Abstract
CdSe quantum dots (QDs) are potential fluorescent reagents, but leakage of Cd and Se often induces cytotoxicity. Here we prepared CdSe-based QDs with glass to reduce their leakage and examined their cytotoxicity using keratinocyte cells. The cytotoxicity of the QDs with glass was obviously lower than that of the commercial QDs with polymer, suggesting their safety for biological applications.
Key words:
Photoluminescent quantum dots (QDs), such as those based on CdSe and CdTe have attracted much interest owing to their bright photoluminescence (PL), widely tunable PL wavelength and excellent photostability.Citation1–5) In particular, CdSe-based core/shell-type QDs, such as CdSe/ZnS are considered to be one of the best QD phosphors. Bright PL of CdSe-based QDs originates from high PL quantum efficiency, large absorption coefficient and fast decay of PL. Tunability of PL wavelength reflects the quantum confinement effect that brings about particle size-dependent PL wavelength. These unique features offer potential applicability of CdSe-based QDs in various fields from fluorescent labels to electro-optical displays and devices. Among these applications, CdSe-based QDs are most expected as fluorescent reagents for labeling biological tissues and cells.Citation3–6) Compared with organic fluorescent dyes, these QDs have superior photostability and higher degree of freedom of excitation light wavelength. However, when CdSe-based QDs are in contact with aqueous media, Cd and Se ions are partially released from the QDs, deteriorating the PL properties and inducing cytotoxicity.Citation7,8) There have been a number of reports on the cytotoxicity of CdSe-based QDs in vitro and in vivo, but their cytotoxic effects vary from case to case.Citation6,8–13) Several reasons are considered for such variations as follows: different core/shell composition of QDs, size of QDs, surface ligands, shell material and thickness, surface charge, types of cells that are exposed to QDs, etc. Another challenge in the exploitation of QDs as biological labels, especially for the single particle tracking, is the stochastic fluctuation of PL, also called blinking. In the case of commercially available polymer-coated CdSe-based QDs (QD-polymerNP), as well as single QD overlaid with silica, blinking is unavoidable, because the QDs are isolated from each other.Citation6,14)
In order to reduce the cytotoxicity caused by the release of Cd and Se ions from QDs and to avoid blinking, we have developed a series of QD-incorporated glass nanoparticles (QD-glassNPs), as shown in Fig. (A). The size (diameter, 20–100 nm) of glass nanoparticle and the number of QDs embedded in QD-glassNPs are well-controlled.Citation7,15–17) Successively, the potentials of QD-glassNPs for fluorescence imaging of living cells was confirmed.Citation18) Although we observed that the Cd leakage from the QD-glassNPs in HEPES buffer (pH 7.4) was lowered by 1–3 orders of magnitude than that from the commercially available QD-polymerNPs (Fig. (B)),Citation7) their cytotoxic effects had not been examined. In this study, we utilized human keratinocyte HaCaT cells, as a skin exposure model, and confirmed the lower cytotoxicity of QD-glassNPs, compared with QD-polymerNPs.
Fig. 1. (A) The structure and transmission electron microscopy (TEM) image of a small glass particle incorporating multiple CdSe/ZnS QDs (prepared at AIST) and (B) The structure of a CdSe/ZnS QD coated with polymer (commercially available).
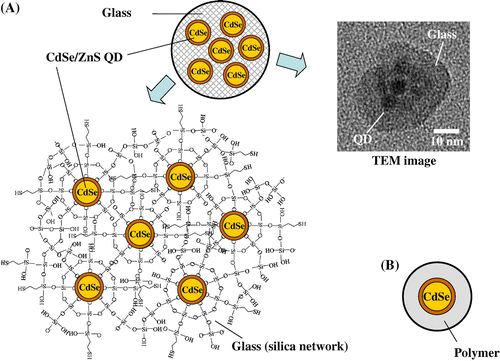
Two types of CdSe-based QD-glassNPs (sample No. 1 and 2 in Table ) were prepared as described previously.Citation15,17) Also, for comparison of cytotoxicity results, four types of QD-polymerNPs (sample No. 3–6 in Table ) were purchased from InvitrogenTM (MA, USA). The main component of the organic polymer component in the commercial QD-polymerNPs is nontoxic polyethylene glycol (PEG).Citation19) The size of these QD-glassNPs and QD-polymerNPs is about 10–50 nm. As shown in Table , the size of QDs incorporated in glass NPs and polymer NPs ranged from approximately 4 to 12 nm. For comparison, two different glass NPs without any QD (QD-free glass NPs) (sample No. 7 and 8 in Table ) were also prepared. The size of QD-free glass NPs was almost the same as that of QD-glassNPs. All NPs tested in this study were hydrophilic and therefore dispersed uniformly in the aqueous media. The cytotoxic effects of QD-glassNPs, QD-polymerNPs and QD-free glassNPs on human keratinocyte HaCaT cells were evaluated using MTT assay as described previously.Citation20,21) Human keratinocyte HaCaT cells were purchased from the German Cancer Research Center (DKFZ, Heidelberg, Germany). These cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Life Technologies) and supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Life Technologies), 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 250 ng/mL of amphotericin B (Nacalai Tesque Inc., Japan). Cells were cultured in a 75 cm2 flask (Corning Japan) at 37 °C in a 5% CO2 atmosphere. For the cell viability assay, cells were seeded on 96-well plates (Corning Japan) at 1 x 105 cells/mL, incubated for 24 h, removed from the culture medium, applied with the NP dispersion, and then incubated for another 6 or 24 h. For the determination of cell viability, MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide, Nacalai Tesque, Inc.) assay was conducted. The cells labeled using QD-glassNPs, QD-polymerNPs or QD-free glassNPs samples were incubated with 0.5 mg/mL MTT at 37 °C for 2 h. Isopropyl alcohol containing 40 mM HCl (150 μL) was added to the culture medium (100 μL), and the cells were lysed until the formazan was completely dissolved. The optical density of formazan was measured at 570 nm using a microplate reader (Multiskan Ascent, Thermo Scientific, Helsinki, Finland). HaCaT cells were incubated with different concentrations of QD-glassNPs, QD-polymerNPs and QD-free glassNPs. The concentrations of QDs tested were set at 0.1, 1.0 and 10 nM by considering the standard concentrations of commercial QD samples for cell and tissue staining (0.5–15 nM) and single particle tracking (less than 0.5 nM).Citation19,22) When one NP contained multiple QDs, these concentrations in culture media were calculated from the number of QDs. The concentration of QD-free glassNPs was adjusted to be the same as that of QD-glassNPs.
Table 1. Nanoparticle samples used for testing their cytotoxicity.
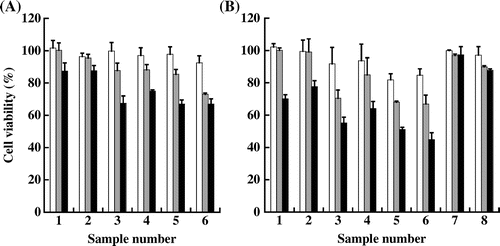
The viability of HaCaT cells exposed to QD-glassNPs (sample No. 1 and 2), QD-polymerNPs (sample No. 3–6) and QD-free glassNPs (sample No. 7 and 8) is shown in Fig. (A) (6 h exposure) and (B) (24 h exposure). When QDs were treated at 0.1 nM, the samples other than QD-polymerNPs (sample No. 5 and 6, 24 h exposure, about 15% inhibition) did not significantly affect the viability of cells, even after 6 or 24 h exposures. At 1 nM concentration, significant cytotoxic effect was induced by all QD-polymerNPs samples. Longer exposure to QD-polymerNPs (24 h) exhibited more inhibitory effects than shorter one (6 h). On the other hand, either QD-glassNPs or QD-free glassNPs showed negligible effects on the viability of HaCaT cells, even after 24 h exposure. At 10 nM concentration, all QD-glassNPs and QD-polymerNPs, exhibited much more inhibitory effects on the viability of HaCaT cells after treatments for 6 or 24 h. Especially, sample 6, one of the QD-polymerNP samples severely suppressed the cell viability of HaCaT cells to approximately 45% after 24 h exposure; whereas, the inhibitory effects of QD-glassNP samples were much lower even at the highest concentration tested, which is 10 nM.
Fig. 2. Cell viability of HaCaT cells after exposure to the nanoparticle samples listed in Table for 6 h (A) and 24 h (B).
In summary, we examined the cytotoxic effects of homemade CdSe-based QD-glassNPs and commercial CdSe-based QD-polymerNPs on cultured HaCaT cells, a skin exposure model. The cytotoxicity of the QD-glassNPs was significantly lower than that of commercial QD-polymerNPs. Since glass is more rigid and dense than polymer, the leakage of Cd and Se ions from QDs seemed to be blocked by the glass layer more effectively than organic polymer layer. Further studies, such as cytotoxic effects on different cell lines and tissues and pharmacokinetics in animal models are needed before any value can be endorsed to our CdSe-based QD-glassNPs for biological applications. etc. At this stage, our data might be useful as a first step since HaCaT cells have been often utilized to evaluate the cytotoxicity of various kinds of NPs.Citation20,21)
Author contributions
M. Ando and Y. Shigeri conceptualized and designed the study. M. Horie, Y. Akazawa-Ogawa, Y. Hagihara and N. Murase analyzed and interpreted the data. M. Ando and Y. Shigeri wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
We are grateful to Dr Kazunori Kawasaki for helpful discussion. We thank Dr Vasudevanpllai Biju for his careful reading of the manuscript.
References
- Weller H. Colloidal semiconductor Q-particles: chemistry in the transition region between solid state and molecules. Angew. Chem. Int. Ed. 1993;32:41–53.10.1002/(ISSN)1521-3773
- Murray CB, Norris DJ, Bawendi MG. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993;115:8706–8715.10.1021/ja00072a025
- Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM. Biological applications of quantum dots. Biomaterials. 2007;28:4717–4732.10.1016/j.biomaterials.2007.07.014
- Rosenthal SJ, Chang JC, Kovtun O, McBride JR, Tomlinson ID. Biocompatible quantum dots for biological applications. Chem. Biol. 2011;18:10–24.10.1016/j.chembiol.2010.11.013
- Biju V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014;43:744–764.10.1039/C3CS60273G
- Chen F, Gerion D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Lett. 2004;4:1827–1832.10.1021/nl049170q
- Yang P, Murase N, Suzuki M, et al. Bright, non-blinking, and less-cytotoxic SiO2 beads with multiple CdSe/ZnS nanocrystals. Chem. Commun. 2010;46:4595–4597.10.1039/c002243h
- Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–18.10.1021/nl0347334
- Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006;114:165–172.10.1289/ehp.8284
- Hauck TS, Anderson RE, Fischer HC, Newbigging S, Chan WCW. In vivo quantum-dot toxicity assessment. Small. 2010;6:138–144.10.1002/smll.v6:1
- Bottrill M, Green M. Some aspects of quantum dot toxicity. Chem. Commun. 2011;47:7039–7050.10.1039/c1cc10692a
- Peng L, He M, Chen B, et al. Cellular uptake, elimination and toxicity of CdSe/ZnS quantum dots in HepG2 cells. Biomaterials. 2013;34:9545–9558.10.1016/j.biomaterials.2013.08.038
- Brunetti V, Chibli H, Fiammengo R, et al. InP/ZnS as a safer alternative to CdSe/ZnS core/shell quantum dots: in vitro and in vivo toxicity assessment. Nanoscale. 2013;5:307–317.
- https://www.lifetechnologies.com/jp/ja/home/references/molecular-probes-the-handbook/ultrasensitive-detection-technology/qdot-nanocrystal-technology.html
- Yang P, Ando M, Taguchi T, Murase N. Highly luminescent CdSe/CdxZn1–xS quantum dots with narrow spectrum and widely tunable wavelength. J. Phys. Chem. C. 2011;115:14455–14460.10.1021/jp201214k
- Wang S, Li C, Yang P, Ando M, Murase N. Silica encapsulation of highly luminescent hydrophobic quantum dots by two-step microemulsion method. Colloids Surf. A. 2012;395:24–31.
- Li C, Murase N. Formation mechanism of highly luminescent silica capsules incorporating multiple hydrophobic quantum dots with various emission wavelengths. J. Colloid Interface Sci. 2013;411:82–91.10.1016/j.jcis.2013.08.053
- Hosokawa C, Onishi E, Murase N, Ando M, Kawasaki K, Taguchi T. Photoluminescence analysis of neurons using glass beads with multiple CdSe/ZnS quantum dots. Proc. 8th Annual Meeting of Soc. of Nano Sci. and Tech. 2010;85:S2-S7.
- https://tools.lifetechnologies.com/content/sfs/manuals/mp10198.pdf
- Horie M, Nishio K, Fujita K, et al. Protein adsorption of ultrafine metal oxide and its influence on cytotoxicity toward cultured cells. Chem. Res. Toxicol. 2009;22:543–553.10.1021/tx800289z
- Horie M, Nishio K, Fujita K, et al. Ultrafine NiO particles induce cytotoxicity in vitro by cellular uptake and subsequent Ni(II) release. Chem. Res. Toxicol. 2009;22:1415–1426.10.1021/tx900171n
- Seo D, Farlow J, Southard K, Jun Y, Gartner ZJ. Production and targeting of monovalent quantum dots. J. Vis. Exp. 2014;92:e52198. doi:10.3791/52198
