?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
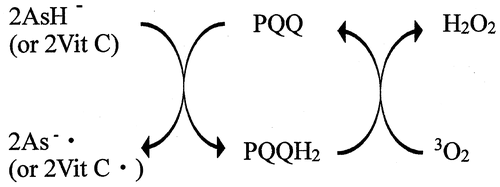
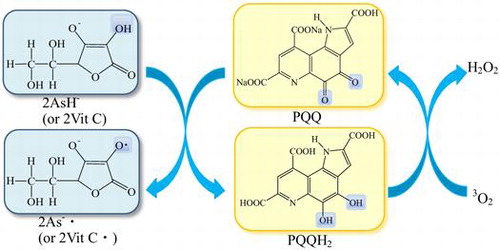
Measurements of the reaction of sodium salt of pyrroloquinoline quinone (PQQNa2) with vitamin C (Vit C) were performed in phosphate-buffered solution (pH 7.4) at 25 °C under nitrogen atmosphere, using UV–vis spectrophotometry. The absorption spectrum of PQQNa2 decreased in intensity due to the reaction with Vit C and was changed to that of pyrroloquinoline quinol (PQQH2, a reduced form of PQQ). One molecule of PQQ was reduced by two molecules of Vit C producing a molecule of PQQH2 in the buffer solution. PQQH2, thus produced, was recycled to PQQ due to air oxidation. PQQ and Vit C coexist in many biological systems, such as vegetables, fruits, as well as in human tissues. The results obtained suggest that PQQ is reduced by Vit C and functions as an antioxidant in biological systems, because it has been reported that PQQH2 shows very high free-radical scavenging and singlet-oxygen quenching activities in buffer solutions.
Graphical abstract
Recycling of PQQH2 and PQQ under the coexistence of Vit C.
Pyrroloquinoline quinone (PQQ) has attracted much attention in the recent years owing to its several interesting physiological functions.Citation1,2) PQQ is a water-soluble quinone compound first identified as a cofactor of alcohol and glucose dehydrogenases in bacteria.Citation3,4) PQQ also functions as a growth factor and contributes to mammalian cell growth.Citation5–7) It has been also reported to show neuroprotective effects.Citation8–10) A recent study suggests that PQQ protects the spinal cord against secondary damage by attenuating the inducible nitric oxide synthase expression following a primary physiological injury.Citation11)
The reduced form of PQQ, i.e., PQQH2 (pyrroloquinoline quinol, see Fig. ) exhibits antioxidative properties in in vitro examinations.Citation12,13) In experiments using cultured cells, it has been reported that PQQ prevents oxidative stress-induced neuronal death.Citation10,14) Moreover, a marked decrease in ischemia damage was observed for in vivo models such as in the case of cardiovascular or cerebral ischemia.Citation9,15) PQQ preserves mitochondrial function and prevents oxidative injury in adult rat cardiac myocytes.Citation16) Furthermore, it has been reported that PQQ prevents cognitive deficit caused due to oxidative stress in rats.Citation17,18)
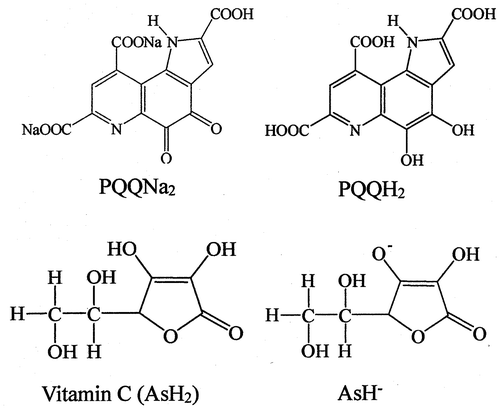
Fig. 1. Molecular structures of PQQNa2, PQQH2, vitamin C (ascorbic acid, AsH2), and ascorbate monoanion (AsH−).
Small amounts of PQQ have been found in many kinds of foods such as fruits and vegetables.Citation2,19–21) Furthermore, the presence of small amounts of free PQQ has also been found in eight of the human organs, in plasma and urine and in three of the rat organs.Citation22,23)
Earlier PQQH2 has been prepared by the reaction of PQQ with thiols (RSH) (such as thiophenol, mercaptoethanol, cysteine, and 1,4-butanedithiol)) in 0.05 M acetate buffer (including 20% CH3CN) under anaerobic conditions (see reaction (Equation1(1)
(1) )).Citation24) Reduction of PQQ to PQQH2 has also been performed using dithiothreitol with two SH groups in the same molecule.Citation25) Furthermore, we found that PQQNa2 (disodium salt of PQQ) could be easily reduced to PQQH2 by reacting it with cysteine and glutathione (RSH) in 0.05 M phosphate-buffered solution (pH 7.4) under nitrogen atmosphere (Fig. ).Citation26) These results suggest that PQQ exists as a reduced form within the cells and plays an important role as an antioxidant (AO).
(1)
(1)
It is well known that lipid peroxyl radical () and singlet oxygen (1O2) are two major representative reactive oxygen species generated in biological systems. In a previous work, aroxyl radical (
)-scavenging activities of PQQH2 and water-soluble AOs (such as vitamin C (Vit C) and uric acid) were measured in 5.0 wt% Triton X-100 micellar solution (pH 7.4) using stopped-flow spectrophotometry (see reaction (Equation2
)).Citation26) A stable aroxyl radical (
) (2,6-di-t-butyl-4-(4′-methoxyphenyl)phenoxyl) was used as a model for
radical.Citation27,28) The second-order rate constant (ks) (1.86 × 103 M−1 s−1) for the reaction of
with PQQH2 was found to be 7.4 times larger than that of Vit C (2.51 × 102 M−1 s−1), which is known as the most active water-soluble AO.
(2)
(2)
Furthermore, a kinetic study of the quenching reaction of 1O2 with PQQH2 and other seven natural AOs (Vit C, uric acid, epicatechin, epigallocatechin, α-tocopherol (α-TocH), ubiquinol-10 (UQ10H2), and β-carotene) (reaction (Equation3(3)
(3) )) has been performed in 5.0 wt% Triton X-100 micellar solution (pH 7.4), indicating that PQQH2 shows high 1O2-quenching activity.Citation29)
(3)
(3)
These results suggest that PQQH2 may contribute to the protection of oxidative damage in biological systems by scavenging the free-radicals and quenching 1O2.
As described above, Vit C is well known as a representative water-soluble AO. Vit C (ascorbic acid (AsH2)) will take the ascorbate monoanion (AsH−) structure at pH 7.4 (see Fig. ).Citation30) Hydrophilic Vit C (AsH−) enhances the AO activity of α-TocH by regenerating α-tocopheroxyl () radical to α-TocH (reaction (Equation4
))
(4)
(4)
where is ascorbate free-radical. The reaction of
with Vit C is well known as the usual tocopherol-regeneration reaction in biomembrane systems.
PQQ and Vit C are found in a number of foodsCitation2,19–21,31) in addition to human and rat tissues.Citation22,23,32–34) High concentrations of Vit C are included in human plasma.Citation32) In this study, the UV–vis absorption measurements for the reaction of PQQNa2 (that is, PQQ) with Vit C were performed in 0.05 M phosphate-buffered solution (pH 7.4) at 25.0 °C. Absorption spectrum of PQQNa2 was decreased by the reaction with Vit C and changed to that of PQQH2 (see reaction (Equation5(5)
(5) )). Our results suggest that PQQ is reduced by Vit C lacking the SH group and functions as an AO in biological systems.
(5)
(5)
Materials and methods
Materials
Commercial Vit C (Wako Chemicals, Japan) was used as received. Powdered sample of PQQNa2 was supplied by Mitsubishi Gas Chemical Co., Inc., Japan. The results for the elemental analysis, thermogravimetry, and titration of H2O using the Karl-Fischer’s reagent indicated that PQQNa2 was present in the monohydrate form ().Citation26 The buffer solution was prepared using the distilled water treated with a Millipore Q system, and the pH was adjusted to 7.4 using 0.05 M KH2PO4–Na2HPO4 solution.
Methods
Measurements of the UV–vis absorption spectrum for the reaction of PQQNa2 with Vit C (reaction (Equation5(5)
(5) )) were performed using a Shimadzu UV-2100S spectrophotometer by mixing equal volumes of 0.05 M phosphate-buffered solutions (pH 7.4) of PQQNa2 and Vit C. Generally, 0.01 M phosphate-buffered (pH 7.4) solution is used for the biochemical experiments. Vit C is a weak acid, as it is well known. However, in the present work, comparatively high concentrations of Vit C (5.36 × 10−4 and 5.36 × 10−3 M) were used for the measurements. Consequently, 0.05 M phosphate-buffered (pH 7.4) solution was used for the measurements to avoid the shift of pH value.
PQQNa2 is stable, and Vit C is comparatively stable in the buffer solution (pH 7.4) under air. On the other hand, as reported in previous works, PQQH2 is unstable in the buffer solution (pH 7.4) under air and easily oxidized to PQQ.Citation26,35,36) Consequently, the reduction of PQQNa2 to PQQH2 by Vit C was performed under strictly de-aerated and nitrogen-substituted conditions using a Hamilton 1000 series gas-tight syringe and sealing cap to avoid oxidation of PQQH2. Air oxidation of PQQH2 was performed by taking off the above sealing cap from quartz cell, and then, quartz cell containing the reaction mixture was covered with sponge rubber cap to avoid the evaporation of buffer solution. The details of the UV–vis absorption measurements are the same as reported in a previous study.Citation26) All the measurements were performed at 25.0 ± 0.5 °C.
Results
UV–vis absorption spectra of PQQNa2, PQQH2, and Vit C in buffer solution at pH 7.4
UV–vis absorption spectra of PQQNa2 and Vit C were measured in 0.05 M phosphate-buffered solution at pH 7.4, as shown in Fig. . The maximum value of the absorption peaks (λmax) and values of molar extinction coefficient (εmax) obtained for PQQNa2 and Vit C are listed in Table (where “sh” stands for shoulder).
Fig. 2. The UV–vis absorption spectra of PQQNa2 (–––), PQQH2 (·····), and Vit C (AsH−) (- - -) with the same concentrations of 4.45 × 10−5 M in 0.05 M phosphate-buffered solution (pH 7.4) at 25.0°.
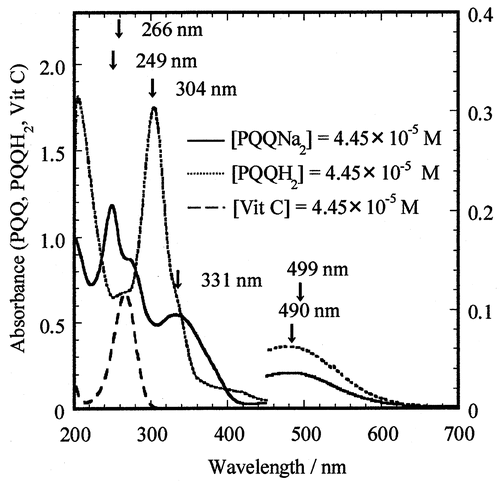
Table 1. Values of UV–vis absorption maxima (
 ) and molar extinction coefficients (
) and molar extinction coefficients (
 ) for PQQNa2, PQQH2, and Vit C in 0.05 M phosphate-buffered solution (pH 7.4).
) for PQQNa2, PQQH2, and Vit C in 0.05 M phosphate-buffered solution (pH 7.4).
In a previous work, the reduction of PQQNa2 to PQQH2 was performed using cysteine and glutathione in 0.05 M phosphate-buffered solution at pH 7.4 (reaction (Equation1(1)
(1) )).Citation26) The UV–vis absorption spectra of PQQH2 obtained by the above reductions were similar to each other. The absorption spectrum of PQQH2 obtained by the reduction using cysteine is shown in Fig. , together with those of PQQNa2 and Vit C. The spectra of PQQH2, PQQNa2, and Vit C with the same concentration of 4.45 × 10−5 M in buffer solution are shown in Fig. . The values of λmax and εmax reported for PQQH2 are listed in Table .
The values of the molar extinction coefficients (ε304.0 and ε307.5) at λ = 304.0 and 307.5 nm for PQQH2, PQQNa2, and Vit C are also listed in Table , as these values are necessary to analyze the changes in the UV–vis absorption spectra observed by the reaction between PQQNa2 and Vit C (reaction (Equation5(5)
(5) )).
PQQNa2 is reduced to PQQH2 by the reaction with Vit C in buffer solution at pH 7.4
The measurements for the reaction of PQQNa2 with Vit C were performed in a buffer solution. Fig. , panels (A) and (B), shows an example of the reaction between PQQNa2 (4.45 × 10−5 M) and Vit C (5.36 × 10−4 M) in 0.05 M phosphate-buffered solution (pH 7.4) at 25.0 °C. The spectra were recorded at (A) 6 min (t = 0–90 min) and (B) 3 h (t = 1.5–15.5 h) intervals. As shown in the Fig. (A) and (B), notable changes in the absorption spectrum of PQQNa2 were observed indicating the reaction of PQQNa2 with Vit C. Two isosbestic points were clearly observed at 342 and 396 nm, suggesting that the reaction is simple and the contribution of any side reactions is negligible.
Fig. 3. Changes in the absorption spectrum of PQQNa2 and PQQH2 during the reaction of PQQNa2 with Vit C in 0.05 M phosphate-buffered solution (pH 7.4) at 25.0 °C.
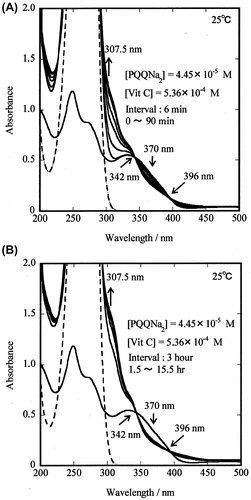
As reported in a previous study, a decrease (↓) in the absorbance of PQQNa2 at ~370 nm and an increase in the absorbance of PQQH2 at 304 nm (see Fig. in Ref. [Citation26]) was observed in the reactions of PQQNa2 with cysteine and glutathione in buffer solution (pH 7.4). This was because these reducing agents do not show any absorption at ~304 nm. Two isosbestic points (348 and 410 nm) were observed for the reaction of PQQNa2 with glutathione. It was clear that PQQH2 was produced as a result of the reaction of PQQNa2 with cysteine and glutathione. Itoh et al. had reported similar results for the reaction of PQQ with thiophenol in acetate buffer (pH 4).Citation24,36)
Fig. 4. Formation of PQQH2 due to the reaction of PQQNa2 with Vit C, and decay of PQQH2 in the presence of air.
On the other hand, Vit C shows an absorption maximum at λmax = 266 nm (εmax = 15,200 M−1 cm−1) and a weak absorption (shoulder) at 304 nm (ε304 = 193 M−1 cm−1) (see Table and Fig. ). When the same concentrations (4.45 × 10−5 M) of PQQNa2 and Vit C were used for the reaction, the absorption of Vit C at 304 nm is observed to be weak or negligible in comparison with that of PQQH2. This is because the ε304 value of Vit C (193 M−1 cm−1) is more than two orders of magnitude smaller than the ε304 of PQQH2 (40,000 M−1 cm−1), as shown in Fig. . However, in the present work, the concentration of Vit C (5.36 × 10−4 M) that was used for the reaction was 12 times higher than that of PQQNa2 (4.45 × 10−5 M). The result indicates that strong absorption of Vit C, which was shown by dotted line (- - - - -) in Fig. (A) and (B), overlaps to that of PQQH2. Consequently, we could not observe an absorption peak of PQQH2 at 304 nm.
On the other hand, the changes in the absorption spectra observed at wavelength >304 nm, such as, a decrease (↓) in the absorbance of PQQNa2 at ~370 nm and an increase (↑) in the absorbance of PQQH2 at 307.5 nm with time (Fig. (A) and (B)) are similar to that observed for the reaction of PQQNa2 with glutathione. Furthermore, two isosbestic points (at 342 and 396 nm) were observed for the reaction of PQQNa2 with Vit C, and these data are also similar to the one (348 and 410 nm) observed for the reaction of PQQNa2 with glutathione in a previous work.Citation26) These results suggest that PQQNa2 was reduced to PQQH2 by the reaction with Vit C. Detailed reason was explained in the Discussion section.
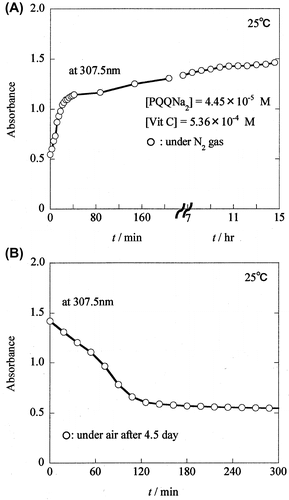
By reacting PQQNa2 with Vit C, a most notable increase in the absorbance of PQQH2 was observed at 307.5 nm, as shown in Fig. (A) and (B). Fig. (A) shows the time course of the increase in absorbance at 307.5 nm of PQQH2. This increase was observed when a 0.05 M phosphate-buffered solution (pH 7.4) containing PQQNa2 (2 × 4.45 × 10−5 M) was mixed with a 0.05 M buffer solution of Vit C (2 × 5.36 × 10−4 M) (1:1, v/v; final concentrations of PQQNa2 (4.45 × 10−5 M) and Vit C (5.36 × 10−4 M)). As shown in Fig. (A), the absorbance of PQQH2 at 307.5 nm increases rapidly from 0.544 at t = 0 min to ~1.1 at t = 30 min, and then increases gradually to 1.460 at t = 14 h. A detailed analysis of the change in the absorbance observed for PQQH2 is discussed in the Discussion section.
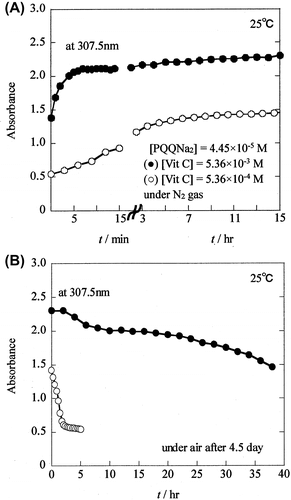
The reaction between PQQNa2 and Vit C was performed by keeping the concentration of PQQNa2 ([PQQNa2] = 4.45 × 10−5 M) constant and using a 10 times higher concentration of Vit C ([Vit C] = 5.36 × 10−3 M). Interestingly, the absorption spectrum of PQQNa2 disappeared rapidly and changed to that of PQQH2 (data not shown).
Time dependence of the absorbance (●) observed at 307.5 nm for PQQH2 is shown in Fig. (A), together with that (○) observed for the reaction of PQQNa2 with lower concentration of Vit C (Fig. (A)). As shown in Fig. (A), the absorbance at 307.5 nm increased rapidly from 1.378 at t = 0 min to ~2.10 at t = 10 min and further increased slowly to 2.302 at t = 15 h. The increase in absorbance from t = 0 to 10 min is much faster than that for the solution containing a lower concentration of Vit C (5.36 × 10−4 M). PQQ molecules are considered to be reduced easily to PQQH2 in the presence of higher concentration of Vit C.
Fig. 5. Formation of PQQH2 due to the reaction of PQQNa2 with Vit C, and decay of PQQH2 in the presence of air.
The reaction mixtures obtained after the reaction of PQQNa2 ([PQQNa2] = 4.45 × 10−5 M) with Vit C ([Vit C] = 5.36 × 10−4 M (or 5.36 × 10−3 M)) were kept for 4.5 days at 5 °C under refrigeration. During these 4.5 days, no change in the spectrum was observed indicating that PQQH2 produced during the above reaction is stable under the nitrogen atmosphere.
Air oxidation of PQQH2 under the coexistence of Vit C in buffer solution at pH 7.4
As described in one of the previous sections, PQQH2 is stable in the presence of Vit C in buffer solution under nitrogen gas atmosphere. On the other hand, exposing the solution to air (i.e. molecular oxygen (3O2)), remarkable changes in the absorption spectra were observed, as shown in Fig. . A downward arrow indicates a decrease (↓) in the absorbance of PQQH2 at 307.5 nm and an upward arrow suggests an increase (↑) in the absorbance of PQQ at 370 nm with time. As shown in Fig. , two isosbestic points were observed at 342 and 396 nm. These wavelengths are the same as those (342 and 396 nm) observed for the reduction reaction of PQQ with Vit C (see Fig. )). The result indicates that the oxidation of PQQH2 by 3O2 is also a simple reaction, and the contribution of side reactions is negligible. Furthermore, the absorption of PQQ was clearly observed at t = 300 min, as shown in Fig. . It is clear that PQQ was regenerated by the reaction of PQQH2 with 3O2 (reaction (Equation6(6)
(6) )).Citation36) The nature of the changes in the absorption spectra observed around the wavelength region (~300–500 nm) is very similar to those shown in Fig. . The result clearly indicates that PQQH2 is recycled to PQQ due to the reaction with 3O2 in the buffer solution at pH 7.4 (see Fig. ).Citation25,36)
(6)
(6)
Fig. 6. PQQH2 solution produced due to the reaction of PQQNa2 ([PQQNa2]t = 0 = 4.45 × 10−5 M) with Vit C ([Vit C]t = 0 = 5.36 × 10−4 M) was exposed to air, after keeping the solution under nitrogen atmosphere for 4.5 day.
![Fig. 6. PQQH2 solution produced due to the reaction of PQQNa2 ([PQQNa2]t = 0 = 4.45 × 10−5 M) with Vit C ([Vit C]t = 0 = 5.36 × 10−4 M) was exposed to air, after keeping the solution under nitrogen atmosphere for 4.5 day.](/cms/asset/ec26a0ea-6ba5-493d-b1aa-a733138ebad3/tbbb_a_1072462_f0006_b.gif)
By reacting PQQH2 with air, the most notable decrease in the absorbance of PQQH2 was observed at 307.5 nm, as shown in Fig. . Fig. (B) shows the time course of the decrease in the absorbance at 307.5 nm for PQQH2 observed when the solution containing PQQH2 was exposed to air. As shown in Fig. (B), the absorbance of PQQH2 at 307.5 nm decreases gradually from 1.420 at t = 0 min to ~0.60 at t = 120 min and further decreases very slowly to 0.544 at t = 300 min.
Similarly, by exposing the reaction mixture containing PQQH2 and a high concentration of Vit C ([Vit C] = 5.36 × 10−3 M) to air, the change in the absorption spectrum was observed. However, the observed change was not very remarkable (data not shown). Fig. (B) shows the time course of the decrease in the absorbance of PQQH2 at 307.5 nm observed when the solution was exposed to air. The absorbance of PQQH2 at 307.5 nm (indicated by ●) decreases slowly from 2.305 at t = 0 min to 1.465 at t = 38 h. The decrease in the absorbance of PQQH2 was much slower in comparison with the reaction mixture containing lower concentration of Vit C ([Vit C] = 5.36 × 10−4 M) (indicated by ○). Not only the PQQ produced by the air oxidation of PQQH2, but also the residual Vit C in the reaction mixture contribute to the absorbance observed at 307.5 nm. The changes in the absorbance of PQQH2 are discussed in greater details in the Discussion section.
Discussion
UV–vis absorption spectra of 
 (or
(or 
 ) and
) and 
 radicals in buffer solution at pH 7.4
radicals in buffer solution at pH 7.4
In the previous studies, the acid dissociation constants (Ka) for the three COOH groups and one NH group in PQQ were determined by the measurement of the pH dependence of oxidation–reduction potentials (E1/2).Citation37–39) The three COOH groups in PQQ molecule will take a monoanion form (COO−) at pH 7.4 (Fig. ), because the pKa1, pKa2, and pKa3 values of the three COOH groups and the pKa4 value of the NH group in the PQQ molecule are reported to be 1.60, 2.20, 3.30, and 10.30, respectively. Consequently, both PQQNa2 and PQQ should take a similar molecular structure in the buffer solution with pH 7.4 and exhibit similar UV–vis absorption spectra. Therefore, we have used an abbreviation, “PQQ,” instead of “PQQNa2” as needed (see reactions (Equation1(1)
(1) ), (Equation5
(5)
(5) ), etc.).
Vit C (ascorbic acid, AsH2) is a dibasic acid and can exist in three different molecular forms, i.e., the undissociated form (AsH2), monoanion form (AsH−), and dianion form (As2−), depending on the pH value (Fig. ). Vit C will take a monoanion form (AsH−) at pH 7.4, as the pKa1 and pKa2 values of Vit C (AsH2) are 4.17 and 11.57, respectively.Citation30,40) Hence, we have used an abbreviation, “AsH−,” instead of Vit C as needed (see reactions (Equation5(5)
(5) ), (Equation7
(7)
(7) ), and (Equation8
(8)
(8) )).
As described in the Introduction, the reaction between PQQNa2 and Vit C (AsH−) could simply be expressed as shown in reaction (Equation5(5)
(5) ), which is very similar to the reaction between PQQNa2 and RSH (reaction (Equation1
(1)
(1) )).Citation26) However, by reacting PQQNa2 with AsH−, the following reactions (Equation7
(7)
(7) )–(Equation10
(10)
(10) ) will proceed competitively in the buffer solution (pH 7.4). First, reaction (Equation7
(7)
(7) ) will occur producing
and
, followed by reaction (Equation8
(8)
(8) ) producing PQQH2 and
. Furthermore,
radicals are considered to be consumed by the disproportionation reaction producing PQQ and PQQH2 (reaction (Equation9
(9)
(9) )), as described below.
(7)
(7)
(8)
(8)
(9)
(9)
(10)
(10)
If the radical is stable in the buffer solution (pH 7.4), the absorption of
radical would overlap with those of PQQ, PQQH2, and Vit C in the process of the reaction. Bielski et al.Citation41) had measured the UV–vis absorption spectrum of
radical using the pulse radiolysis technique. In this study, the
radical showed an absorption peak at λmax = 360 nm (εmax = 5000 M−1 cm−1) at pH 7.4. Furthermore, they reported that
radicals decay rapidly, following reaction (Equation10
(10)
(10) ), with a second-order rate constant ((7.00 ± 0.62) × 104 M−1 s−1) at pH 7.4. We tried to measure the UV–vis absorption spectrum of
radical, by oxidizing AsH− with air (3O2) in the buffer solution at pH 7.4. However, only a decrease in the UV–vis absorption spectrum of AsH− at 266 nm was observed, while the spectrum of
radical was not observed at 360 nm (data not shown). As shown in Fig. (A), the absorption peak due to
radicals was not observed at ~360 nm.
To our knowledge, the UV–vis absorption spectrum of radical has not been reported earlier, as
radical is considered to be unstable. In fact, the absorption which will be due to
radicals was not observed for the reaction of PQQ with Vit C, as shown in Fig. . PQQH2 and (−)-epicatechin (or (+)-catechin) are the molecules possessing a catechol structure. (−)-Epicatechin and (+)-catechin radicals, which are the transient products due to their AO action, are known to be unstable. Jovanovic et al.Citation42,43) have measured the absorption spectra of these radicals using pulse radiolysis technique. It has been reported that (−)-epicatechin and (+)-catechin radicals disappear by disproportionation reaction, producing ortho-quinone products of (+)-catechin and (−)-epicatechin.Citation44,45) Similarly,
radicals generated by the reaction (Equation7
(7)
(7) ) will disappear rapidly by (i) reaction (Equation8
(8)
(8) ) and/or (ii) disproportionation reaction (Equation9
(9)
(9) ). However, the details of the decay mechanism of
radicals are not well understood at present.
PQQNa2 is reduced by Vit C producing PQQH2, which is further recycled to PQQ by air oxidation in buffer solution at pH 7.4
As described in the Results section, by adding the 0.05 M phosphate-buffered solution (pH 7.4) of Vit C (2 × 5.36 × 10−4 M) to the solution of PQQNa2 (2 × 4.45 × 10−5 M) (1:1 in volume) at 25 °C, the absorption spectrum of PQQNa2 disappeared rapidly, and finally changed to that of PQQH2 (see Fig. (A) and (B)). The time dependence of the absorbance at 307.5 nm is shown in Fig. (A).
The reaction mixture includes PQQNa2 and Vit C in the buffer solution. If only PQQNa2 and Vit C coexist in the buffer solution (see reaction (Equation5(5)
(5) )), the total absorbance (
) of PQQNa2 and Vit C at the beginning of the reaction could be expressed as given in Equation (Equation11
(11)
(11) ).
(11)
(11)
where and εVit C are the molar extinction coefficients of PQQNa2 and Vit C, respectively, at 307.5 nm in the buffer solution (pH 7.4) (see Table ). [PQQNa2]initial and [Vit C]initial are the initial concentrations of the reactants PQQNa2 and Vit C, respectively, which were used for reaction (Equation5
(5)
(5) ) (with low conc. of Vit C), as listed in Table . The
value (0.525) at t = 0 min calculated using Equation (Equation11
(11)
(11) ) is in good agreement with the observed value (0.544) listed in Table . These results suggest that the method of analysis performed in the present study could be considered reliable.
Table 2. Changes in the absorbance of PQQH2 at 307.5 nm due to the reaction of PQQNa2 with vitamin C in the buffer solution (pH 7.4) at 25.0 °C under nitrogen atmosphere.
By reacting PQQNa2 with Vit C under nitrogen atmosphere, PQQNa2 is reduced and produces PQQH2 (see Fig. (A) and (B)). As described in a previous section, and
radicals are unstable in the buffer solution at pH 7.4 and do not contribute to the change in the absorption spectra observed due to the reaction between PQQNa2 and Vit C. Therefore, if the reduction reaction of PQQNa2 with Vit C is completed at t = 15 h (see Fig. (A)), the
value of PQQH2 and Vit C in solution could be calculated using Equation (Equation12
(12)
(12) ) as follows:
(12)
(12)
where [PQQH2]final is the final concentration of PQQH2 produced as a result of the reduction of Vit C. In order to reduce one molecule of PQQ to one molecule of PQQH2, two molecules of Vit C are required (see reaction (Equation5(5)
(5) )). Consequently, [Vit C] changes from [Vit C]initial (5.36 × 10−4 M) at t = 0 min to [Vit C]final (5.36 × 10−4 – 2 × 4.45 × 10−5 = 4.47 × 10−4 M) at t = 15 h, where [PQQNa2]initial is equal to 4.45 × 10−5 M.
The values of (calcd.),
(calcd.), and
(calcd.) at t = 15 h were found to be 1.678, 0.037, and 1.715, respectively, according to Equation (Equation12
(12)
(12) ) and are listed in Table . The contribution of
(calcd.) to
(calcd.) is small (~2.2%) and seems to be almost negligible. The value of
(obsd.), i.e., 1.423 was estimated by subtracting the contribution of Vit C (
(calcd.) (0.037)) from the total
(obsd.) (1.460). A comparison of the
(obsd.) (1.423) with
(calcd.) (1.678) suggests that the former value is 0.85 times smaller than the latter. The
(obsd.) value (1.460) (or
(obsd.) (1.423)) at t = 15 h increases gradually and seems to approach the
(calcd.) (1.715) (or
(calcd.) (1.678)) values, as shown in Fig. (A).
On the other hand, by introducing air into the reaction mixture containing PQQH2 and Vit C (with residual PQQ in mixture), rapid change in the absorption spectrum was observed due to the oxidation of PQQH2 to PQQ (Fig. ). As shown in Fig. (B), the (obsd.) value (1.420) decreases gradually from t = 0 to 120 min and slowly approaches to a constant value of 0.544 (see Table ). This value is very similar to the values of
(obsd.) (0.544) and
(calcd.) (0.525) that are obtained at t = 0 min for the reaction between PQQNa2 and Vit C (Table ). As listed in Table , the ratio of
(obsd.) to
(calcd.) (=0.507/0.481) is 1.05. This result indicates that PQQH2 is completely recycled to the original PQQ due to air oxidation, as shown in Fig. .
Table 3. Changes in the absorbance of PQQH2 at 307.5 nm due to the reaction of PQQH2 with air (molecular oxygen) in the buffer solution (pH 7.4) at 25.0 °C.
As described in the Results section, the reaction between PQQNa2 and Vit C was performed by keeping the concentration of PQQNa2 ([4.45 × 10−5 M]) constant and using a 10 times higher concentration of Vit C ([5.36 × 10−3 M]) (see reaction (Equation5(5)
(5) ) with high conc. of Vit C) (Table and Fig. (A) and (B)). The initial and final values of AbsPQQH2 (calcd.), AbsVit C (calcd.), and Abstotal (calcd.) are listed in Table , together with the values of AbsPQQH2 (obsd.), AbsVit C (obsd.), and Abstotal (obsd.) observed.
As described above, the (obsd.) value (0.544) observed for the reactants showed a good agreement with the
(calcd.) value (0.525), when [Vit C]initial was low (5.36 × 10−4 M). However, when a 10 times higher concentration of Vit C ([Vit C]initial = 5.36 × 10−3 M) was used for the reaction, the
(obsd.) value (1.378) at t = 0 min was 1.49 times larger than the
(calcd.) one (0.924). This is because it takes about 3 min (dead time) before (i) mixing the two buffer solutions containing PQQNa2 and Vit C, and (ii) in recording the first UV–vis spectrum. As shown in Fig. (A), the rate of the increase in the absorbance of PQQH2 for the reaction (Equation5
(5)
(5) ) (high conc. of Vit C) (indicated by ●) is much faster than that for the reaction (Equation5
(5)
(5) ) (low conc. of Vit C) (indicated by ○). Consequently, the increase in the
(obsd.) value from 0.924 to 1.378 at t = 0 min may be explained by considering that the reaction occurred during the dead time.
As shown in Fig. (A), for the reaction of PQQNa2 with Vit C ([Vit C] = 5.36 × 10−3 M), the Abstotal (obsd.) value (indicated by ●) increases rapidly from 1.378 at t = 0 min and shows a constant value (~2.10) at t = 7–12 min, suggesting that the reduction reaction is completed at around t = 7 min. In fact, the Abstotal (obsd.) value (~2.10) is similar to the Abstotal (calcd.) (2.120) (Table ). However, the Abstotal (obsd.) value increases gradually from ~2.10 to 2.302 at t = 15 h. As listed in Table , the ratio of (obsd.) to
(calcd.) (=2.302/2.120) is 1.09. The reason for such an increase of the
(obsd.) value is not clearly understood at present.
Furthermore, by introducing air into the reaction mixture, the Abstotal (obsd.) value (indicated by ●) decreases slowly (see Fig. (B)). At t = 38 h in the presence of air, (obsd.) value (1.465) is 1.59 times larger than
(calcd.) (0.923). The high concentration of Vit C included in the solution could suppress the oxidation of PQQH2 to PQQ. A notable difference in the time dependence of Abstotal (obsd.) value is clear from the plots shown in Fig. (B). As listed in Table , the ratio of
(obsd.) to
(calcd.) (1.030/0.481) is 2.14, indicating that about one half of PQQH2 is not oxidized and is left unconsumed in the solution. Vit C, which coexists in the reaction mixture, functions as an AO and prevents the oxidation of PQQH2 to PQQ.
PQQ is reduced to PQQH2 by Vit C, which may function as an AO in biological systems
As described in the Introduction section, PQQ and Vit C coexist in many foodsCitation2,19–21,31) and human and rat tissues.Citation22,23,32–34) High concentrations of Vit C are also present in human plasmaCitation32) and coexist with PQQ.Citation22) The results obtained in the present study suggest that PQQ is reduced to PQQH2 by Vit C, which functions as an AO in many biological systems. This is because it has been reported that PQQH2 shows very high free-radical scavenging and singlet-oxygen quenching activities in buffer solution at pH 7.4.Citation26,29) Furthermore, the results obtained in the present study suggest that PQQ and PQQH2 may be recycled, for example, in the human plasma, as shown in Fig. .
In a recent study, the measurement of -scavenging rate constants (ks) for AOs (such as PQQH2, α-TocH, UQ10H2, and three catechins) were performed in dimethyl sulfoxide (DMSO) solution using stopped-flow spectrophotometry (see reaction (Equation2
)).Citation46) In this study, the values for ks were not only measured for each of the AOs, but also for the mixture of two AOs, i.e., (i) α-TocH and PQQH2 and (ii) α-TocH and UQ10H2. A notable synergistic effect was observed which suggested that the ks values increase by 1.72, 2.42, and 2.50 times for α-TocH, PQQH2, and UQ10H2, respectively, for the solutions including two kinds of AOs. The measurements of the regeneration rate constants of α-tocopheroxyl radical (
) to α-TocH in the presence of PQQH2 and UQ10H2 were performed in DMSO using a double-mixing stopped-flow spectrophotometry technique (reaction (Equation13
(13)
(13) )). The second-order rate constant (kr) (1.08 × 105 M−1 s−1) obtained for PQQH2 was found to be 3.0 times larger than that (3.57 × 104) for UQ10H2. This result indicates that the pro-oxidant effect of
is suppressed by the coexistence of PQQH2 or UQ10H2.
(13)
(13)
As reported in the previous studies, PQQ coexists with α-TocH in foods and biological systems (such as plasma, blood, and various tissues).Citation2,19,20,22) Consequently, the above-mentioned synergistic effects, i.e., an increase in the free-radical scavenging rate and the suppression of the pro-oxidant reaction may also function in foods and biological systems. Recently, in light of its beneficial physiological effects, PQQ has been identified as a potential candidate for its use in the dietary supplements.Citation47) The results obtained in the present study suggest that the use of supplements including PQQNa2, Vit C, and α-TocH at the same time is preferable in maintaining a good health.
Author contributions
K. Mukai designed research and wrote the manuscript. A. Ouchi performed experiment and analyzed the data. S. Nagaoka helped to draft the manuscript and supervised the research. K. Ikemoto and M. Nakano prepared sodium salt of PQQ and provided valuable advice.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was partly supported by the Challenging Exploratory Research from the Japan Society for the Promotion of Science [grant number 24658123].
References
- Rucker R, Chowanadisai W, Nakano M. Potential physiological importance of pyrroloquinoline quinone. Altern. Med. Rev. 2009;14:268–277.
- Stites TE, Mitchell AE, Rucker RB. Physiological importance of quinoenzymes and the o-quinone family of cofactors. J. Nutr. 2000;130:719–727.
- Duine JA, Frank JJ, Van Zeeland JK. Glucose dehydrogenase from Acinetobacter calcoaceticus. A ‘quinoprotein’. FEBS Lett. 1979;108:443–446.10.1016/0014-5793(79)80584-0
- Salisbury SA, Forrest HS, Cruse WBT, Kennard O. A novel coenzyme from bacterial primary alcohol dehydrogenases. Nature. 1979;280:843–844.10.1038/280843a0
- Killgore J, Smidt C, Duich L, et al. Nutritional importance of pyrroloquinoline quinone. Science. 1989;245:850–852.10.1126/science.2549636
- Steinberg F, Stites TE, Anderson P, et al. Pyrroloquinoline quinone improves growth and reproductive performance in mice fed chemically defined diets. Exp. Biol. Med. 2003;228:160–166.
- Kimura K, Takada M, Ishii T, Tsuji-Naito K, Akagawa M. Pyrroloquinoline quinone stimulates epithelial cell proliferation by activating epidermal growth factor receptor through redox cycling. Free Radical Biol. Med. 2012;53:1239–1251, and references are cited therein.10.1016/j.freeradbiomed.2012.07.015
- Jensen FE, Gardner GJ, Williams AP, Gallop PM, Aizenman E, Rosenberg PA. The putative essential nutrient pyrroloquinoline quinone is neuroprotective in a rodent model of hypoxic/ischemic brain injury. Neuroscience. 1994;62:399–406.10.1016/0306-4522(94)90375-1
- Zhang Y, Feustel PJ, Kimelberg HK. Neuroprotection by pyrroloquinoline quinone (PQQ) in reversible middle cerebral artery occlusion in the adult rat. Brain Res. 2006;1094:200–206.10.1016/j.brainres.2006.03.111
- Hara H, Hiramatsu H, Adachi T. Pyrroloquinoline quinone is a potent neuroprotective nutrient against 6-hydroxydopamine-induced neurotoxicity. Neurochem. Res. 2007;32:489–495.10.1007/s11064-006-9257-x
- Hirakawa A, Shimizu K, Fukumitsu H, Furukawa S. Pyrroloquinoline quinone attenuates iNOS gene expression in the injured spinal cord. Biochem. Biophys. Res. Commun. 2009;378:308–312.10.1016/j.bbrc.2008.11.045
- Miyauchi K, Urakami T, Abeta H, et al. Action of pyrroloquinolinequinol as an antioxidant against lipid peroxidation in solution. Antioxid. Redox Signaling. 1999;1:547–554.10.1089/ars.1999.1.4-547
- He K, Nukada H, Urakami T, Murphy MP. Antioxidant and pro-oxidant properties of pyrroloquinoline quinone (PQQ): implications for its function in biological systems. Biochem. Pharmacol. 2003;65:67–74.10.1016/S0006-2952(02)01453-3
- Nunome K, Miyazaki S, Nakano M, Iguchi-Ariga S, Ariga H. Pyrroloquinoline quinone prevents oxidative stress-induced neuronal death probably through changes in oxidative status of DJ-1. Biol. Pharm. Bull. 2008;31:1321–1326.10.1248/bpb.31.1321
- Zhu B, Simonis U, Cecchini G, et al. Comparison of pyrroloquinoline quinone and/or metoprolol on myocardial infarct size and mitochondrial damage in a rat model of ischemia/reperfusion injury. J. Cardiovasc. Pharmacol. Ther. 2006;11:119–128.10.1177/1074248406288757
- Tao R, Karliner JS, Simonis U, et al. Pyrroloquinoline quinone preserves mitochondrial function and prevents oxidative injury in adult rat cardiac myocytes. Biochem. Biophys. Res. Commun. 2007;363:257–262.10.1016/j.bbrc.2007.08.041
- Ohwada K, Takeda H, Yamazaki M, et al. Pyrroloquinoline quinone (PQQ) prevents cognitive deficit caused by oxidative stress in rats. J. Clin. Biochem. Nutr. 2008;42:29–34.10.3164/jcbn.2008005
- Takatsu H, Owada K, Abe K, Nakano M, Urano S. Effect of vitamin E on learning and memory deficit in aged rats. J. Nutr. Sci. Vitaminol. 2009;55:389–393.10.3177/jnsv.55.389
- Van Der Meer RA, Groen BW, Van Kleef MAG, et al. Isolation, preparation, and assay of pyrroloquinoline quinone. Meth. Enzyme. 1990;188:260–283, and references are cited therein.10.1016/0076-6879(90)88043-A
- Kumazawa T, Sato K, Seno H, Ishii A, Suzuki O. Levels of pyrroloquinoline quinone in various foods. Biochem J. 1995;307:331–333.
- Noji N, Nakamura T, Kitahata N, et al. Simple and sensitive method for pyrroloquinoline quinone (PQQ) analysis in various foods using liquid chromatography/electrospray-ionization tandem mass spectrometry. J. Agric. Food Chem. 2007;55:7258–7263.10.1021/jf070483r
- Kumazawa T, Seno H, Urakami T, Matsumoto T, Suzuki O. Trace levels of pyrroloquinoline quinone in human and rat samples detected by gas chromatography/mass spectrometry. Biochim. Biophys. Acta. 1992;1156:62–66.10.1016/0304-4165(92)90096-D
- Mitchell AE, Jones AD, Mercer RS, Rucker RB. Characterization of pyrroloquinoline quinone amino acid derivatives by electrospray ionization mass spectrometry and detection in human milk. Anal. Biochem. 1999;269:317–325.10.1006/abio.1999.4039
- Itoh S, Kato N, Mure M, Ohshiro Y. Kinetic studies on the oxidation of thiols by coenzyme PQQ. Bull. Chem. Soc. Jpn. 1987;60:420–422.10.1246/bcsj.60.420
- Toyama H, Nishibayashi E, Saeki M, Adachi O, Matsushita K. Factors required for the catalytic reaction of PqqC/D which produces pyrroloquinoline quinone. Biochem. Biophys. Res. Commun. 2007;354:290–295.10.1016/j.bbrc.2007.01.001
- Ouchi A, Nakano M, Nagaoka S, Mukai K. Kinetic study of the antioxidant activity of pyrroloquinolinequinol (PQQH2, a reduced form of pyrroloquinolinequinone) in micellar solution. J. Agric. Food Chem. 2009;57:450–456.10.1021/jf802197d
- Mukai K, Daifuku K, Okabe K, Tanigaki T, Inoue K. Structure-activity relationship in the quenching reaction of singlet oxygen by tocopherol (vitamin E) derivatives and related phenols. Finding of linear correlation between the rates of quenching of singlet oxygen and scavenging of peroxyl and phenoxyl radicals in solution. J. Org. Chem. 1991;56:4188–4192.10.1021/jo00013a021
- Mukai K, Tokunaga A, Itoh S, et al. Structure–activity relationship of the free-radical-scavenging reaction by vitamin E (α-, β-, γ-, δ-tocopherols) and ubiquinol-10: pH dependence of the reaction rates. J. Phys. Chem. B. 2007;111:652–662.10.1021/jp0650580
- Mukai K, Ouchi A, Nakano M. Kinetic study of the quenching reaction of singlet oxygen by pyrroloquinolinequinol (PQQH2, a reduced form of pyrroloquinolinequinone) in micellar solution. J. Agric. Food Chem. 2011;59:1705–1712.10.1021/jf104420y
- Mukai K, Nishimura M, Kikuchi S. Stopped-flow investigation of the reaction of vitamin C with tocopheroxyl radical in aqueous Triton X-100 micellar solutions. J. Biol. Chem. 1991;266:274–278.
- Finley JW, Kong A-N, Hintze KJ, Jeffery EH, Ji LL, Lei XG. Antioxidants in foods: state of the science important to the food industry. J. Agric. Food Chem. 2011;59:6837–6846.10.1021/jf2013875
- de Rijke YB, Demacker PNM, Assen NA, Sloots LM, Katan MB, Stalenhoef AFH. Red wine consumption does not affect oxidizability of low-density lipoproteins in volunteers. Am. J. Clin. Nutr. 1996;63:329–334.
- Colomé C, Artuch R, Vilaseca M-A, et al. Lipophilic antioxidants in patients with phenylketonuria. Am. J. Clin. Nutr. 2003;77:185–188.
- Homma Y, Kondo Y, Kaneko M, et al. Promotion of carcinogenesis and oxidative stress by dietary cholesterol in rat prostate. Carcinogenesis. 2004;25:1011–1014.10.1093/carcin/bgh105
- Duine JA, Frank JJ, Verwiel PEJ. Characterization of the second prosthetic group in methanol dehydrogenase from hyphomicrobium X. Eur. J. Biochem. 1981;118:395–399.
- Itoh S, Ohshiro Y, Agawa T. Reaction of reduced PQQ (PQQH2) and molecular oxygen. Bull. Chem. Soc. Jpn. 1986;59:1911–1914.10.1246/bcsj.59.1911
- Kano K, Mori K, Uno B, Kubota T, Ikeda T, Senda M. Voltammetric and spectroscopic studies of pyrroloquinoline quinone coenzyme under neutral and basic conditions. Bioelectrochem. Bioenergy. 1990;23:227–238.10.1016/0302-4598(90)80012-8
- Kano K, Mori K, Uno B, Kubota T, Ikeda T, Senda M. Voltammetric determination of acid dissociation constants of pyrroloquinoline quinone and its reduced form under acidic conditions. Bioelectrochem. Bioenergy. 1990;24:193–201.10.1016/0302-4598(90)85021-9
- Zhang Z, Tillekeratne LMV, Kirchhoff JR, Hudson RA. High-performance liquid chromatographic separation and pH-dependent electrochemical properties of pyrroloquinoline quinone and three closely related isomeric analogues. Biochem. Biophys. Res. Commun. 1995;212:41–47.10.1006/bbrc.1995.1933
- Mitani S, Ouchi A, Watanabe E, Kanesaki Y, Nagaoka S, Mukai K. Stopped-flow kinetic study of the aroxyl radical-scavenging action of catechins and vitamin C in ethanol and micellar solutions. J. Agric. Food Chem. 2008;56:4406–4417.10.1021/jf703770m
- Bielski BHJ, Comstock DA, Bowen RA. Ascorbic acid free radicals. I. Pulse radiolysis study of optical absorption and kinetic properties. J. Am. Chem. Soc. 1971;93:5624–5629.10.1021/ja00751a006
- Jovanovic SV, Steenken S, Tosic M, Marjanovic B, Simic MG. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994;116:4846–4851.10.1021/ja00090a032
- Jovanovic SV, Hara Y, Steenken S, Simic MG. Antioxidant potential of gallocatechins. A pulse radiolysis and laser photolysis study. J. Am. Chem. Soc. 1995;117:9881–9888.10.1021/ja00144a014
- Sawai Y, Sakata K. NMR analytical approach to clarify the antioxidative molecular mechanism of catechins using 1,1-diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 1998;46:111–114.10.1021/jf970342x
- Nakanishi I, Fukuhara K, Ohkubo K, et al. Superoxide anion generation via electron-transfer oxidation of catechin dianion by molecular oxygen in an aprotic medium. Chem. Lett. 2001;1152–1153.10.1246/cl.2001.1152
- Ouchi A, Ikemoto K, Nakano M, Nagaoka S, Mukai K. Kinetic study of aroxyl radical scavenging and α-tocopheroxyl regeneration rates of pyrroloquinolinequinol (PQQH2, a reduced form of pyrroloquinolinequinone) in dimethyl sulfoxide solution: finding of synergistic effect on the reaction rate due to the coexistence of α-tocopherol and PQQH2. J. Agric. Food Chem. 2013;61:11048–11060.10.1021/jf4040496
- Nakano M, Takahashi H, Koura S, Chung C, Tafazoli S, Roberts A. Acute and subchronic toxicity studies of pyrroloquinoline quinone (PQQ) disodium salt (BioPQQ™) in rats. Regul. Toxicol. Pharmacol. 2014;70:107–121.10.1016/j.yrtph.2014.06.024