Abstract
Alopecia impairs the physical and mental health of patients. We have previously shown that 8-week-old ob/ob mice have no reactivity to depilation, which is a stimulus that induces anagen transition in normal mice, while no hair cycle abnormalities have been reported in other studies until mice reach 7 weeks of age. Therefore, we hypothesized that ob/ob mice have abnormalities in hair cycle progression beyond 7 weeks of age. We examined 6- to 24-week-old ob/ob and 6- to 10-week-old normal mice. After acclimation, the dorsal skin was harvested and the hair cycle phase was identified histologically and immunohistochemically. Normal mice showed catagen–telogen and telogen–anagen transitions at 6 and 8–9 weeks old, respectively. In contrast, the anagen–catagen transition was observed in 7-week-old mice and the telogen phase was maintained from 10 to 24 weeks in most ob/ob mice. These results suggests that ob/ob mice are a possible model animal for telogen effluvium.
Graphical abstract
In this study, the elongated telogen of hair cycle in ob/ob mice was identified through the comparison with wild-type mice.
Key words:
Introduction
Hair is a skin appendage—similar in role to sweat glands, sebaceous glands, and nails—supporting skin functions. In particular, the hair plays unique and important functions on the head to intercept sunlight exposure, buffer external forces, and maintain body temperature.Citation1) Hair follicles support the mechanical strength of the skin by their deeply invigilated structure into the dermis, and the skin barrier functions as a route of sebum to the skin surface.Citation2) Furthermore, hair plays an important role in self-expression and the formation of self-image.
Hairs repeat growth and falling off via the hair cycle, which consists of anagen, catagen, and telogen phases. During anagen, hair matrix cells rapidly proliferate and differentiate to hair shafts. During catagen, hair matrix cells decrease by apoptosis and hair follicles regress. Telogen is a resting phase, in which hair bulbs locate near the hair bulge. Hairs shed following the transition from the telogen to anagen phase.Citation1)
Alopecia involves the loss of hair follicles and a reduction in hair density owing to the loss or thinning of hair shafts. Alopecia is classified as anagen effluvium or telogen effluvium. Anagen effluvium, including alopecia areata and alopecia medicamentosa, is a type of hair loss due to the damage of proliferative hair follicle cells in the anagen phase.Citation3) Telogen effluvium occurs due to abnormalities in the hair cycle such as anagen shortening and telogen elongation.Citation4) Hair does not grow fully in the shortened anagen phase, resulting in reduced hair density. Typical telogen effluviums are androgenetic alopecia and senile alopecia. Since alopecia decreases not only the buffering function of hair but also the skin barrier function, the scalp becomes vulnerable to external stimuli, and alopecia often causes pain and itching of the scalp.Citation5–8) To date, many reports have shown the effects of alopecia on social and mental quality of life, including lower self-esteem, poorer body image, and lower quality of life.Citation9–12) Therefore, alopecia is a crucial problem in health science that impairs the physical and mental health of patients.
The incidence of alopecia has increased. The average incidence of androgenic alopecia has been reported to be about 30% in men.Citation13) Alopecia areata, with an incidence and lifetime risk of approximately 0.1–0.7% and 1.7%, respectively, is a hereditary disease that frequently develops in family members of patients.Citation14–18) The incidence of alopecia medicamentosa is 65%, on average, in cancer patients receiving chemotherapy, and can increase to almost 100% for certain drugs.Citation19)
The elucidation of the pathophysiology and molecular mechanisms of alopecia is essential for the establishment of treatments. The overexpression of 5α-reductase is important in androgenic alopecia.Citation20) Therefore, an inhibitor of 5α-reductase, finasteride, is the first choice for the treatment of androgenic alopecia.Citation21) However, five-year follow-up study showed approximately 13% of patients received finasteride treatment showed insufficient improvement, suggesting that other factors are involved in its development and progression.Citation22) Alopecia areata is recognized as an autoimmune disease and is mainly treated by steroid pulse therapy and local immunotherapy.Citation23, 24) However, clinicians experience many cases in which these treatments do not improve symptoms. Thus, the development of additional reagents and techniques is required for the treatment of alopecia.
Recently, our research group identified the hair growth-promoting effect of acyl homoserine lactone in ob/ob mice, which are widely used as obesity and diabetes model mice.Citation25) In this study, our group used 8-week-old male ob/ob mice after anagen induction by depilation. Vehicle-treated ob/ob mice showed delayed transitions to anagen after stimulation relative to the wild-type mice. On the other hand, Sumikawa et al. observed normal hair cycle progression in ob/ob mice until postnatal day 49 in a comparison with wild-type mice.Citation26) Therefore, we hypothesized that ob/ob mice have hair cycle abnormalities after 7 weeks of age. The hair cycle has not been fully characterized in ob/ob mice.
The purpose of this study was to identify hair cycle abnormalities in ob/ob mice. We histologically observed the transition from the telogen to the anagen phase after 6 weeks of age on the dorsal skin of ob/ob and wild-type mice. This study provides a biological foundation for the development of new treatments for alopecia patients with reduced physical and mental quality of life.
Materials and methods
Animal and sample collection
C57BL/6JHamSlc-ob/ob (ob/ob) mice were purchased from Japan SLC (Shizuoka, Japan); they have a single-base mutation in the leptin gene. C57BL/6JHamSlc- +/+ (+/+) littermates (Japan SLC) were used as the control. Mice were individually maintained at 23 ± 2 °C and 55 ± 10% humidity with free access to food and water.
Thirty-six male ob/ob mice that were 6–24 weeks old (including 3 mice each at 6, 7, 8, 9, 10, 15, 18, and 24 weeks, 2 mice at 23 weeks, and single animal at 11, 12, 13, 14, 16, 17, 19, 20, 21, and 22 weeks) and 15 (3 each) male +/+ mice that were from 6 to 10 weeks old were sacrificed after a 1-week acclimation. After shaving, skin tissue samples (1 × 1 cm) were harvested from the center of the back. Experimental protocols were approved by the Animal Research Committee of the University of Tokyo.
Hematoxylin and eosin staining and immunohistochemical analyses
The macroscopic appearances of all mice were recorded with a color chart (Casmatch, Bear Medic Co. Tokyo. Japan) weekly with digital camera (Ricoh imaging, Tokyo, Japan). White balance of images was performed by calibrating the white color of chart with image processing software, Photoshop Element (Adobe Systems, San Jose, CA).
The skin tissue samples were fixed in 4% paraformaldehyde at 4 °C overnight. Tissue was divided into two pieces. One piece was frozen in optimal cutting temperature compound (Sakura Finetek, Tokyo, Japan) and cut into 40-μm-thick sections for hematoxylin and eosin (HE) staining. Briefly, sections were stained with Mayer’s hematoxylin (Muto Pure Chemicals, Tokyo, Japan) for 3 min, and with 20% eosin (Muto Pure Chemicals) for 4 min. Finally, sections were dehydrated with a series of ethanol and xylene washes, and were embedded with mounting agent (Matsunami Glass, Osaka, Japan).
The second piece of tissue was embedded in paraffin and cut into 3-μm-thick sections for immunohistochemistry for Ki67, which is a cell proliferation marker. After deparaffinization, endogenous peroxidase was inactivated with 0.3% H2O2/methanol for 30 min and autoclaved in citrate buffer (pH 6.0) for antigen retrieval. Sections were serially incubated with the rabbit polyclonal anti-Ki67 antibody (Novus Biologicals, Littleton, CO) diluted at 1:100 at 4°C overnight, biotin-conjugated anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) diluted at 1:1000 at room temperature for 30 min, and avidin-biotin complex (Vectastain ABC Standard Kit, Vector Labs, Burlingame, CA) at room temperature for 30 min. The immunoreactivity was visualized by 3,3′-diaminobenzidine tetrahydrochloride (Wako Pure Chemical, Osaka, Japan). Finally, sections were counterstained with hematoxylin.
Identification of the hair cycle phase
The gold standard method to identify the hair cycle phase is histological observations in HE-stained paraffin serial sections.Citation27) In this study, we used an all-in-one inverted microscope (BIORE-VO BZ-9000, Keyence, Tokyo, Japan) that recognizes multiple depth observations in a bright field. A total of 15–20 images were captured from HE-stained 40-μm-thick frozen sections with shifting focal points of 2–3-μm pitches. The hair follicle is a cylindrical structure with an outer diameter of 30 μm, and the hair cycle phase can be identified at the section in which the morphology of the dermal papilla and hair bulb are fully observed. So, the hair cycle phase of a few follicles can be identified in each section by conventional methods. However, the hair cycle phase of most follicles could be identified in each section by our method. Moreover, observations of three non-serial sections prevent multiple observations of follicles, resulting in accurate and stable evaluation.
Hair follicles in which the morphology of the dermal papilla and hair bulb were fully observed were defined as countable follicles. The phases of the hair cycle were defined according to Dry’s classification,Citation28) and ratio of total follicles at each phase to total countable follicles was calculated. The anagen transition was also assessed by Ki67 staining. Since the proliferation of hair matrix cells is activated in anagen, the anagen transition can be reliably identified by a combination of histological and immunohistochemical observations. Three non-serial paraffin sections were stained and observed. Sections were classified as follows: +, all follicles are positive; +/–, a portion of follicles are positive; and –, all follicles are negative.
Results
Identification of the hair cycle phase
There was no difference in the morphology and immunoreactivity for Ki67 of hair follicles in each phase between ob/ob and +/+ mice. In anagen, the hair follicle extended into the subcutaneous fat layer and the dermal papilla was enveloped with hair bulb (Fig. (A)). The hair follicles were regressing in catagen, and the hair bulb was located near the bulge in telogen. Many hair matrix cells were Ki67-positive in anagen (Fig. (B)), whereas a few cells were positive in the outer root sheath of catagen and telogen follicles.
Fig. 1. Identification of anagen hair follicles by HE staining (A) and immunohistochemistry for Ki67 (B).
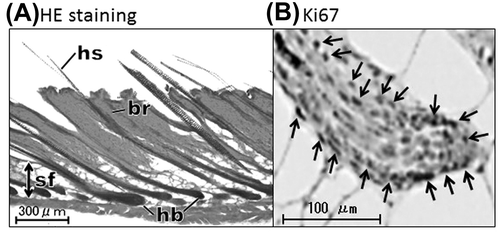
The anagen phase was consistently identified by morphological and immunohistochemical examinations in all specimens.
Progression of the hair cycle
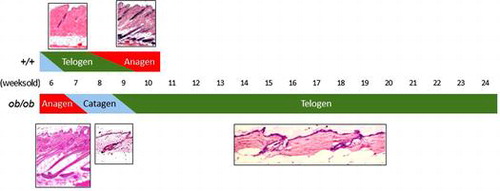
In +/+ mice, catagen and telogen follicles coexisted at 6 weeks of age, and all were telogen at 7 weeks. Telogen and anagen follicles coexisted at 8 and 9 weeks and all were anagen at 10 weeks (Table ).
Table 1. Histologically identified hair cycle phases and positivity for Ki67 in +/+ mice.
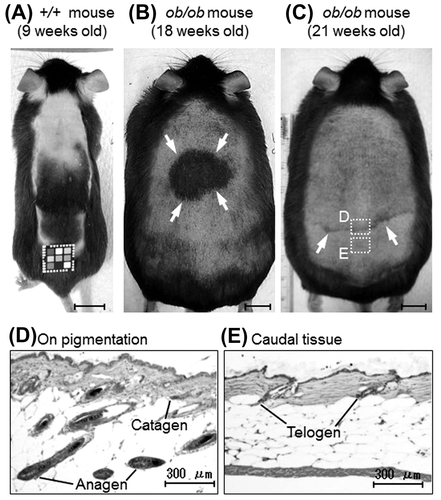
In ob/ob mice, all follicles were anagen at 6 weeks of age, anagen and catagen follicles coexisted at 7 weeks, and all were catagen at 8 weeks old. More than half of all follicles were telogen at 9 weeks, and all were telogen at 10 weeks. From 11 weeks on, all follicles were telogen in all animals except for one 18- and one 21-week-old individual (Table ). The anagen transition is generally identified by skin pigmentation, which reflects the accumulation of melanin in the hair follicle, in wild-type mice (Fig. (A)). One 18- and one 21-week-old ob/ob mice showed a localized and weak linear pigmentation (Fig. (B) and (C)). Histological analysis of these mice was performed on the pigmentation site and the caudal site. On the pigmentation site, coexistence of the anagen and catagen follicles was observed, whereas only the telogen follicles were observed in the caudal tissue (Fig. (D) and (E)). These results indicated that the hair cycle progressed from anagen to catagen in 7 weeks, from catagen to telogen in 9 weeks, and remained telogen until at least 18 weeks in ob/ob mice. In addition, the anagen phase was shortened in the third hair cycle of ob/ob mice.
Table 2. Histologically identified hair cycle phases and Ki67 positivity in ob/ob mice.
Fig. 2. Anagen transition in +/+ and ob/ob mice.
Discussion
To our knowledge, this is the first study to show the abnormality of the hair cycle in 7- to 24-week-old ob/ob mice. ob/ob mice showed the elongation of telogen from 10 to 18 weeks old or more, whereas the telogen period was approximately one week in control mice. A portion of the 18- and 21-week-old ob/ob mice showed a localized or weak anagen transition. These results suggested that ob/ob mice are a model for telogen effluvium including telogen elongation, inhibition of anagen transition and anagen shortening.
Sumikawa et al. reported that ob/ob and control mice had anagen follicles at 5 weeks old and the catagen transition occurred at 6 weeks old, indicating no abnormalities in the progression of the hair cycle in ob/ob mice until 7 weeks old.Citation26) Although our findings are consistent with the previous report with respect to +/+ mice, ob/ob mice showed approximately 1-week delayed progression of the hair cycle. This inconsistency was probably due to the difference in breeding colony (C57BL/6JHamSlc-ob/ob vs. B6.V-Lepob/J).
In contrast, db/db mice lacking functional leptin receptors showed an elongated first telogen phase.Citation26) Since leptin contributes to the regulation of the hair cycle,Citation29) the elongation of telogen is thought to be caused by the leptin deficiency. However, ob/ob mice, which express N-terminal fragments of leptin including receptor binding domain, showed no abnormality until postnatal day, suggesting compensation by N-terminal leptin.Citation26) Therefore, hair cycle abnormalities of ob/ob mice found in this study were thought to be caused by factors other than the leptin mutation.
Leptin is an adipokine that suppresses food intake in response to elevated blood glucose levels.Citation30) ob/ob mice with a leptin mutation show a remarkable increase in body weight and blood glucose level from around 6 weeks old.Citation31, 32) Obesity and hyperglycemia are candidate factors leading to abnormalities of the second hair cycle in ob/ob mice. Recently, epidemiological studies have revealed metabolic syndrome as an associating factor with androgenetic alopecia. The prevalence of obesity, hyperglycemia, and insulin resistance was higher in androgenetic alopecia patients than in the control group,Citation33, 34) and the severity of androgenetic alopecia is positively correlated with body mass index.Citation35) Our research group has revealed elevated oxidative stress in metabolic syndrome patients and model mice, and mutual upregulation of skin oxidative stress and mineralocorticoid receptor (MR) expression in hair follicles of mice.Citation37, 38) Overexpression of MR in epidermal keratinocytes induces hypoplasia of fetal hair follicles and progression of postnatal alopecia.Citation39) Therefore, we hypothesized that the elevated oxidative stress is a factor to modify the development or progression of alopecia via MR in metabolic syndrome, although the trigger of androgenetic alopecia is the enhanced expression/activation of 5α-reductase. The higher oxidative stress in the scalp or hair matrix cells of androgenetic alopecia and alopecia areataCitation40, 41) supports this hypothesis. Further studies of the improving effects of decreasing oxidative stress and body weight in ob/ob mice are required.
The elongation of telogen and the shortening of anagen beyond 10 weeks old in ob/ob mice resemble the pathology of telogen effluvium, suggesting that ob/ob mice are a possible model animal for telogen effluvium. Since alopecia often causes scalp pain and itching owing to the condition or treatment,Citation5, 6, 8, 42) the development of scalp care methods is required to prevent or to attenuate patient discomfort. The elucidation of the pathology and molecular mechanisms of alopecia and hair cycle abnormalities in ob/ob mice can be the basis for such efforts.
A limitation of this study is that the hair cycle phase was identified in different individuals in each age class. Therefore, the anagen transition in ob/ob mice might be underestimated. The development of non-invasive methods to identify hair cycle phases is required for the accurate evaluation of the hair cycle.
In conclusion, ob/ob mice show telogen elongation and inhibition of the anagen transition in the second hair cycle after the mice reach 10 weeks of age, resembling the pathology of telogen effluvium. It is expected that ob/ob mice can be used to elucidate the underlying mechanisms and evaluate scalp care for individuals with alopecia as a new animal model of telogen effluvium.
Author contribution
Natsumi Tasaki and Takeo Minematsu involved in study conception, acquisition of data, analysis and interpretation of data, and manuscript drafting and revision. Gojiro Nakagami and Hiromi Sanada involved in study conception, interpretation of data, and critical reading of draft. Yuko Mugita and Shin-ichi Ikeda performed the experiments.
Disclosure statement
This study was performed as part of a research project titled “Research on Scalp Care Science” in collaboration with Aderans Co. Ltd. (Tokyo, Japan). The authors freely borrowed an all-in-one inverted microscope from Keyence Co. Ltd. (Tokyo, Japan). The companies did not contribute to the design and performance of the experiment, data analysis, or writing of this manuscript.
References
- Alonso L, Fuchs E. The hair cycle. J. Cell Sci. 2006;119:91–393.
- O’goshi K, Iguchi M, Tagami H. Functional analysis of the stratum corneum of scalp skin: studies in patients with alopecia areata and androgenetic alopecia. Arch. Dermatol. Res. 2000;292:605–611.10.1007/s004030000185
- Kanwar AJ, Narang T. Anagen effluvium. Indian J. Dermatol. Venereol. Leprol. 2013;79:604–612.10.4103/0378-6323.116728
- Grover C, Khurana A. Telogen effluvium. Indian J. Dermatol. Venereol. Leprol. 2013;79:591–603.10.4103/0378-6323.116731
- Bin Saif GA, McMichael A, Kwatra SG, Chan YH, Yosipovitch G. Central centrifugal cicatricial alopecia severity is associated with cowhage-induced itch. Br. J. Dermatol. 2013;168:253–256.
- Cutrer FM, Pittelkow MR. Cephalalgic alopecia areata: a syndrome of neuralgiform head pain and hair loss responsive to botulinum A toxin injection. Cephalalgia. 2006;26:747–751.10.1111/cha.2006.26.issue-6
- Willimann B, Trüeb RM. Hair pain (trichodynia): frequency and relationship to hair loss and patient gender. Dermatology. 2002;205:374–377.10.1159/000066437
- Yamakoshi T, Andoh T, Makino T, Kuraishi Y, Shimizu T. Clinical and histopathological features of itch in patients with alopecia areata. Acta Derm. Venereol. 2013;93:575–576.10.2340/00015555-1613
- de Hollanda TR, Ramos-e-Silva M, Sodre CT, Brasil M. Quality of life in alopecia areata: a case-control study. Int. J. Trichology. 2014;6:8–12.10.4103/0974-7753.136748
- Han SH, Byun JW, Lee WS, et al. Quality of life assessment in male patients with androgenetic alopecia: result of a prospective, multicenter study. Ann. Dermatol. 2012;24:311–318.10.5021/ad.2012.24.3.311
- McGarvey EL, Baum LD, Pinkerton RC, Rogers LM. Psychological sequelae and alopecia among women with cancer. Cancer Pract. 2001;9:283–289.10.1046/j.1523-5394.2001.96007.x
- Pickard-Holley S. The symptom experience of alopecia. Semin. Oncol. Nurs. 1995;11:235–238.10.1016/S0749-2081(05)80003-8
- Takashima I, Iju M, Sudo M. Alopecia androgenetica—its incidence in Japanese and associated condition. In: Orfanos CE, Montagna W, Stüttgen G, editors. Hair research. Berlin: Springer-Verlag; 1981. p. 287–293.10.1007/978-3-642-81650-5
- Jackow C, Puffer N, Hordinsky M, Nelson J, Tarrand J, Duvic M. Alopecia areata and cytomegalovirus infection in twins: genes versus environment? J. Am. Acad. Dermatol. 1998;38:418–425.10.1016/S0190-9622(98)70499-2
- Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ 3rd. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin. Proc. 1995;70:628–633.10.4065/70.7.628
- Sharma VK, Dawn G, Kumar B. Profile of alopecia areata in Northern India. Int. J. Dermatol. 1996;35:22–27.
- van der Steen P, Traupe H, Happle R, Boezeman J, Sträter R, Hamm H. The genetic risk for alopecia areata in first degree relatives of severely affected patients. An estimate. Acta Derm. Venereol. 1992;72:373–375.
- Yang S, Yang J, Liu JB, et al. The genetic epidemiology of alopecia areata in China. Br. J. Dermatol. 2004;151:16–23.10.1111/bjd.2004.151.issue-1
- Trüeb RM. Chemotherapy-induced alopecia. Curr. Opin. Support Palliat. Care. 2010;4:281–284.10.1097/SPC.0b013e3283409280
- Piraccini BM, Alessandrini A. Androgenetic alopecia. G. Ital. Dermatol. Venereol. 2014;149:15–24.
- Blumeyer A, Tosti A, Messenger A, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J. Dtsch. Dermatol. Ges. 2011;9Suppl 6:S1–S57.10.1111/ddg.2011.9.issue-s6
- Yoshitake T, Takeda A, Ohki K, et al. Five-year efficacy of finasteride in 801 Japanese men with androgenetic alopecia. J. Dermatol. 2015;42:735–738. doi:10.1111/1346-8138.12890.
- Gilhar A, Ullmann Y, Berkutzki T, Assy B, Kalish RS. Autoimmune hair loss (alopecia areata) transferred by T lymphocytes to human scalp explants on SCID mice. J. Clin. Invest. 1998;101:62–67.10.1172/JCI551
- Gilhar A, Landau M, Assy B, Shalaginov R, Serafimovich S, Kalish RS. Melanocyte-associated T cell epitopes can function as autoantigens for transfer of alopecia areata to human scalp explants on prkdcscid mice. J. Invest. Dermatol. 2001;117:1357–1362.10.1046/j.0022-202x.2001.01583.x
- Minematsu T, Nishijima Y, Huang L, et al. HSL attenuates the follicular oxidative stress and enhances the hair growth in ob/ob mice. Plast. Reconstr. Surg. Glob. Open. 2013;1:e60.10.1097/GOX.0000000000000000
- Sumikawa Y, Inui S, Nakajima T, Itami S. Hair cycle control by leptin as a new anagen inducer. Exp. Dermatol. 2014;23:27–32.10.1111/exd.12286
- Paus R, Müller-Röver S, van der Veen C, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J. Invest. Dermatol. 1999;113:523–532.10.1046/j.1523-1747.1999.00740.x
- Dry FW. The coat of the mouse (Mus musculus). J. Genet. 1926;16:287–340.10.1007/BF02983004
- Yang CC, Sheu HM, Chung PL, et al. Leptin of dermal adipose tissue is differentially expressed during the hair cycle and contributes to adipocyte-mediated growth inhibition of anagen- phase vibrissa hair. Exp. Dermatol. 2014;24:57–60.
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432.10.1038/372425a0
- Dubuc PU. The development of obesity, hyperinsulinemia, and hyperglycemia in ob/ob mice. Metabolism. 1976;25:1567–1574.10.1016/0026-0495(76)90109-8
- Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J. Hered. 1950;41:317–318.
- Acibucu F, Kayatas M, Candan F. The association of insulin resistance and metabolic syndrome in early androgenetic alopecia. Singapore Med. J. 2010;51:931–936.
- Arias-Santiago S, Gutiérrez-Salmerón MT, Castellote-Caballero L, Buendía-Eisman A, Naranjo-Sintes R. Androgenetic alopecia and cardiovascular risk factors in men and women: a comparative study. J. Am. Acad. Dermatol. 2010;63:420–429.10.1016/j.jaad.2009.10.018
- Yang CC, Hsieh FN, Lin LY, Hsu CK, Sheu HM, Chen W. Higher body mass index is associated with greater severity of alopecia in men with male-pattern androgenetic alopecia in Taiwan: a cross-sectional study. J. Am. Acad. Dermatol. 2014;70:297–302.e1.10.1016/j.jaad.2013.09.036
- Ibuki A, Akase T, Nagase T, et al. Skin fragility in obese diabetic mice: possible involvement of elevated oxidative stress and upregulation of matrix metalloproteinases. Exp. Dermatol. 2012;21:178–183.10.1111/exd.2012.21.issue-3
- Matsumoto M, Ibuki A, Minematsu T, et al. Structural changes in dermal collagen and oxidative stress levels in the skin of Japanese overweight males. Int. J. Cosmet. Sci. 2014;36:477–484.10.1111/ics.2014.36.issue-5
- Nagase T, Akase T, Sanada H, et al. Aging-like skin changes in metabolic syndrome model mice are mediated by mineralocorticoid receptor signaling. Aging Cell. 2013;12:50–57.10.1111/acel.12017
- Sainte Marie Y, Toulon A, Paus R, et al. Targeted skin overexpression of the mineralocorticoid receptor in mice causes epidermal atrophy, premature skin barrier formation, eye abnormalities, and alopecia. Am. J. Pathol. 2007;171:846–860.10.2353/ajpath.2007.060991
- Abdel Fattah NS. Ebrahim AA, El Okda ES. Lipid peroxidation/antioxidant activity in patients with alopecia areata. J. Eur. Acad. Dermatol. Venereol. 2011;25:403–408.
- Bahta AW, Farjo N, Farjo B, Philpott MP. Premature senescence of balding dermal papilla cells in vitro is associated with p16 (INK4a) expression. J. Invest. Dermatol. 2008;128:1088–1094.10.1038/sj.jid.5701147
- Katagiri K, Arakawa S, Hatano Y, Fujiwara S. Fexofenadine, an H1-receptor antagonist, partially but rapidly inhibits the itch of contact dermatitis induced by diphenylcyclopropenone in patients with alopecia areata. J. Dermatol. 2006;33:75–79.10.1111/jde.2006.33.issue-2
