Abstract
Sirt 1 plays a critical role in stress responses. We determined the deregulation of Sirt 1 activity, p53 acetylation, Bcl-2 expression, and mitochondria-dependent apoptosis in mouse osteoblast MC3T3-E1 cells which were exposed to H2O2. And then we investigated the protective role of Sirt 1 activator, Resveratrol (RSV), against the H2O2-induced apoptosis. Results demonstrated that Sirt 1 and Bcl-2 were inhibited, whereas p53 acetylation, Bax, and caspase 9 were promoted by H2O2, as was aggravated by the Sirt 1 inhibitor, EX-527. Instead, RSV inhibited the H2O2-induced both p53 acetylation and the caspase 9 activation, whereas ameliorated the H2O2-induced Bcl-2 inhibition and apoptosis. In conclusion, Sirt 1 was downregulated during the H2O2-induced apoptosis in MC3T3-E1 cells. And the chemical activation of Sirt 1 inhibited the H2O2-induced apoptosis via the downregulation of p53 acetylation. Our results suggest that Sirt 1 upregulation appears to be an important strategy to inhibit the oxidative stress-induced apoptosis.
Graphical abstract
Reseratrol inhibits the hydrogen dioxide-induced apoptosis
The progressive loss of bone with aging is characterized by an increased osteoblast apoptosis and a decreased osteoblast number and bone formation rate.Citation1) Furthermore, the age-related bone mass loss is associated with a progressive increase in the bone levels of reactive oxygen species (ROS),Citation2) which is the characteristic of oxidative stress. The increased ROS leads the cause of cellular damage in pathological conditions including osteoporosis, in which there is an increased bone resorption and low bone mass.Citation3–5) The blood 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress, has been reported to be associated with osteoporosis in postmenopausal women.Citation6) Oxidative stress independently links osteoporosis to the increase of superoxide dismutase/glutathione peroxidase in elderly Mexicans.Citation4) H2O2 treatment or oxidative stress increased osteoclast activityCitation6) or resulted in mitochondrial DNA deletion in severe male osteoporosis.Citation5) And chemicals promoting ROS, such as rotenone and H2O2, decreases cell viability and reduce mitochondrial membrane potential and further caused osteoblast apoptosis.Citation7) The association of oxidative stress with osteoporosis was also reconfirmed by the oxidative stress inhibition-mediated protection against osteoporosis.Citation8,9) Thus, the oxidative stress might be one of the causes to osteoporosis.
Since increased oxidative damage has been reported in aging-related disease, understanding the molecular mechanisms that control the development of oxidative stress-induced damage of osteoblast cells is therefore fundamental for gaining insight into aging and age-related diseases such as osteoporosis. Sirt 1, standing for sirtuin (silent mating type information regulation 2 homolog) 1, is a NAD+-dependent Class III histone deacetylase and plays a critical role in stress responsesCitation10) by deacetylating proteins, including p53.Citation11,12) Acetylation is a post-translational modification that can activate apoptosis-associated p53.Citation13–15) Downregulation of Sirt 1 increases acetylation of p53Citation16,17) and promotes the p53-dependent apoptosis.Citation17) On the other side, the Sirt 1 activator, Resveratrol (RSV) attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through Sirt 1-mediated deacetylation of p53.Citation18) Thus, Sirt 1 represses p53 activity and prevents p53-dependent senescencing of oxidative stress. However, the molecular mechanism that underlines the promoted p53 activity by the Sirt 1 repression under such conditions as oxidative stress is not well understood. And the reduced Sirt 1 activity and promoted p53 activity under oxidative stress in osteoblast has rarely been investigated.
In this study, we determined the deregulation of Sirt 1 activity, p53 acetylation, expression of Bcl-2 family, and the mitochondria-dependent apoptosis in mouse osteoblast MC3T3-E1 cells which were exposed to hydrogen dioxide. And then we investigated the protective role of Sirt 1 activator against the hydrogen dioxide-induced p53 acetylation, deregulation of Bcl-2 family, and the induction of mitochondria-dependent apoptosis.
Materials and methods
Cell lines and culture
Mouse osteoblast MC3T3-E1 cells were purchased from the American Type Culture Collection (ATCC) and were cultured with the ATCC-formulated α-Minimal essential medium (α-MEM) (GIBCO, Rockville, MD, USA), supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA), 100 units/mL penicillin, and 100 mg/mL streptomycin in a humidified incubator under 5% CO2 at 37 °C. For the hydrogen dioxide treatment, cells were subject to 0, 30, 60, or 120 μM H2O2 for various hours. The chemical Sirt 1 activator, RSV (Sigma-Aldrich, St. Louis, MO, USA) and the chemical Sirt 1 inhibitor, EX-527 (Sigma-Aldrich, St. Louis, MO, USA) were utilized to promote or inhibit the Sirt 1 activity in the MC3T3-E1 cells, subject to 120 μM H2O2.
Fluorescence-activated cell sorting (FACS) assay for apoptotic cells and caspase 3 activity assay
The apoptosis of MC3T3-E1 cells was assayed with the Annexin V-FITC apoptosis detection kit (Sigma-Aldrich, St. Louis, MO, USA). In brief, 1 × 106 MC3T3-E1 cells post-treatment were collected and were resuspended in 1000 μL 1 × Binding Buffer at a concentration of 1 × 106 cells/mL and were stained with Annexin V-FITC and Propidium Iodide at RT for 5–15 min (placed in the dark). Then, the FACS was performed for the analysis of apoptotic cells on a FACScan cytometer (BD Biosciences, San Jose, CA, USA). The results were expressed as the percentage of apoptotic cells from the total cells.
The caspase-3 activity was analyzed using the EnzChek Caspase-3 Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Briefly, the MC3T3-E1 cells, post-treatment, were collected. Then, the cell pellets were resuspended in 50 μL of the 1X Cell Lysis Buffer and were centrifuged at 5000 rpm for 5 min to pellet the cellular debris. Add 50 μL of the 2x substrate working solution to each sample and incubate the samples at room temperature for approximately 30 min. Then, measure the fluorescence with excitation/emission of 342/441 nm using appropriate excitation and emission filters or settings.
Western blotting analysis
The treated MC3T3-E1 cells were lysed in a cell lysis reagent (Sigma-Aldrich, St. Louis, MO, USA) according to the product’s manual and supplemented with a protease inhibitor cocktail (Pierce, Rockford, IL,USA). For the cytochrome c release assay, the cytoplasmic protein was isolated by the Mitochondria/Cytosol Fractionation Kit (Abcam, Cambridge, UK). The cell lysates of all samples were subjected to SDS-PAGE and were blotted onto PVDF membrane, and the specific blots were blocked with 5% non-fat milk. The membrane was incubated with corresponding first antibody at 1:500 to 1:1000 dilutions for one hour and subsequently reacted with the second Goat anti-rabbit IgG conjugated to horseradish peroxidase (Pierce, Rockford, IL, USA) at 1:2000 dilutions for 30 min. Finally, the ECL detection systems (Super Signal West Femto; Pierce, Rockford, IL, USA) were used for blot detection. Membranes were washed with blocking buffer after each step. The following first antibodies were used in this study: Polyclone rabbit anti-mouse β-actin, anti-mouse Bcl-2, or cytochrome c were purchased from Sinobio (Beijing, China). The polyclone rabbit anti-mouse Sirt 1, p53, p53 (acetylated K382), or Bax were purchased from Abcam (Cambridge, UK). The polyclone rabbit anti-mouse cleaved caspase 9, cleaved caspase 3, or lysed Poly ADP ribose polymerase (PARP) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Sirt 1 activity assay
SIRT 1 Activity Assay Kit (Fluorometric) (Abcam, Cambridge, UK) was used to measure the Sirt 1 deacetylase activity, according to the product’s manual. Briefly, MC3T3-E1 cells were lysed with 50 μL 1x ice-cold Cell Lysis Buffer (Abcam, Cambridge, UK). Then, the lysate was successively added with SIRT 1 Assay Buffer (10 μL), NAD (5 μL), FluoroSubstrate Peptide (5 μL), or FluoroDeacetylated Peptide (5 μL). Then, the sample was supplemented with ddH2O to a final volume of 100 μL. Read the fluorescence intensity for 30–60 min at 1–2 min intervals using microtiter plate fluorometer with excitation at 340–360 nm and emission at 440–460 nm using a microplate reader (Bio Red, Hercules, CA, USA). Measure and calculate the rate of reaction while the reaction velocity remains constant.
Cell viability assay
The viability of MC3T3-E1 cells was measured using MTT method. In brief, MC3T3-E1 cells, after various treatment, were added with MTT in the final concentration of 0.5 mg/mL and were incubated for 3 h at 37 °C. Then, the supernatants were carefully removed, and each well was added 100 μL DMSO and was pipetted up and down to dissolve crystals. Then, the plate was put into the 37 °C for another incubation for 15 min and then was measured at 570 nm wavelength using a microplate reader (Bio-Rad, Laboratories, Hercules, CA, USA).
Statistical analysis
Analysis of variance was applied to determine the statistical difference between two groups. All analyses were performed using a two-tailed t-test, when with a homogeneity test of variance, and a p-value less than 005 was considered statistically significant
Results
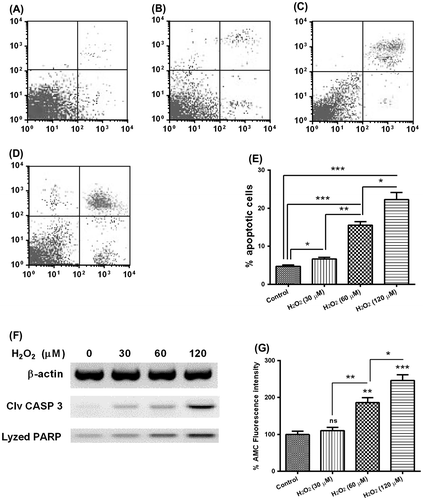
Hydrogen dioxide induces apoptosis in mouse osteoblast MC3TC-E1 cells
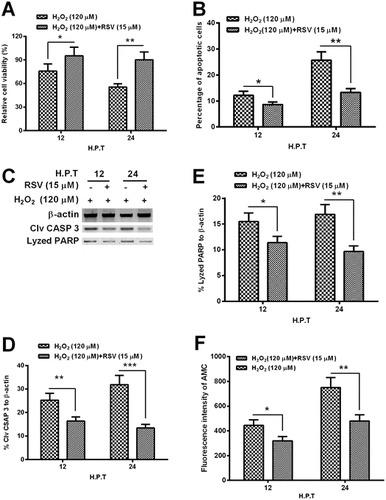
To investigate the mechanism of the apoptosis induction by hydrogen dioxide in mouse osteoblast MC3TC-E1 cells, we treated the approximately 85%-confluent MC3TC-E1 cells with H2O2 of 0, 30, 60, or 120 μM for 24 h. And then the cells were stained with Annexin V and propiumiodine and were analyzed for apoptosis by FACS. As shown in Fig. , compared to the MC3TC-E1 cells without H2O2 treatment, 30, 60, or 120 μM H2O2 significantly promoted a significantly high level of apoptosis of MC3TC-E1 cells (Fig. (E); p < 0.05 or p < 0.001). And there was a dose-dependence of such promotion, there was significant difference between the groups of 30 and 60 μM (6.643 ± 0.447 vs 15.540 ± 0.952; p < 0.01), or between the groups of 60 and 120 μM (15.540 ± 0.952 vs 22.29 ± 1.893; p < 0.05). We then analyzed with Western blot assay the activation of caspase 3, which is the executioner caspase of apoptosis, and the lysis of PARP, which was catalyzed by the activated caspase 3, in the MC3TC-E1 cells treated with 0, 30, 60, or 120 μM H2O2 for 24 h. And Fig. (F) indicated the upregulation of both cleaved CASP 3 and lysed PARP by the H2O2 treatment. In addition, the caspase 3 activity assay also confirmed the apoptosis induction by the H2O2 treatment with 60 or 120 μM H2O2 for 24 h. Taken together, our results confirmed the apoptosis induction by the H2O2 treatment in MC3TC-E1 cells.
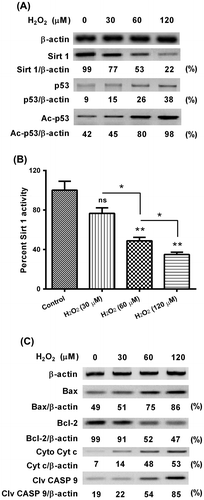
Deregulation of Sirt 1, acetylated p53, Bax, Bcl-2, and caspase 9 in the hydrogen dioxide-treated MC3TC-E1 cells
As the mammalian ortholog of Sir2, which is an NAD+-dependent protein deacetylase and a founding member of the sirtuin family,Citation19) Sirt 1 provides the protection against apoptosis induced by ROS stress.Citation19) And we next investigated the regulation on Sirt 1 by the condition of excessive oxidative stress (H2O2) in MC3TC-E1 cells. As shown in Fig. (A), the Sirt 1 level was significantly inhibited by the H2O2 treatment for 24 h, whereas the p53 level, particularly the acetylated p53, was promoted by the H2O2 treatment for 24 h. And the Sirt 1 downregulation was also confirmed by the Sirt 1 activity assay. H2O2 treatment with 60 or 120 μM significantly inhibited the Sirt 1 activity (Fig. (B); p < 0.01, respectively) dose-dependently (p < 0.05 for the difference between the groups of 60 and 120 μM). Bcl-2 family governs mitochondrial outer membrane permeabilization (MOMP) and regulates apoptosis either via being pro-apoptotic (Bax, BAD, Bak, and Bok among others) or via being anti-apoptotic (Bcl-2, Bcl-xL, and Bcl-w, among an assortment of others).Citation20,21) To confirm the role of mitochondria-dependent apoptosis and particularly the role of Bcl-2 family in oxidative stress in MC3TC-E1 cells, we then analyzed the expression of Bcl-2, Bax, cytochrome c release from mitochondria, caspase 9 activation in the H2O2-treated MC3TC-E1 cells. As shown in Fig. (C), the Bax was promoted, whereas the Bcl-2 was inhibited in the H2O2-treated MC3TC-E1 cells, dose-dependently. And the cytosolic cytochrome c (released from mitochondria) and activated caspase 9 (cleaved caspase 9) were also upregulated by the H2O2 treatment. Thus, we confirmed the deregulation of Sirt 1, acetylated p53, Bax, Bcl-2, and caspase 9 by oxidative stress in MC3TC-E1 cells.
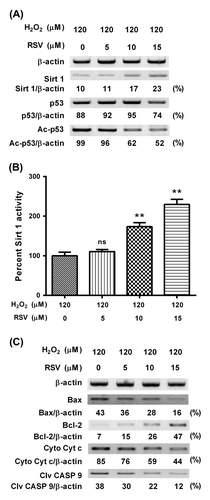
Sirt 1 activator inhibits the p53 acetylation and ameliorates the Bcl-2 inhibition in MC3T3-E1 cells subject to hydrogen dioxide
To further identify the role of Sirt 1 in the H2O2-induced deregulation of acetylated p53, Bax, Bcl-2, and caspase 9 in MC3TC-E1 cells, we then utilized RSV, one chemical Sirt 1 activator, to reverse the Sirt 1 downregulation by H2O2 treatment, and then re-evaluated the level of p53 acetylation, the Bcl-2 inhibition, and caspase 9 activation in MC3T3-E1 cells subject to H2O2. Fig. (A) indicated that RSV reversed the H2O2-induced Sirt 1 decreasing, dose-dependently. And such reversal was reconfirmed by the Sirt 1 activity assay (Fig. (B); p < 0.01, respectively, for 10 or 15 μM). What is more, the H2O2-induced p53 acetylation was also inhibited by 10 or 15 μM RSV, which had no influence on the H2O2-promoted p53 (Fig. (A)). Then, we re-evaluated the regulation on Bcl-2 family and caspase 9 by the H2O2 treatment, in the presence of RSV. And Fig. (C) demonstrated that RSV with 10 or 15 μM inhibited the Bax upregulation and Bcl-2 downregulation by the H2O2 treatment. And the inhibition by the RSV treatment (10 or 15 μM) on the cytochrome c release and caspase 9 promotion was also confirmed (Fig. (C)). Therefore, our results confirmed the inhibition by Sirt 1 activator on the H2O2-induced deregulation of acetylated p53, Bax, Bcl-2, and caspase 9 in MC3TC-E1 cells.
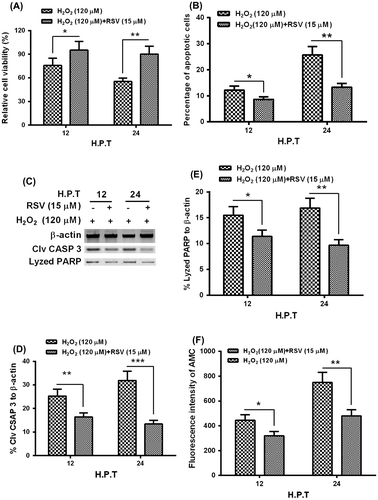
RSV inhibits the hydrogen dioxide-induced apoptosis in MC3T3-E1 osteoblast cells
To reconfirm the role of Sirt 1 in the hydrogen dioxide-induced apoptosis in MC3T3-E1 cells, we then re-examined the hydrogen dioxide-induced apoptosis in the MC3T3-E1 cells, in the presence of RSV. It was demonstrated that the RSV with 15 μM significantly ameliorated the cellular viability decreasing induced by H2O2 with 120 μM (Fig. (A); p < 0.05 or p < 0.01 for 12 or 24 h). And the H2O2-induced apoptosis was also blocked by the RSV; there were significantly reduced apoptotic cells in the group with the treatment with both RSV- and H2O2 than in the group with singular H2O2 treatment (Fig. (B); p < 0.05 or p < 0.01). And the Western blot assay demonstrated that the activated caspase 3 and its lysed PARP in the H2O2-treated MC3T3-E1 cells were significantly downregulated by RSV (Fig. (C)-(E); p < 0.05, p < 0.01 or p < 0.01). In addition, the caspase 3 activity assay reconfirmed the reduced caspase 3 activity, by 15 μM RSV, which was inducted by the H2O2 treatment with 120 μM for either 12 or 24 h. Therefore, we confirmed the inhibition, by the Sirt 1 activator, of the apoptosis induction by the H2O2 treatment in MC3TC-E1 cells.
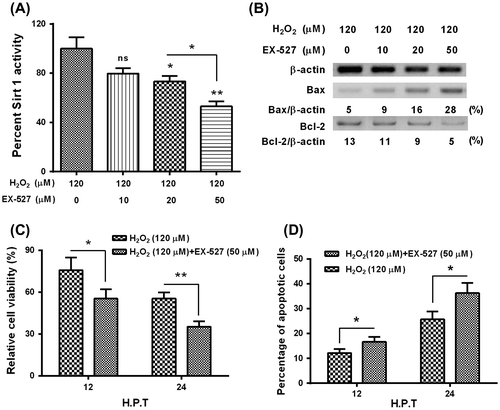
In addition, to reconfirm the protective role of Sirt 1 in the hydrogen dioxide-induced apoptosis in MC3T3-E1 osteoblast cells, we then added the Sirt 1 inhibitor, EX-527, and then re-evaluated the Sirt 1 activity, the Bcl-2 signaling, and the apoptosis induction in the H2O2-treated MC3TC-E1 cells. As shown in Fig. (A), the EX-527 with 20 or 50 μM significantly reduced the Sirt 1activity in the H2O2-treated MC3TC-E1 cells, and both the promoted Bax level and the reduced Bcl-2 level were significantly aggravated by the EX-527 treatment with 20 or 50 μM. Moreover, the EX-527 treatment deteriorated the cellular viability reduction (Fig. (C)) and the apoptosis induction (Fig. (D)) by the H2O2 treatment in MC3TC-E1 cells. Thus, we reconfirmed the protective role of Sirt 1 in the hydrogen dioxide-induced apoptosis in MC3T3-E1 osteoblast cells.
Fig. 5. Sirt 1 inhibitor, EX-527 deteriorates the apoptosis induction by H2O2 in MC3T3-E1 osteoblast cells.
Notes: (A) EX-527 deteriorated the Sirt 1 activity in the H2O2-treated MC3T3-E1 cells; (B) EX-527 aggravated the H2O2-promoted Bax level, and deteriorated the Bcl-2 inhibition by the H2O2 treatment in MC3T3-E1 cells; (C) Influence of EX-527 on the viability (C) and apoptosis (D) of MC3T3-E1 cells subject to both H2O2 and EX-527 treatments. All experiments were performed in independent triplicate. *p < 0.05, **p < 0.01, vs. control.
Discussion
Members of sirtuin family, such as Sirt 1, function in a wide array of cellular processes, including gene silencing, longevity, muscle differentiation, and DNA damage repair.Citation22,23) Stimulation of Sir2 is sufficient to induce prolonged life span in Caenorhabditis elegans, Drosophila melanogaster,Citation24,25) or mice.Citation26) Sirt 1 provides protection against apoptosis and plays an essential role in mediating survival of various types of cells under stress in vitro.Citation27–29) And the in vivo mice model also indicates the aging regulatory role and the resistance to oxidative stress in heart.Citation30) And the protective role of Sirt 1 against oxidative injury has also been confirmed in the neurodegenerative diseases.Citation31,32) Moreover, the age-related bone mass loss, which is associated with a progressive increase in the bone levels of ROS, is also associated with a deregulated Sirt 1 activity, the Sirt 1 activity in peripheral blood mononuclear cells of patients with osteoporosis negatively correlated with the serum C-terminal cross-linking telopeptide of type I collagen (CTX), a marker of bone resorption.Citation33) And the RSV-activated Sirt 1 plays pivotal roles in regulating the balance between the osteoclastic vs. osteoblastic activity result in bone formation in vitro.Citation34) Thereby, it highlights the therapeutic potential of Sirt 1 activator, RSV, for treating osteoporosis and rheumatoid arthritis-related bone loss. In this study, we determined the apoptosis induction by hydrogen dioxide in mouse osteoblast MC3TC-E1 cells. And we confirmed the inhibition of Sirt 1 and Bcl-2, whereas the promotion to acetylated p53, Bax, and caspase 9 in the hydrogen dioxide-treated MC3TC-E1 cells. The level of Sirt 1 and Bcl-2 was significantly inhibited by the H2O2 treatment for 24 h, whereas the p53 level, particularly the acetylated p53, Bax, and activated caspase 9, was promoted by the H2O2 treatment. It implied the regulatory role of Sirt 1 and mitochondria-dependence of H2O2-induced the apoptosis in MC3TC-E1 cells. On the other side, our results demonstrated that the Sirt 1 activator, RSV, inhibits the p53 acetylation and ameliorates the Bcl-2 inhibition in MC3T3-E1 cells subject to hydrogen dioxide. The H2O2-induced both p53 acetylation and the caspase 9 activation were inhibited by RSV treatment, whereas the Bcl-2 inhibition by the H2O2 treatment was ameliorated by the RSV treatment. Therefore, our results confirmed the inhibition by Sirt 1 activator on the H2O2-induced deregulation of acetylated p53, Bax, Bcl-2, and caspase 9 in MC3TC-E1 cells. In addition, the treatment with the Sirt 1 activator, RSV, could inhibit the hydrogen dioxide-induced apoptosis in MC3T3-E1 osteoblast cells.
The role of RSV in the apoptosis induction in various types of cells has been controversial. The agent was shown to induce apoptosis in such cancer cells as skin cancer cells,Citation35) colon cancer cells,Citation36) and neuroblastoma Cells,Citation37) whereas was indicated to exert the protective role against apoptosis in the human tenocytes,Citation38) in endothelial cellsCitation39) and in other types of cells,Citation40) particularly, under the pressure of oxidative stress.Citation40–42) And in this study, we confirmed the protective role of RSV and the Sirt 1 activation against the H2O2-induced apoptosis in MC3T3-E1 osteoblast cells. Moreover, based on the above results, we speculated that activation of p53 acetylation by H2O2-induced downregulation of Sirt 1 could increase the Bax, whereas could reduce the Bcl-2, at protein level. The activated pro-apoptotic protein Bax and the inhibited Bcl-2 initiated the release of cytochrome c from mitochondrial membranes and thereby triggers apoptosis.Citation43) Sirt 1 can cause inhibition of Bax expressionCitation44) and promote the Bcl-2Citation45) via deacetylating p53.Citation44) The Western blot analysis confirmed that the chemical activation of Sirt 1 by RSV led to a reduced apoptosis under oxidative stress, suggesting the protective role of Sirt 1 activation in MC3T3-E1 osteoblast cells under oxidative stress.
In conclusion, we found the downregulation of Sirt 1 during the hydrogen dioxide-induced apoptosis in MC3T3-E1 osteoblast cells, as was followed by the upregulated p53 acetylation, Bax expression, and caspase 9 activation, whereas by the downregulation of Bcl-2. And the chemical activation of Sirt 1 by RSV inhibited the hydrogen dioxide-induced apoptosis via downregulating the p53 acetylation, the Bax promotion and the caspase 9 activation and via upregulating the Bcl-2 level. Our results suggest that upregulation of Sirt 1 appears to be an important strategy to inhibit the oxidative stress-induced apoptosis in MC3T3-E1 osteoblast cells.
Authors contribution
Na He and Chunlei Zhu conceived and designed the experiments. Na He, Xuewei Zhu, Wei He, Shiwei Zhao, and Weiyan Zhao performed the experiments. Na He, Xuewei Zhu, and Chunlei Zhu analyzed the data. Na He and Chunlei Zhu wrote the paper.
Disclosure statement
Authors declare no conflict of interests.
Funding
Supported by the Health Department research plan of Jilin Province (No:20132030); Changchun city of Jilin province science and technology plan projects (No:201339017).
References
- Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010;31:266–300.10.1210/er.2009-0024
- Almeida M, Han L, Martin-Millan M, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007;282:27285–27297.10.1074/jbc.M702810200
- Yılmaz N, Eren E. Homocysteine oxidative stress and relation to bone mineral density in post-menopausal osteoporosis. Aging Clin. Exp. Res. 2009;21:353–357.10.1007/BF03324927
- Sánchez-Rodríguez MA, Ruiz-Ramos M, Correa-Muñoz E, Mendoza-Núñez VM. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet. Disord. 2007;8:124–130.10.1186/1471-2474-8-124
- Varanasi SS, Francis RM, Berger CE, Papiha SS, Datta HK. Mitochondrial DNA deletion associated oxidative stress and severe male osteoporosis. Osteoporos. Int. 1999;10:143–149.10.1007/s001980050209
- Baek KH, Oh KW, Lee WY, et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010;87:226–235.10.1007/s00223-010-9393-9
- Dawson TL, Gores GJ, Nieminen AL, Herman B, Lemasters JJ. Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. Am. J. Physiol. 1993;264:C961–C967.
- Yin H, Shi ZG, Yu YS, et al. Protection against osteoporosis by statins is linked to a reduction of oxidative stress and restoration of nitric oxide formation in aged and ovariectomized rats. Eur. J. Pharmacol. 2012;674:200–206.10.1016/j.ejphar.2011.11.024
- Chavan SN, More U, Mulgund S, Saxena V, Sontakke AN. Effect of supplementation of vitamin C and E on oxidative stress in osteoporosis. Indian J. Clin. Biochem. 2007;22:101–105.10.1007/BF02913324
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell Biol. 2005;6:298–305.10.1038/nrm1616
- Langley E, Pearson M, Faretta M, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21:2383–2396.10.1093/emboj/21.10.2383
- Vaziri H, Dessain SK, Eaton NG, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159.10.1016/S0092-8674(01)00527-X
- Sykes SM, Mellert HS, Holbert MA, et al. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol. Cell. 2006;24:841–851.10.1016/j.molcel.2006.11.026
- Sykes SM, Stanek TJ, Frank A, Murphy ME, McMahon SB. Acetylation of the DNA binding domain regulates transcription-independent apoptosis by p53. J. Biol. Chem. 2009;284:20197–20205.10.1074/jbc.M109.026096
- Kusio-Kobialka M, Wolanin K, Podszywalow-Bartnicka P, et al. Increased acetylation of lysine 317/320 of p53 caused by BCR-ABL protects from cytoplasmic translocation of p53 and mitochondria-dependent apoptosis in response to DNA damage. Apoptosis. 2012;17:950–963.10.1007/s10495-012-0739-9
- Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J. Mol. Cell Cardiol. 2007;43:571–579.10.1016/j.yjmcc.2007.08.008
- Yuan F, Xie Q, Wu J, et al. MST1 promotes apoptosis through regulating Sirt1-dependent p53 deacetylation. J. Biol. Chem. 2011;286:6940–6945.10.1074/jbc.M110.182543
- Zhang C, Feng Y, Qu S, et al. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc. Res. 2011;90:538–545.10.1093/cvr/cvr022
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800.
- Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009;122:437–441.10.1242/jcs.031682
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911.10.1101/gad.13.15.1899
- Buck SW, Gallo CM, Smith JS. Diversity in the Sir2 family of protein deacetylases. J. Leukoc. Biol. 2004;75:939–950.10.1189/jlb.0903424
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004;73:417–435.10.1146/annurev.biochem.73.011303.073651
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230.10.1038/35065638
- Howitz KT, Bitterman kJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196.10.1038/nature01960
- Baur JA, Pearson kJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342.10.1038/nature05354
- Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ. Res. 2004;95:971–980.10.1161/01.RES.0000147557.75257.ff
- Parker AJ, Arango M, Abderrahmane S, et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Med. Sci. (Paris). 2005;21:556–557.10.1051/medsci/2005215556
- Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J. Biol. Chem. 2005;280:43121–43130.10.1074/jbc.M506162200
- Alcendor RR, Gao S, Zhai P, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 2007;100:1512–1521.10.1161/01.RES.0000267723.65696.4a
- Cornelius C, Trovato Salinaro A, Scuto M, et al. Cellular stress response, sirtuins and UCP proteins in Alzheimer disease: role of vitagenes. Immun. Ageing. 2013;10:41.10.1186/1742-4933-10-41
- Salminen A, Kauppinen A, Suuronen T, Kaarniranta K. SIRT1 longevity factor suppresses NF-kappaB -driven immune responses: regulation of aging via NF-kappaB acetylation? BioEssays. 2008;30:939–942.10.1002/bies.v30:10
- Godfrin-Valnet M, Khan KA, Guillot X, et al. Sirtuin 1 activity in peripheral blood mononuclear cells of patients with osteoporosis. Med. Sci. Monit. Basic Res. 2014;20:142–145.
- Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011;286:11492–11505.10.1074/jbc.M110.198713
- Dun J, Chen X, Gao H, Zhang Y, Zhang H, Zhang Y. Resveratrol synergistically augments anti-tumor effect of 5-FU in vitro and in vivo by increasing S-phase arrest and tumor apoptosis. Exp. Biol. Med ( Maywood). 2015.
- Miki H, Uehara N, Kimura A, et al. Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. Int. J. Oncol. 2012;40:1020–1028.
- Ren X, Bai X, Zhang X, et al. Quantitative nuclear proteomics identifies that miR-137-mediated EZH2 reduction regulates resveratrol-induced apoptosis of neuroblastoma cells. Mol. Cell Proteomics. 2015;14:316–328.10.1074/mcp.M114.041905
- Busch F, Mobasheri A, Shayan P, Stahlmann R, Shakibaei M. Sirt-1 is required for the inhibition of apoptosis and inflammatory responses in human tenocytes. J. Biol. Chem. 2012;287:25770–25781.10.1074/jbc.M112.355420
- Csiszar A, Csiszar A, Pinto JT, et al. Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:303–313.10.1093/gerona/glu029
- Chan CM, Huang CH, Li HJ, et al. Protective effects of resveratrol against UVA-induced damage in ARPE19 cells. Int. J. Mol. Sci. 2015;16:5789–5802.10.3390/ijms16035789
- Zhuge CC, Xu JY, Zhang J, et al. Fullerenol protects retinal pigment epithelial cells from oxidative stress-induced premature senescence via activating SIRT1. Invest. Ophthalmol. Vis. Sci. 2014;55:4628–4638.10.1167/iovs.13-13732
- Peritore CS, Ho A, Yamamoto BK, Schaus SE. Resveratrol attenuates L-DOPA-induced hydrogen peroxide toxicity in neuronal cells. NeuroReport. 2012;23:989–994.10.1097/WNR.0b013e32835a4ea4
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331.10.1016/S0092-8674(00)81871-1
- Lee JT, Gu W. SIRT1: Regulator of p53 Deacetylation. Genes Cancer. 2013;4:112–117.10.1177/1947601913484496
- Hassan MK, Watari H, Salah-eldin AE, et al. Histone deacetylase inhibitors sensitize lung cancer cells to hyperthermia: involvement of Ku70/SirT-1 in thermo-protection. PLoS ONE. 2014;9:e94213.10.1371/journal.pone.0094213