Abstract
Chamaecyparis obtusa essential oil (COE) has been widely used to treat allergic diseases and was suggested to exert anti-inflammatory, antioxidant, and antimicrobial effects. This study evaluated the effects of COE on pain-related behavior and pro-inflammatory cytokines in rats with carrageenan (CGN)-induced arthritis. Reduced dynamic weight load on inflamed joint in voluntarily walking rats was used as the behavior test for arthritic pain; 10% COE-treated group was significantly attenuated pain (6–8 h post-CGN injection) compared to VEH (mineral oil)-treated group. In addition, the protein levels of interleukin (IL)-1β, tumor necrosis factor-α, IL-6 (6–8 h), and cyclooxygenase (COX)-2 (8 h) within the synovial membrane, as well as IL-1β, COX-2 (6–8 h), and IL-6 (5–7 h) within the meniscus, of 10% COE-treated group were significantly reduced. The current results implicate that COE has anti-inflammatory and anti-nociceptive effects on arthritis in rats.
Graphical abstract
Chamaecyparis obtusa have inhibitory effects on pain and inflammation in acute arthritis in rats.
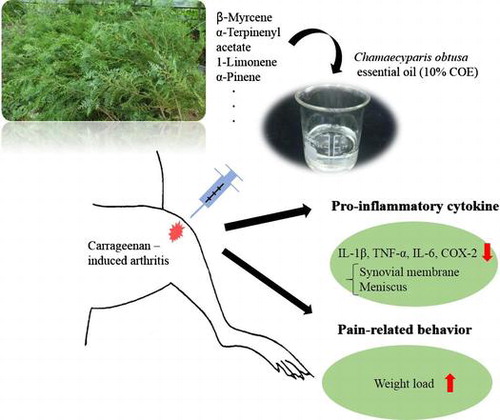
Carrageenan (CGN)-induced arthritis in rats is known to resemble the acute inflammatory phase of human osteoarthritis (OA), which includes cartilage damage, synovial hyperplasia, and synovial fluid exudation.Citation1) Residual and infiltrated immune cells in the CGN-injected tissue release pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 which can promote the activity of matrix metalloproteinases and sensitize the afferent fibers innervating knee joint tissues.Citation2,3) Therefore, as a therapy for arthritis, anti-cytokine drugs have been suggested to counteract this degenerative mechanism and attenuate nociceptive pain. In particular, plant essential oils were used as an alternative to anti-cytokine compounds in inhibiting the inflammatory response associated with arthritis.
Chamaecyparis obtusa is a slow-glowing tree found throughout South Korea and Japan (up to 40 m tall). Its leaves have egg-shaped ridges and a blunt tip.Citation4) C. obtusa essential oil (COE) contains limonene, isobornyl acetate, and terpenes, which have been reported to have anti-inflammatory, antioxidant, and antimicrobial effects.Citation4) In one study, COE ameliorated atopic dermatitis-like symptoms in mice with lipopolysaccharide (LPS)-induced inflammation by inhibiting serum TNF-α and cyclooxygenase (COX)-2.Citation5) In particular, α-pinene, one of the primary components of COE, remarkably decreased the expression of TNF-α in rodents with CGN-induced arthritis.Citation4,6) Nevertheless, the efficacy of COE on nociceptive processing in arthritis is not well understood. This study investigated whether an intra-articular injection of 10% COE in rats with CGN-induced arthritis could reduce the expression of pro-inflammatory cytokines, such as TNF-α, interleukin IL-1β, and IL-6 and COX2, an enzyme that produces prostaglandins (PG), and alleviate reduced dynamic weight load on inflamed joint in voluntarily walking rats.
Materials and methods
Plant material and identification
COE was supplied by the manufacturer (FOSTO) in South Korea. C. obtusa leaves were collected from the Gyeongsangnam-do and identified by the Korea Native Plant Institute (Dongbu Research Council, South Korea). A voucher sample was deposited at the Korea National Arboretum (KNKA201106232011).
Preparation and analysis of the essential oil
COE was extracted from the fresh leaves by steam distillation using the Clevenger-type apparatus and was supplied by the manufacturer (FOSTO) in South Korea. The chemical composition of COE was analyzed using gas chromatography/mass spectrometry (JMS 700 mass spectrometer equipped with a fused silica capillary column; 30 m × 0.25 mm, coating thickness 0.25 μm) at the Korea Testing & Research Institute. The oven temperature was maintained at 250 °C for 10 min. Component identification of COE was based on a comparison of retention times and mass spectra using standard library data (WILEY7).
Animals
Male Sprague–Dawley rats (200–230 g; Orient Bio, South Korea) were housed in a temperature- and light-controlled room (22–25 °C, 12 h light/dark cycle). Food (5L79, PMI Nutrition, USA) and water were available ad libitum. All experimental procedures were conducted in accordance with the guidelines set by the Korea University College of Medicine Animal Research Policies Committee (KUIACUC-2014-6).
CGN-induced arthritis and the application of essential oil
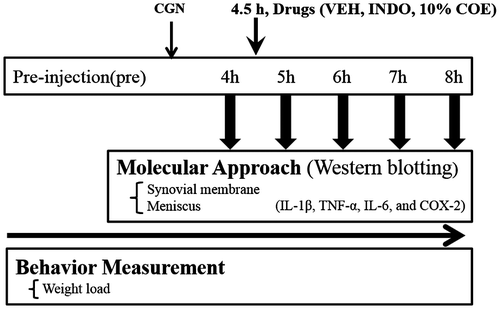
To induce arthritis, 1% lambda-CGN (50 µL, Sigma-Aldrich, USA) was injected into the right knee joint of each rat under inhalation anesthesia consisting of isoflurane (0.5–2%). The injected leg was flexed and extended manually for approximately 2 min. COE was diluted to 10% in mineral oil (VEH). Drugs (10% COE, VEH, and indomethacin; 50 µL) were administered intra-articularly to the knee joint 4.5 h after CGN injection under inhalation anesthesia. Indomethacin (INDO, 99% purity) was used as the positive control (10 mg/kg, 50 µL). Both Western blot and behavior tests were performed by an investigator who was blind to drug treatments. Experimental flowchart is shown in Fig. .
Fig. 1. Experimental flowchart for carrageenan (CGN)-induced arthritis in rats. Drugs were administered intra-articularly to the knee joint 4.5 h after CGN injection.
Dynamic weight load test
Weight loaded onto the side of an inflamed joint can generate pain. Using a dynamic weight load device made by our laboratory, the weight load on limbs of rats (n = 9 in each group) was measured while freely walking.Citation7) The bottom of the path was equipped with a load cell sensor (DACELL, CB1-K2, Korea), and output signals were fed into a digital amplifier (DACELL, DN-AM 300, Korea) for appropriate amplification and filtering. The signal was digitized via an analog–digital converter (DACELL, 1716, Korea) and plotted as a time–weight curve on a personal computer (WBT, Korea Univ., Korea). An investigator identified the plates that the rat stepped on, and the output signals were selected. The test was repeated 3–4 times to obtain at least 8 time–weight curves for a given limb. According to the time curve of weight load present between normal and CGN (1% lambda, 50 µL)-induced rats in our previous study, we decided the time course for the weight load test (pre and 4, 5, 6, 7, and 8 h after CGN injection)Citation8); the weight load values of the affected leg showed the most notable decrease 4 h after CGN injection and drugs were administered intra-articularly under isoflurane (0.5–2%) anesthesia 4.5 h after the CGN injection.
Isolation of tissues
The times for tissue extraction were determined on the basis of the time course of weight load test. The synovial membranes and menisci of 10% COE-, VEH-, and INDO-treated groups were obtained at 5–8 h after CGN injection (0.5–3.5 h after drugs injection) (n = 3 for each group). Extracted tissues were snap-frozen in liquid nitrogen and stored at −80 °C before experimentation. Total cellular protein was extracted from the samples on ice and homogenized in lysis buffer (50 mM Tris–HCl containing 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 1% NP-40, and 1 mM phenylmethylsulfonyl fluoride) using a homogenizer. Homogenates were centrifuged at 12,000 rpm for 20 min at 4 °C, and the supernatants were collected in a new tube. Protein concentrations were determined using the Bradford protein assay, with bovine serum albumin as a standard.
Western blot analysis
The protein (40 µg) was separated on a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and transferred to a nitrocellulose membrane (Whatman Protran, 0.45 μm, Thermo-Fisher Scientific, USA) at 55 V for 4 h using a Mini-PROTEAN 3 (Bio-Rad, USA) device. The membranes were blocked with 5% nonfat milk in TBST (0.5% Tween-20 in 20 mM Tris, 137 mM NaCl) for 1 h at room temperature and probed with antibodies against IL-1β (1:1000, ab9787, Abcam, United Kingdom), TNF-α (1:1000, ab66579, Abcam, United Kingdom), IL-6 (1:1000, sc-57315, Santa Cruz, USA), and COX-2 (1:1000, ab6665, Abcam, United Kingdom) overnight at 4 °C, with rocking. The membranes were washed thrice for 15 min each in TBST. A goat anti-rabbit or anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000–1:5000, sc-2004, sc-2031, Santa Cruz, USA) was then added in a 5% blocking buffer for 2 h at room temperature, with rocking. The protein bands were detected by chemiluminescence (Pierce®EC, Thermo Scientific, USA) on medical X-ray film (Agfa, CP-BU, Belgium), with β-actin (1:1000, sc-130656, Santa Cruz, USA) used as a loading control. The densities of specific bands were measured using ImageJ software and normalized against the loading control.
Statistical analysis
The density of the bands obtained in Western blot analysis was measured using ImageJ software. One-way analysis of variance (ANOVA) with post hoc Tukey test was used to compare the Western blot data acquired from the drug-treated groups. The weight load was normalized and expressed as a percentage of body weight on a given day. Repeated measures ANOVA was used to identify group or interaction effects with regard to the time course of weight load value. One-way ANOVA with post hoc Tukey test was used to compare the data obtained from drug-treated groups at individual time points after CGN injection. All data are presented as means ± standard errors of the means. For all data, p < 0.05 was considered statistically significant.
Results
Chemical components of COE
The relative percentages of constituents (total 23) in COE by using peak area normalization were expressed in Table . The major constituents of the oil were β-Myrcene (26.4%), α-Terpinenyl acetate (12.6%), and l-Limonene (11.2%).
Table 1. Chemical components of Chamaecyparis obtusa essential oil (COE) and the relative percentages using peak area normalization.
Effects of COE on dynamic weight load in arthritic pain
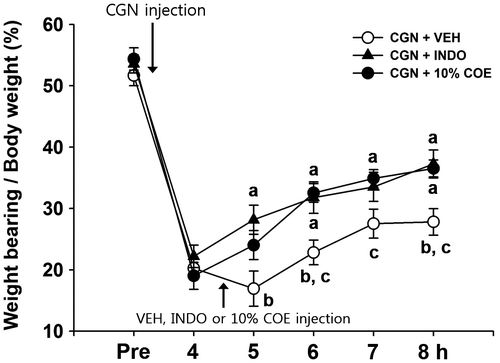
The value of the dynamic weight load was measured at various time intervals after the intra-articular injection of CGN. Injection of CGN led to a notably reduced weight load in the affected hindlimb at 4 h after injection; this phenomenon was partially recovered at 8 h (Fig. ). Four hours and thirty minutes after CGN injection, 10% COE, INDO, and VEH were injected intra-articularly, and the anti-nociceptive effects of these drugs were measured from 5–8 h after CGN injection. The group treated with 10% COE showed a significant attenuation in reduced weight load compared to the VEH-treated group at 6, 7, and 8 h after CGN injection (p < 0.05, one-way ANOVA). In addition, the INDO-treated group showed a significant attenuation of reduced weight load compared to VEH-treated group at 5, 6, and 8 h after CGN injection (p < 0.05, one-way ANOVA).
Fig. 2. Inhibitory effects of 10% Chamaecyparis obtusa essential oil (COE; 50 μL, intra-articularly) and indomethacin (INDO; 10 mg/kg, 50 μL, i.a.) on weight load in carrageenan (CGN)-induced arthritic rats.
The expression of pro-inflammatory cytokines and COX in inflamed synovial membrane and meniscus
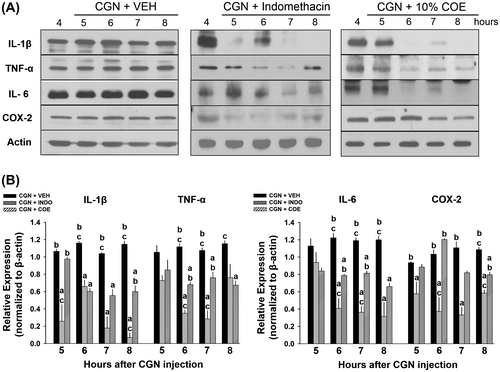
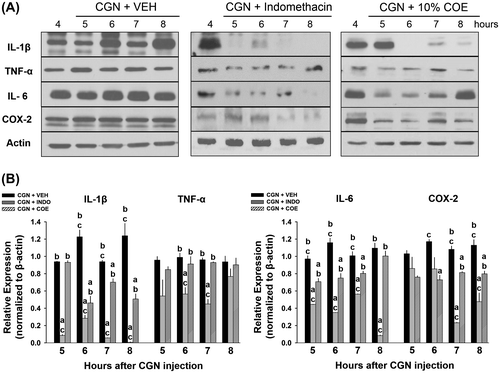
In order to investigate the anti-inflammatory effects of 10% COE, we compared IL-1β, TNF-α, IL-6, and COX-2 expressions in inflamed synovial membrane and meniscus among 10% COE-, INDO-, and VEH-treated groups 5–8 h post-CGN injection. In the inflamed synovial membrane tissue, there was no significant difference in the levels of all cytokines and COX-2 5 h after CGN injection between 10% COE- and VEH-treated group (Fig. (B)). However, the 10% COE-treated group showed a significant reduction in the levels of IL-1β, TNF-α, and IL-6 (at 6–8 h), and COX-2 (at 8 h) when compared to VEH-treated group (p < 0.05, one-way ANOVA). INDO is known to prevent the biosynthesis of PG and prostacyclins via the COX enzyme in the arachidonic acid pathway, thus suppressing IL-6 secretion and IL-1β/TNF-α-induced inflammatory hypernociception.Citation9,10) Compared to VEH-treated group, INDO-treated group (positive control) showed a significant reduction in IL-1β (at 5–8 h), TNF-α (at 6–7 h), IL-6 (at 6–8 h), and COX-2 (at 5–8 h) after CGN injection (p < 0.05, one-way ANOVA). In addition, the INDO-treated group showed a significant decrease in the levels of IL-1β (at 5–8 h), TNF-α (at 6–7 h), COX-2 (at 6–8 h), and IL-6 (at 6–7 h) compared to 10% COE-treated group (p < 0.05, one-way ANOVA).
Fig. 3. Inhibitory effects of 10% Chamaecyparis obtusa essential oil (COE; 50 μL, intra-articularly) and indomethacin (INDO; 10 mg/kg, 50 μL, i.a.) on inflamed synovial membrane 5–8 h after carrageenan (CGN) injection.
In the inflamed meniscal tissue, the levels of IL-1β and COX-2 (at 6–8 h), and IL-6 (at 5–7 h) in 10% COE-treated group significantly decreased compared to their corresponding levels in the VEH-treated group (p < 0.05, one-way ANOVA), but there was no difference in TNF-α levels between the two groups 5–8 h after CGN injection (Fig. (B)). The expression of IL-1β and IL-6 (at 5–8 h), TNF-α (at 6–7 h), and COX-2 (at 7–8 h) in INDO-treated group significantly decreased compared to their corresponding levels in VEH-treated group (p < 0.05, one-way ANOVA). In addition, the INDO-treated group showed a significant decrease in the levels of IL-1β and IL-6 (at 5–8 h), TNF-α (at 6–7 h) and COX-2 (at 7–8 h) compared to 10% COE-treated group (p < 0.05, one-way ANOVA).
Fig. 4. Inhibitory effects of 10% Chamaecyparis obtusa essential oil (COE; 50 μL, intra-articularly) and indomethacin (INDO; 10 mg/kg, 50 μL, i.a.) on inflamed meniscus 5–8 h after carrageenan (CGN) injection.
Discussion
In this study, we demonstrated that the intra-articular application of COE had remarkable inhibitory effects on pain-related behavior (e.g. reduced weight load), as well as the expression of pro-inflammatory cytokines and COX-2 in both the synovial membrane and meniscus of the inflamed knee joint in rats.
CGN-induced arthritis in rats can be characterized as an inflamed synovial membrane with synovial hyperplasia and synovial fluid exudation due to the high osmotic gradient of carrageenan.Citation11) The release of proinflammatory cytokines from residual and infiltrated immune cells is upregulated, giving rise to acute joint swelling. In particular, TNF-α and IL-1β are considered the main pro-inflammatory cytokines that initiate and develop inflammation in the knee joint. TNF-α and IL-1β can work in concert to degrade joint tissues,Citation12) stimulate the nociceptive fibers innervating them, and enhance the activity of IL-6 in inflammatory responses. There was relatively less attention given to the association of meniscus and synovial membrane in the inflamed knee joints of animal models although inflammation or angiogenesis of these two tissues were found to be present in patients with OA.Citation13–15) The current results showed that the increased expression of TNF-α, IL-1β, and IL-6 was maintained in both the meniscus and the synovial membrane of the inflamed knee joint 5–8 h post-CGN injection (Figs. (A) and (A)). These evidences of upregulated proinflammatory cytokine expression in these two tissues might reflect structural damage or symptom progression shown in OA.
Plant essential oils have been suggested to be a therapeutic compound for inflammation or arthritis. Some studies revealed that Cleistocalyx operculatus and Artemisia fukudo extracts have inhibitory effects on the secretion of pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6 in LPS-treated cells.Citation16,17) The essential oils of Cordia verbenacea and Pterodon emarginatus attenuated TNF-α and IL-1β expressions in a CGN-induced inflammatory paw of rats.Citation6) In case of COE which contains limonene, isobornyl acetate, and terpenes, a reduction in TNF-α expression via a COX-2-mediated pathway in the serum of rats with LPS-induced intraperitoneal inflammation was found after application.Citation18) With regard to the efficacy of COE on arthritis in our study, we initially found that there was a significant reduction in TNF-α and IL-6 proteins after applying 10% COE compared to 1 and 20% COE (figure not shown). A previous study also reported that the treatment with 5 and 10% COE significantly decreased mast cell infiltration in (2,4-dinitrochlorobenzene)-induced skin lesions of miceCitation5) although a percentage of components in COE was different from that in COE we used.Citation19) In this study, we showed that the expression of IL-1β, TNF-α, and IL-6 in the inflamed synovial membrane, as well as IL-1β and IL-6 in inflamed meniscus, was significantly inhibited by COE. However, contrary to our expectation, the expression of TNF-α in the inflamed meniscus were almost unaffected. This result is inconsistent with previous studies showing that the herbal formula and C. verbenacea essential oil notably inhibit the expression level of TNF-α in ankle and paw tissues in LPS- or CGN-induced arthritis of rats.Citation20,21) We cannot completely explain the non-efficacy of COE on the release of TNF-α in inflamed meniscus; however, it was observed that a strong (or synergistic) inflammatory action of IL-1β, IL-6, and COX-2 in inflamed meniscus was decreased.
Our study also revealed that CGN-induced inflamed knee joint increased COX-2, and the application of COE attenuated COX-2 expression in inflamed synovial membrane (at 6–8 h) and meniscus (at 7 h). The upregulation of COX-2 induced by IL-1β and TNF-α in the macrophages, synoviocytes, fibroblasts, and chondrocytes of inflamed tissue lead to the synthesis of PG which is responsible for the increased excitability of nociceptors in the knee joint. As a result, this sensitization can cause nociceptive behavior. The reduction of dynamic weight load in voluntarily walking rats indicates pain-related behavior typically expressed by the inflamed joint; hence, the recovery of this behavior can represent the efficacy of analgesics.Citation7,8) Our results showed that COE had remarkable anti-nociceptive effects on reduced weight load in the inflamed joint 5–8 h after CGN injection, which supports the hypothesis that COE has the ability to alleviate nociceptive processing through the COX-2/PG-mediated pathway.
Taken together, our results demonstrated that COE has anti-inflammatory and anti-nociceptive effects on arthritis, including the inhibition of pro-inflammatory cytokines and COX-2 in inflamed joint tissues and also the decrease in nociceptive behavior such as reduced weight load onto affected joint. Further studies are required to explore which component(s) of COE contribute to its anti-inflammatory and anti-nociceptive effects in arthritis.
Author contributions
H.R.S., H.J.C., E.H.P., S.W.M., and H.C.H. conceptualized and designed the study; H.R.S. and H.J.C. analyzed and interpreted the data; H.R.S., E.H.P., and H.C.H. wrote the manuscript; H.R.S., E.H.P., S.W.M., and H.C.H. supported statistical analysis; Y.I.K., S.J.P., and C.W.P. supported materials/reagents tools; H.R.S. and H.J.C. contributed equally to this work; and H.C.H. supervised the study.
Disclosure statement
None of the authors have any conflict of interest related to this work.
Funding
This work was supported by the Korea Forest Research Institute, Republic of Korea [grant number FM 362 0400-2010-01]; The Chung Yang, Cha Young Sun, M.D. and Jang Hi Joo Yeo Sa Memorial Fund to Hee Chul Han.
References
- Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Mol. Pain. 2007;3:8. 10.1186/1744-8069-3-8
- Sakaki H, Matsumiya T, Kusumi A, et al. Interleukin-1beta induces matrix metalloproteinase-1 expression in cultured human gingival fibroblasts: role of cyclooxygenase-2 and prostaglandin E2. Oral. Dis. 2004;10:87–93. 10.1046/j.1354-523X.2003.00982.x
- LeGrand A, Fermor B, Fink C, et al. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001;44:2078–2083. 10.1002/(ISSN)1529-0131
- Yang JK, Choi MS, Seo WT, Rinker DL, Han SW, Cheong GW. Chemical composition and antimicrobial activity of Chamaecyparis obtusa leaf essential oil. Fitoterapia. 2007;78:149–152. 10.1016/j.fitote.2006.09.026
- Joo SS, Yoo YM, Ko SH, et al. Effects of essential oil from Chamaecypris obtusa on the development of atopic dermatitis-like skin lesions and the suppression of Th cytokines. J. Dermatol. Sci. 2010;60:122–125. 10.1016/j.jdermsci.2010.08.008
- Passos GF, Fernandes ES, da Cunha FM, et al. Anti-inflammatory and anti-allergic properties of the essential oil and active compounds from Cordia verbenacea. J. Ethnopharmacol. 2007;110:323–333. 10.1016/j.jep.2006.09.032
- Min SS, Han JS, Kim YI, et al. A novel method for convenient assessment of arthritic pain in voluntarily walking rats. Neurosci. Lett. 2001;308:95–98. 10.1016/S0304-3940(01)01983-8
- Lee KS, Park EH, Cho HY, Kim YI, Han HC. Peripheral group II and III metabotropic glutamate receptors in the knee joint attenuate carrageenan-induced nociceptive behavior in rats. Neurosci. Lett. 2013;542:21–25. 10.1016/j.neulet.2013.03.006
- Higgs GA. Arachidonic acid metabolism, pain and hyperalgesia: the mode of action of non-steroid mild analgesics. Br. J. Clin. Pharmacol. 1980;10:233S–235S. 10.1111/bcp.1980.10.issue-S2
- Cunha TM, Verri WA, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc. Natl. Acad. Sci. USA. 2005;102:1755–1760. 10.1073/pnas.0409225102
- Hansra P, Moran EL, Fornasier VL, Bogoch ER. Carrageenan-induced arthritis in the rat. Inflammation. 2000;24:141–155. 10.1023/A:1007033610430
- Henderson B, Pettipher ER. Arthritogenic actions of recombinant IL-1 and tumour necrosis factor alpha in the rabbit: evidence for synergistic interactions between cytokines in vivo. Clin. Exp. Immunol. 1989;75:306–310.
- Hardy MM, Seibert K, Manning PT, et al. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002;46:1789–1803. 10.1002/(ISSN)1529-0131
- Haywood L, McWilliams DF, Pearson CI, et al. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003;48:2173–2177. 10.1002/(ISSN)1529-0131
- Ashraf S, Wibberley H, Mapp PI, Hill R, Wilson D, Walsh DA. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann. Rheum. Dis. 2011;70:523–529. 10.1136/ard.2010.137844
- Yoon WJ, Moon JY, Song G, et al. Artemisia fukudo essential oil attenuates LPS-induced inflammation by suppressing NF-kappaB and MAPK activation in RAW 264.7 macrophages. Food Chem. Toxicol. 2010;48:1222–1229. 10.1016/j.fct.2010.02.014
- Dung NT, Bajpai VK, Yoon JI, Kang SC. Anti-inflammatory effects of essential oil isolated from the buds of Cleistocalyx operculatus (Roxb.) Merr and Perry. Food. Chem. Toxicol. 2009;47:449-453. 10.1016/j.fct.2008.11.033
- An BS, Kang JH, Yang H, et al. Anti-inflammatory effects of essential oils from Chamaecyparis obtusa via the cyclooxygenase-2 pathway in rats. Mol. Med. Rep. 2013;8:255–259.
- Hong EJ, Na kJ, Choi IG, Choi KC, Jeung EB. Antibacterial and antifungal effects of essential oils from coniferous trees. Biol. Pharm. Bull. 2004;27:863–866. 10.1248/bpb.27.863
- Zhang RX, Fan AY, Zhou AN, et al. Extract of the Chinese herbal formula Huo Luo Xiao Ling Dan inhibited adjuvant arthritis in rats. J. Ethnopharmacol. 2009;121:366–371. 10.1016/j.jep.2008.11.018
- Medeiros R, Passos GF, Vitor CE, et al. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br. J. Pharmacol. 2007;151:618–627. 10.1038/sj.bjp.0707270
