Abstract
HpaBC monooxygenase was previously reported to hydroxylate resveratrol to piceatannol. In this article, we report a novel catalytic activity of HpaBC for the synthesis of a pentahydroxylated stilbene. When Escherichia coli cells expressing HpaBC were incubated with resveratrol, the resulting piceatannol was further converted to a new product. This product was identified by mass spectrometry and NMR spectroscopy as a 5-hydroxylated piceatannol, 3,4,5,3′,5′-pentahydroxy-trans-stilbene (PHS), which is a reportedly valuable biologically active stilbene derivative. We attempted to produce PHS from piceatannol on a flask scale. After examining the effects of detergents and buffers on PHS production, E. coli cells expressing HpaBC efficiently hydroxylated piceatannol to PHS in a reaction mixture containing 1.5% (v/v) Tween 80 and 100 mM 3-morpholinopropanesulfonic acid-NaOH buffer at pH 7.5. Under the optimized conditions, the whole cells regioselectively hydroxylated piceatannol, and the production of PHS reached 6.9 mM (1.8 g L−1) in 48 h.
Graphical abstract
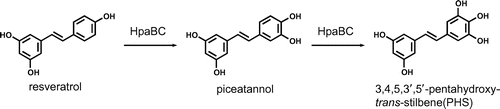
HpaBC regioselectively hydroxylates piceatannol to 3,4,5,3′,5′-pentahydroxy-trans-stilbene, a valuable biologically active stilbene derivative.
Hydroxystilbenes are biologically active molecules that are found in plants.Citation1–3) Many hydroxylated derivatives of stilbene exist that differ based on the number and position of the hydroxy groups attached to the stilbene scaffold. These hydroxy groups play important roles in biological activity. Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is the best-known hydroxystilbene. This compound has been shown to activate sirtuins, therefore it may contribute to lifespan extension.Citation4–Citation6) Resveratrol also has various health-benefiting properties including antioxidant, anticancer, and anti-inflammatory activities.Citation7–Citation9) Additionally, piceatannol (3,4,3′,5′-tetrahydroxy-trans-stilbene) has recently attracted much attention.Citation10–Citation12) A recent report indicates that piceatannol and its metabolite, isorhapontigenin, induce expression of sirtuin 1 in THP-1 human monocytic cell line.Citation10) In addition to having similar biological activities to resveratrol,Citation10–Citation12) piceatannol not only exerts positive effects on human dermal cells through the inhibition of melanogenesis and synthesis of collagen, but also exhibits vasorelaxant effects in rat thoracic aorta.Citation13,14) Interestingly, these effects of piceatannol are more pronounced than those of resveratrol.Citation13,14) These beneficial properties of hydroxystilbenes encourage their use in health and functional foods, as well as in pharmaceuticals and cosmetics. Natural hydroxystilbenes are available through extraction from several plants. However, the yields of these metabolites in extracts are usually low due to their low accumulation in plants. Also, these compounds are often difficult to synthesize by chemical methods.
Biotechnological and biocatalytic processes are promising methods for the synthesis of hydroxystilbenes. In particular, monooxygenases that catalyze the hydroxylation of the stilbene scaffold provide a simple and efficient synthetic approach to hydroxystilbenes. To date, several monooxygenases including P450 monooxygenases and a tyrosinase have been reported to hydroxylate resveratrol to piceatannol.Citation15,16) In addition, we recently found that the two-component flavin-dependent monooxygenase HpaBC from Pseudomonas aeruginosa has high activity for the hydroxylation of resveratrol to piceatannol (Fig. ).Citation17,18) HpaBC consists of a flavin-dependent monooxygenase (HpaB) and an NAD(P)H:flavin oxidoreductase (HpaC). This monooxygenase physiologically functions as a 4-hydroxyphenylacetate 3-hydroxylase in the degradation pathway of 4-hydroxyphenylacetate.Citation19–Citation21) Recombinant cells expressing HpaBC enabled the regioselective hydroxylation of resveratrol to produce 23 mM (5.2 g L−1) piceatannol.Citation18) HpaBC is useful practically because this monooxygenase can efficiently produce the rare and expensive compound piceatannol from resveratrol, which is relatively abundant and inexpensive. HpaBC from Escherichia coli also reportedly catalyzes resveratrol hydroxylation.Citation22) Thus, monooxygenases have catalytic potential for the synthesis of various hydroxystilbenes. So far, however, there have been no reports concerning monooxygenases that catalyze the hydroxylation of stilbene derivatives other than resveratrol.
In this article, we report a novel catalytic activity of HpaBC for the synthesis of a pentahydroxylated stilbene. We found that HpaBC further hydroxylated the tetrahydroxystilbene piceatannol to generate a pentahydroxystilbene. Here, we examined the chemical structure of this pentahydroxystilbene and identified it as the valuable biologically active stilbene derivative 3,4,5,3′,5′-pentahydroxy-trans-stilbene (PHS). Furthermore, we investigated flask-scale production of this compound using recombinant cells expressing HpaBC.
Materials and methods
Chemicals
Resveratrol and piceatannol were purchased from Tokyo Kasei (Tokyo, Japan). Tween 80 and Triton X-100 were purchased from MP Biomedicals (Illkirch, France). Tris(hydroxymethyl)aminomethane (Tris) was purchased from Wako Pure Chemicals (Osaka, Japan). 2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES) and 3-morpholinopropanesulfonic acid (MOPS) were purchased from Dojindo (Kumamoto, Japan). All other chemicals were of analytical grade.
Preparation of whole cells
The pETDhpaBC plasmid carrying the hpaB and hpaC genes, which was previously constructed,Citation17) was introduced into Escherichia coli BL21 Star (DE3) cells (Invitrogen, Carlsbad, CA, USA). The transformed E. coli cells were cultivated at 25 °C in Luria–Bertani medium, which contained (per liter) Bacto tryptone (10 g), Bacto yeast extract (5 g), and NaCl (10 g, pH 7.0), supplemented with ampicillin (100 μg mL−1). After cultivation for 12 h (OD600 = 0.8–1.0), isopropyl-β-D-thiogalactopyranoside (1 mM) was added to the medium and cultivation was continued for an additional 12 h at 25 °C. Cells were harvested by centrifugation and were washed with potassium phosphate buffer (50 mM, pH 7.5) containing glycerol (10% v/v). These cells were used for whole-cell reaction.
Reactions using whole cells
The reaction mixture (100 μL) contained the transformed E. coli cells (32 g of dry cell weight per liter), the substrate resveratrol or piceatannol (10 mM), dimethylsulfoxide (4% v/v), and potassium phosphate buffer (200 mM, pH 7.5) containing glycerol (10% v/v). The reaction mixture was supplemented with Tween 80 or Triton X-100 when required. When the effect of buffers on the reaction was examined, potassium phosphate buffer was replaced with Tris–HCl buffer (100 mM, pH 7.5), HEPES–NaOH buffer (100 mM, pH 7.5), or MOPS–NaOH buffer (100 or 200 mM, pH 7.5). The reactions were performed at 30 °C with vigorous shaking.
Production of PHS on a flask scale
The reaction was performed in a 500-mL flask that contained the transformed E. coli cells (32 g of dry cell weight per liter), piceatannol (10 mM), dimethylsulfoxide (4% v/v), Tween 80 (1.5% v/v), and MOPS–NaOH buffer (100 mM, pH 7.5) containing glycerol (10% v/v) in a volume of 15 mL. The reactions were carried out at 30 °C with reciprocal shaking at a speed of 240 rpm.
Product analysis
High-performance liquid chromatography (HPLC) analysis was performed using an HPLC system (1100 series, Agilent, Palo Alto, CA) with an XTerra MS C18 IS column (4.6 × 20 mm; particle size, 3.5 μm; Waters, Milford, MA), as described previously.Citation17,18) The reaction mixture (100 μL) was acidified by the addition of HCl (pH 2–3), and methanol (500 μL) and water (400 μL) were added to the reaction mixture. After vigorous shaking and centrifugation, the resulting supernatant (10 μL) was injected into the HPLC system. Mobile phases A and B were composed of a mixture of acetonitrile/methanol/potassium phosphate buffer (10 mM, pH 2.7) at a ratio of 2.5:2.5:95 and of acetonitrile, respectively. The samples were eluted with 0% B for 3 min, followed by a linear gradient of 0–70% B for 9 min at a flow rate of 1 mL min−1. Compounds were detected spectrophotometrically at a wavelength of 254 nm. The amounts of resveratrol and piceatannol were calculated from standard calibration curves that were made using the compounds purchased from Tokyo Kasei. The reaction product PHS was isolated using the HPLC system with a fraction collector (1200 series, Agilent) and an XTerra MS C18 column (4.6 × 250 mm; particle size, 3.5 μm; Waters), as described previously.Citation18,23) The amount of PHS was calculated from a standard calibration curve that was made using the compound isolated in this study. Mass analysis was performed using a Thermo Finnigan LCQ (Waltham, MA) with an electrospray ionization, as described previously.Citation18,23) NMR analysis was performed using a Bruker ADVANCE600 (Billerica, MA, USA), as described previously.Citation18,23)
PHS: 1H NMR (600 MHz, [D6]acetone): δ = 6.26 (t, J = 2.1 Hz, 1H; H-4′), 6.52 (d, J = 2.1 Hz, 2H; H-2′, H-6′), 6.64 (s, 2H; H-2, H-6), 6.78 (d, J = 16.2 Hz, 1H; H-b), 6.88 (d, J = 16.2 Hz, 1H; H-a), 7.55 (s, 1H; –OH), 8.03 (s, 2H; –OH), 8.33 (s, 2H; –OH); 13C NMR (600 MHz, [D6]DMSO): δ = 102.7, 105.7, 106,7, 127,1, 129.7, 129.9, 134.0, 140.8, 146.8, 159.6; MS (ESI) (m/z): calculated for C14H11O5 [M−H]−, 259.1; found, 259.2. The data concerning the NMR analyses are shown in the Supplemental Figs. S1 and S2.
Results and discussion
Production of a pentahydroxystilbene from resveratrol via piceatannol by HpaBC
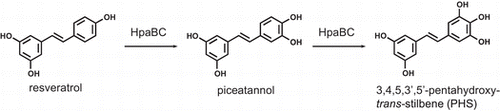
We examined the hydroxylation activity of HpaBC for resveratrol in detail. Whole-cell assays were performed using transformed E. coli cells expressing HpaBC.Citation18) Reaction conditions previously optimized for resveratrol were employed;Citation18) the reaction mixture contained the transformed E. coli cells (32 g of dry cell weight per liter), 10 mM resveratrol, 4% (v/v) dimethylsulfoxide, 1% (v/v) Tween 80, 10% (v/v) glycerol, and 200 mM potassium phosphate buffer (pH 7.5). When the transformed E. coli cells were incubated with resveratrol in a microtube that contained 100 μL of this reaction mixture, the substrate was converted to piceatannol within only 2 h (Figs. and (A)). The initial rate of resveratrol hydroxylation by the whole cells was estimated at 5.4 μmol per g of cells per minute. Interestingly, after resveratrol was almost completely hydroxylated to piceatannol, this product was further converted by E. coli cells expressing HpaBC (Fig. ). A new HPLC peak (retention time, 5.3 min) appeared after 1 h (Fig. (B)). The compound corresponding to this peak was confirmed to be a monooxygenation product of piceatannol based on the determination of its mass value ([M−H]−, m/z = 259.2). Furthermore, the 1H NMR and 13C NMR spectra of this product were in agreement with previously determined spectra of PHS.Citation24) Based on these observations, this product was identified as PHS (Fig. ). E. coli cells expressing HpaBC regioselectively hydroxylated the C-5 position of piceatannol without any detectable by-products (Fig. (B)). When the transformed E. coli cells were incubated with piceatannol as a substrate, 9.8% of 10 mM piceatannol was converted to PHS in 6 h (see Fig. , 1% (v/v) Tween 80). The initial rate of piceatannol hydroxylation by the whole cells was estimated at 0.085 μmol per g of cells per minute, which was 63 times lower than the rate of resveratrol hydroxylation.
Fig. 2. Hydroxylation of resveratrol via piceatannol to PHS by E. coli cells expressing HpaBC.
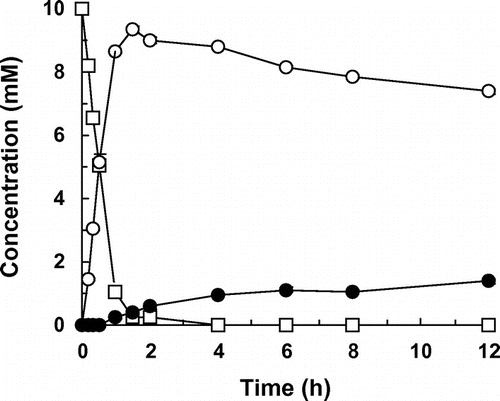
Fig. 3. HPLC chromatograms of the reaction products formed from resveratrol by E. coli cells expressing HpaBC after 0.5 h (A) and 12 h (B).
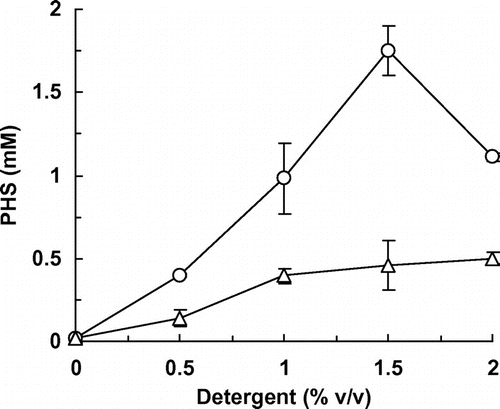
Fig. 4. Effect of detergents on PHS production.
PHS naturally occurs in the seeds of Syagrus romanzoffiana.Citation25) This compound significantly reduces postprandial blood glucose levels.Citation25) It was also reported that PHS induces apoptosis in colorectal carcinoma cells.Citation26) Notably, PHS exhibits the highest cytotoxic activity among hydroxystilbenes including resveratrol and piceatannol.Citation26) Furthermore, PHS inhibits 12-O-tetradecanoylphorbol-13-acetate-induced neoplastic transformation in JB6 P + cells more efficiently than resveratrol, suggesting that PHS has high potential as an agent for the prevention of cancer.Citation27) These reports indicate that PHS is a valuable biologically active stilbene derivative, although the number of reports on PHS is limited, partly due to the difficulty of preparing this commercially unavailable compound. It was reported that PHS was synthesized from the corresponding benzylhalide and methoxybenzaldehyde through several reaction steps including Michaelis–Arbuzov rearrangement, Horner–Wadsworth–Emmons reaction, and demethylation.Citation24) In contrast, HpaBC can provide a biocatalytic synthetic approach to PHS from commercially available piceatannol with just one step.
Effect of detergents on PHS production
E. coli cells expressing HpaBC have high potential as catalysts for the preparation of PHS. We examined reaction conditions to enhance the productivity of PHS by these whole-cell catalysts. Piceatannol was used as a substrate for the production of PHS. Previously, we confirmed that the addition of the non-ionic detergents Tween 80 and Triton X-100 to the reaction mixture significantly enhanced the productivity of piceatannol from resveratrol by the whole-cell catalysts, probably due to increased permeability of E. coli cell membranes to the substrate.Citation18) Thus, we first examined the effect of the concentrations of Tween 80 and Triton X-100 on PHS production by E. coli cells expressing HpaBC. In the absence of any detergent, the whole cells were not able to convert piceatannol to PHS (Fig. ). These results are different from our previous report on conversion of resveratrol to piceatannol, in which the whole cells converted 6.2% of 10 mM resveratrol to piceatannol in the absence of any detergent,Citation18) suggesting that the membrane is less permeable to piceatannol than resveratrol. However, the addition of Tween 80 to the reaction mixture significantly enhanced the yield of PHS from piceatannol (Fig. ). E. coli cells expressing HpaBC converted piceatannol most efficiently in the presence of 1.5% (v/v) Tween 80 and produced 1.7 mM PHS in 6 h. Although the addition of Triton X-100 also enhanced the yield, this detergent was less effective on PHS production than Tween 80. Thus, 1.5% (v/v) Tween 80 was added to the reaction mixture thereafter.
Effect of buffers on PHS production
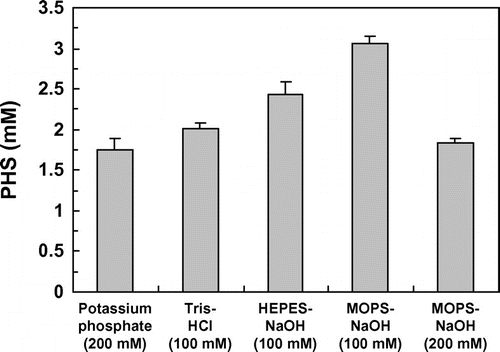
We next examined the effect of buffers on PHS production by E. coli cells expressing HpaBC. Three buffers, Tris–HCl, HEPES–NaOH, and MOPS–NaOH, were compared with potassium phosphate buffer, which was used in the experiments presented above. The pH values of these buffers were adjusted to 7.5. As shown in Fig. , all three buffers enhanced the yield of PHS from piceatannol. In particular, MOPS–NaOH buffer (100 mM) was the most effective; E. coli cells expressing HpaBC produced 3.1 mM PHS in 6 h (Fig. ). The initial rate of piceatannol hydroxylation by the whole cells in the presence of MOPS–NaOH buffer (100 mM) was estimated at 0.26 μmol per g of cells per minute, a rate 1.8 times higher than that in the presence of potassium phosphate buffer (200 mM). When the concentration of MOPS–NaOH buffer was increased from 100 to 200 mM, the amount of PHS produced decreased; the whole cells produced only 1.8 mM PHS in 6 h (Fig. ).
Fig. 5. Effect of buffers on PHS production.
Production of PHS on a flask scale
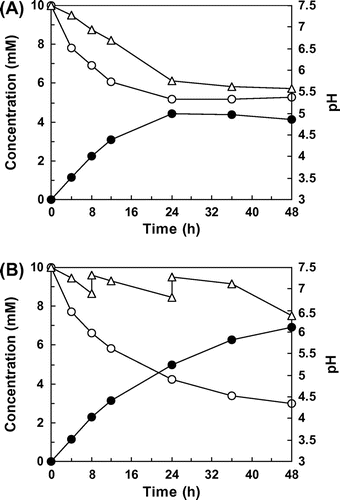
Under the optimized conditions, we attempted to produce PHS from piceatannol on a flask scale. The reaction mixture contained 1.5% (v/v) Tween 80 and 100 mM MOPS–NaOH buffer (pH 7.5). When E. coli cells expressing HpaBC were incubated with 10 mM piceatannol in a 500-ml flask that contained 15 ml of this reaction mixture, the whole cells produced 4.4 mM PHS in 24 h. Following this, however, there was no further increase in accumulation of this product (Fig. (A)). We confirmed that the pH of the reaction mixture decreased to 5.8 after 24 h of the reaction, suggesting that the cessation of PHS production might have been caused by the decrease in pH. Thus, the pH was adjusted to 7.3 using 2 M NaOH when the pH of the reaction mixture decreased below 7.0 at 8 h and 24 h. This adjustment of pH strongly enhanced the yield of PHS; the production of PHS continued to increase after 24 h and reached 6.9 mM (1.8 g L−1) in 48 h (Fig. (B)).
Fig. 6. Production of PHS on a flask scale.
Conclusion
In this study, we found that HpaBC has catalytic activity for the regioselective hydroxylation of piceatannol to PHS. To our knowledge, HpaBC is the first enzyme shown to catalyze this reaction. Furthermore, our experimental setup achieved gram-per-liter production of PHS on a flask scale. The substrate piceatannol also can be synthesized using HpaBC from the relatively inexpensive stilbene derivative resveratrol. The efficient synthetic method presented here might be helpful in the preparation of PHS, leading to new pharmaceutical applications of this compound. More generally, HpaBC should be applicable to the synthesis of various polyhydroxylated derivatives of stilbenes and related compounds.
Author contributions
T.F., M.S., and K.K., designed research. T.F., performed research. T.F., analyzed the data. T.F., M.S., and K.K., wrote the article.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2015.1072463.
Disclosure statement
No potential conflict of interest was reported by the authors.
Furuya_Supplemental_material.pdf
Download PDF (211.4 KB)Notes
Abbreviations: HEPES, 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid; HPLC, high-performance liquid chromatography; MOPS, 3-morpholinopropanesulfonic acid; PHS, 3,4,5,3′,5′-pentahydroxy-trans-stilbene; Tris, tris(hydroxymethyl)aminomethane.
References
- Fulda S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today. 2010;15:757–765.10.1016/j.drudis.2010.07.005
- Kang NJ, Shin SH, Lee HJ, Lee KW. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol. Ther. 2011;130:310–324.10.1016/j.pharmthera.2011.02.004
- Murias M, Jäger W, Handler N, et al. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: structure-activity relationship. Biochem. Pharmacol. 2005;69:903–912.10.1016/j.bcp.2004.12.001
- Baur JA, Pearson kJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342.10.1038/nature05354
- Bhullar KS, Hubbard BP. Lifespan and healthspan extension by resveratrol. Biochim. Biophys. Acta. 2015;1852:1209–1218.10.1016/j.bbadis.2015.01.012
- Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122.10.1016/j.cell.2006.11.013
- Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220.10.1126/science.275.5297.218
- Singh CK, Ndiaye MA, Ahmad N. Resveratrol and cancer: challenges for clinical translation. Biochim. Biophys. Acta. 2015;1852:1178–1185.10.1016/j.bbadis.2014.11.004
- Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health – a comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011;55:1129–1141.10.1002/mnfr.v55.8
- Kawakami S, Kinoshita Y, Maruki-Uchida H, Yanae K, Sai M, Ito T. Piceatannol and its metabolite, isorhapontigenin, induce SIRT1 expression in THP-1 human monocytic cell line. Nutrients. 2014;6:4794–4804.10.3390/nu6114794
- Piotrowska H, Kucinska M, Murias M. Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat. Res. 2012;750:60–82.10.1016/j.mrrev.2011.11.001
- Tang YL, Chan SW. A review of the pharmacological effects of piceatannol on cardiovascular diseases. Phytother. Res. 2014;28:1581–1588.10.1002/ptr.5185
- Matsui Y, Sugiyama K, Kamei M, et al. Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J. Agric. Food Chem. 2010;58:11112–11118.10.1021/jf102650d
- Sano S, Sugiyama K, Ito T, Katano Y, Ishihata A. Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds. J. Agric. Food Chem. 2011;59:6209–6213.10.1021/jf104959t
- Kim DH, Ahn T, Jung HC, Pan JG, Yun CH. Generation of the human metabolite piceatannol from the anticancer-preventive agent resveratrol by bacterial cytochrome P450 BM3. Drug Metab. Dispos. 2009;37:932–936.10.1124/dmd.108.026484
- Lee N, Kim EJ, Kim BG. Regioselective hydroxylation of trans-resveratrol via inhibition of tyrosinase from Streptomyces avermitilis MA4680. ACS Chem. Biol. 2012;7:1687–1692.10.1021/cb300222b
- Furuya T, Kino K. Catalytic activity of the two-component flavin-dependent monooxygenase from Pseudomonas aeruginosa toward cinnamic acid derivatives. Appl. Microbiol. Biotechnol. 2014;98:1145–1154.10.1007/s00253-013-4958-y
- Furuya T, Kino K. Regioselective synthesis of piceatannol from resveratrol: catalysis by two-component flavin-dependent monooxygenase HpaBC in whole cells. Tetrahedron Lett. 2014;55:2853–2855.10.1016/j.tetlet.2014.03.076
- Chakraborty S, Ortiz-Maldonado M, Entsch B, Ballou DP. Studies on the mechanism of p-hydroxyphenylacetate 3-hydroxylase from Pseudomonas aeruginosa: a system composed of a small flavin reductase and a large flavin-dependent oxygenase. Biochemistry. 2010;49:372–385.10.1021/bi901454u
- Ellis HR. The FMN-dependent two-component monooxygenase systems. Arch. Biochem. Biophys. 2010;497:1–12.10.1016/j.abb.2010.02.007
- Galan B, Diaz E, Prieto MA, Garcia JL. Functional analysis of the small component of the 4-hydroxyphenylacetate 3-monooxygenase of Escherichia coli W: a prototype of a new flavin:NAD(P)H reductase subfamily. J. Bacteriol. 2000;182:627–636.10.1128/JB.182.3.627-636.2000
- Lin Y, Yan Y. Biotechnological production of plant-specific hydroxylated phenylpropanoids. Biotechnol. Bioeng. 2014;111:1895–1899.10.1002/bit.25237
- Furuya T, Kino K. Discovery of 2-naphthoic acid monooxygenases by genome mining and their use as biocatalysts. ChemSusChem. 2009;2:645–649.10.1002/cssc.v2:7
- Murias M, Handler N, Erker T, et al. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: synthesis and structure-activity relationship. Bioorg. Med. Chem. 2004;12:5571–5578.10.1016/j.bmc.2004.08.008
- Lam SH, Chen JM, Kang CJ, Chen CH, Lee SS. α-Glucosidase inhibitors from the seeds of Syagrus romanzoffiana. Phytochemistry. 2008;69:1173–1178.10.1016/j.phytochem.2007.12.004
- Li H, Wu WK, Zheng Z, et al. 3,3′,4,5,5′-Pentahydroxy-trans-stilbene, a resveratrol derivative, induces apoptosis in colorectal carcinoma cells via oxidative stress. Eur. J. Pharmacol. 2010;637:55–61.10.1016/j.ejphar.2010.04.009
- Lee KW, Kang NJ, Rogozin EA, et al. The resveratrol analogue 3,5,3′,4′,5′-pentahydroxy-trans-stilbene inhibits cell transformation via MEK. Int. J. Cancer. 2008;123:2487–2496.10.1002/ijc.v123:11