Morimatsu F. Antihypertensive effects and endothelial progenitor cell activation by intake of chicken collagen hydrolysate in pre- and mild-hypertension. Biosci. Biotechnol. Biochem. 2009;73:422–424.10.1271/bbb.80189
- Matsumoto H, Ohara H, Ito K, Nakamura Y, Takahashi S. Clinical effects of fish type I collagen hydrolysate on skin properties. ITE Lett. Batter, New Technol. Med. 2006;7:386–390.
- Ohara H, Ito K, Iida H, Matsumoto H. Improvement in the moisture content of the stratum corneum following 4 Weeks of collagen hydrolysate ingestion. Nippon Shokuhin Kagaku Kogaku Kaishi. 2009;56:137–145.10.3136/nskkk.56.137
- Kouguchi T, Ohmori T, Shimizu M, et al. Effects of a chicken collagen hydrolysate on the circulation system in subjects with mild hypertension or high-normal blood pressure. Biosci. Biotechnol. Biochem. 2013;77:691–696.10.1271/bbb.120718
- Ramshaw JA, Shah NK, Brodsky B. Gly-X-Y tripeptide frequencies in collagen: a context for host–guest triple-helical peptides. J. Struct. Biol. 1998;122:86–91.10.1006/jsbi.1998.3977
- Iwai K, Hasegawa T, Taguchi Y, et al. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005;53:6531–6536.10.1021/jf050206p
- Postlethwaite AE, Seyer JM, Kang AH. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc. Natl. Acad. Sci. USA. 1978;75:871–875.10.1073/pnas.75.2.871
- Laskin DL, Kimura T, Sakakibara S, Riley DJ, Berg RA. Chemotactic activity of collagen-like polypeptides for human peripheral blood neutrophils. J. Leukoc. Biol. 1986;39:255–266.
- Wisniewski K, Artemowicz B, Lutostanska A, Mackowiak J, Koziolkiewicz W. Central activity of peptide Gly-Pro-Hyp-The main component of collagen degradation products mixture. Acta Neurobiol. Exp. 1994;54:33–38.
- Ohara H, Ichikawa S, Matsumoto H, et al. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J. Dermatol. 2010;37:330–338.10.1111/jde.2010.37.issue-4
- Nakatani S, Mano H, Sampei C, Shimizu J, Wada M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthr. Cartil. 2009;17:1620–1627.10.1016/j.joca.2009.07.001
- Kawaguchi T, Nanbu PN, Kurokawa M. Distribution of prolylhydroxyproline and its metabolites after oral administration in rats. Biol. Pharm. Bull. 2012;35:422–427.10.1248/bpb.35.422
- Hata S, Hayakawa T, Okada H, Hayashi K, Akimoto Y, Yamamoto H. Effect of oral administration of high advanced-collagen tripeptide (HACP) on bone healing process in rat. J. Hard Tissue Biol. 2008;17:17–22.10.2485/jhtb.17.17
- Tsuruoka N, Yamato R, Sakai Y, Yoshitake Y, Yonekura H. Promotion by collagen tripeptide of type I collagen gene expression in human osteoblastic cells and fracture healing of rat femur. Biosci. Biotechnol. Biochem. 2007;71:2680–2687.10.1271/bbb.70287
- Pyun HB, Kim M, Park J, et al. Effects of collagen tripeptide supplement on photoaging and epidermal skin barrier in UVB-exposed Hairless mice. Prev. Nutr. Food. Sci. 2012;17:245–253.10.3746/pnf.2012.17.4.245
- Okawa T, Yamaguchi Y, Takada S, et al. Oral administration of collagen tripeptide improves dryness and pruritus in the acetone-induced dry skin model. J. Dermatol. Sci. 2012;66:136–143.10.1016/j.jdermsci.2012.02.004
- Watanabe-Kamiyama M, Shimizu M, Kamiyama S, et al. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J. Agric. Food Chem. 2010;58:835–841.10.1021/jf9031487
- Abe M, Hoshi T, Tajima A. Characteristics of transmural potential changes associated with the proton-peptide co-transport in toad small intestine. J. Physiol. 1987;394:481–499.10.1113/jphysiol.1987.sp016882
- Aito-Inoue M, Lackeyram D, Fan MZ, Sato K, Mine Y. Transport of a tripeptide, Gly-Pro-Hyp, across the porcine intestinal brush-border membrane. J. Pept. Sci. 2007;13:468–474.10.1002/(ISSN)1099-1387
- Ichikawa S, Morifuji M, Ohara H, Matsumoto H, Takeuchi Y, Sato K. Hydroxyproline-containing dipeptides and tripeptides quantified at high concentration in human blood after oral administration of gelatin hydrolysate. Int. J. Food Sci. Nutr. 2010;61:52–60.10.3109/09637480903257711
- Ohara H, Matsumoto H, Ito K, Iwai K, Sato K. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J. Agric. Food Chem. 2007;55:1532–1535.10.1021/jf062834s
- Alpers DH. Digestion and absorption of carbohydrates and proteins. Physiology of the Gastrointestinal Tract. 3rd ed; 1994. p. 1723–1749.
- Ganapathy V, Leibach FH. Role of pH gradient and membrane potential in dipeptide transport in intestinal and renal brush border membrane resicles from the rabbit. Studies with L-carnosine and glycyl-L-proline. J. Biol. Chem. 1983;258:14189–14192.
- Ichimura T, Yamanaka A, Otsuka T, Yamashita E, Maruyama S. Antihypertensive effect of enzymatic hydrolysate of collagen and Gly-Pro in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2009;73:2317–2319.10.1271/bbb.90197
- Iwai K, Zhang Y, Kouguchi T, et al. Blood concentration of food-derived peptides follwing oral intake of chicken collagen hydrolysate and its angiotensin-converting enzyme inhibitory activity in healthy volunteers. Nippon Shokuhin Kagaku Kogaku Kaishi. 2009;56:326–330.10.3136/nskkk.56.326

![Fig. 2. Whole-body autoradiographs 24hr after a single oral administration of Gly-[3H]Pro-Hyp to a fasting male rat.Notes: Autradiographs of orally administrated Gly-[3H]Pro-Hyp to rat. The dose was 0.35 mmol/kg (74 MBq/kg).](/cms/asset/ed9a9494-c092-4439-964b-f42e44cd52d3/tbbb_a_1062711_f0002_b.gif)