Abstract
We show that a rice GRAS family protein, CIGR2, is a bonafide transcriptional activator, and through this function, targets the B-type heat shock protein-encoding gene OsHsf23 (Os09g0456800). CIGR2 (Os07g0583600) is an N-acetylchitooligosaccharide elicitor-responsive gene whose activity, through the direct transcriptional control of OsHsf23, is required for mediating hypersensitive cell death activation during pathogen infection. RNAi lines of CIGR2 and OsHsf23 similarly exhibited the higher level of granulation in the epidermal cells of leaf sheath inoculated with an avirulent isolate of rice blast fungus. Interestingly, we did not observe altered levels of resistance, suggesting that CIGR2 suppresses excessive cell death in the incompatible interaction with blast fungus via activation of OsHsf23.
Graphical abstract
N-Acetylchitooligosaccharides-responsive CIGR2 suppresses cell death of rice in cooperation with OsHsf23.
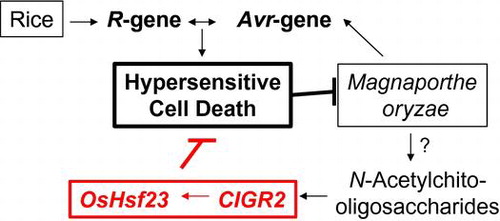
Higher plants employ a suite of complex mechanisms to activate resistance signaling processes in response to pathogen infection. In most cases, these responses can be functionally categorized based on timing, as well as on the mechanism(s) associated with the initial recognition and signaling events associated with their activity. For example, during the initial stages of pathogen perception, the plant innate immunity system is engaged via the recognition of conserved cellular components derived from the pathogen. In brief, pathogen-associated molecular patterns (PAMPs) elicit a rapid basal defense response via the recognition by host-derived pathogen recognition receptors (PRRs). As a result of this interaction, basal resistance—typically referred to as PAMPs-triggered immunity (PTI)—functions in the rapid induction of host responses aimed at abrogating pathogen growth and the elicitation of disease.Citation1) In the second layer of the host immune signaling, termed effector-triggered immunity, the activity of pathogen-derived effectors, whose function is to block PTI, is recognized by the host, and this process leads to the induction of robust immune signaling to prevent pathogen immune subversion and the proliferation.
Among the best-characterized elicitors of PTI are N-acetylchitooligosaccharides (GNn), hydrolyzed fragments of chitin, a major component of the fungal cell wall. As highly potent elicitors of PTI, GNns are perceived by the plasma membrane-localized PRRs CEBiPCitation2) and OsCERK1Citation3) in rice, or by AtCERK1Citation4) and AtLYK5Citation5) in Arabidopsis. Once bound to its receptor, GNns initiate a highly regulated and specific suite of defense responses, including the induction of dynamic changes in host gene expression.Citation2,6)
Members of the plant-specific GRAS gene family encode transcriptional regulators with functions in a wide array of signaling mechanisms, including response to growth and development, hormone signaling, and plant defense. We previously reported that the expression of two genes encoding members of the GRAS family of proteins, CIGR1 and CIGR2 (Os07g0583600), were up-regulated in response to GNn in rice cells, and their respective gene products were localized within the nucleus.Citation7,8) In total, these data support our hypothesis that these PTI-inducible genes may encode proteins that function as transcriptional regulators. Since the first discovery of SCARECROW in Arabidopsis,Citation9) genes encoding plant-specific GRAS family proteins have been identified as key regulators in numerous developmental process, as well as in response to biotic and abiotic stimuli.Citation10,11) As noted above, the GRAS family of proteins is hypothesized to be transcriptional regulators, and numerous efforts have been made to identify GRAS target genes. For example, SHORT-ROOT, a transcription factor associated with developmental processes required for root formation in Arabidopsis, was shown to directly activate eight genes, including SCARECROW, that are responsible for the radial patterning and development of root tissue.Citation12) Similarly, RGA, encoding a DELLA-type GRAS family protein in Arabidopsis that negatively regulates gibberellin (GA) signaling, was shown to induce a set of genes involved in GA-signaling.Citation13)
In addition to growth and developmental processes, GRAS proteins have also been shown to function in a wide array of biotic signaling processes. For example, two Medicago truncatula genes, NSP1 and NSP2, encoding GRAS family proteins that are indispensable to nodule formation, were shown to activate an early nodulin gene, ENOD11, by binding of the heteropolymer of NSP1 and NSP2 to the promoter region of ENOD11.Citation14) Similarly, during pathogenic interactions, a tomato SIGRAS6 was shown to mediate the resistance (R) gene-dependent resistance to Pseudomonas syringae pv. tomato.Citation15) In the current study, we show that CIGR2 activates the expression of OsHsf23 (Os09g0456800), a gene encoding a B-type heat shock transcription factor (HST). Transgenic expression of CIGR2 and OsHsf23 was found to suppress cell death induced in the incompatible interactions of rice with rice blast fungus, Magnaporthe oryzae, suggesting a regulatory role for these proteins during plant–pathogen interactions.
Materials and methods
Fungal materials and preparation of conidia
M. oryzae field isolates Ai79-142 (race 037.7; MAFF #101520;http://www.gene.affrc.go.jp/databases-micro_search_en.php) and P91-15B (race 001.0) were grown on oatmeal agar plates at 26 °C. For plant inoculation assays, a conidial suspension was prepared as reported previously.Citation16) Washed conidia were obtained from the conidial suspension by centrifugation and re-suspended in sterile distilled water as described previously.Citation17)
Pathogen inoculation assays
Oryza sativa cv. Nipponbare BL2, susceptible and resistant to Ai79-142 and P91-15B, respectively, were grown in hydroponic culture for three weeks as previously described.Citation18) The fourth leaves were sprayed with a conidial suspension (1 x 105 conidia/mL) and incubated at 25 °C in a moist chamber for 24 h and then transferred to a growth chamber at 25 °C. In some experiments, noted in the text, the excised fourth leaves from rice plants were spot-inoculated with 5 μL of conidia suspension and incubated in the same way as described above. For the leaf sheath assay, excised leaf sheaths were inoculated with the conidial suspension (1 x 105 conidia/mL) and incubated at 25 °C for the times indicated. Evaluation of hyphal infection in epidermal cells of the leaf sheaths was carried out as described previously.Citation18) The resultant vector was introduced into rice via Rhizobium radiobacter strain EHA105 as previously described.Citation19)
Histological techniques
To measure the longitudinal length of lesions, inoculated leaves were treated with a solution of lactophenol/trypan blue followed by destaining with chloral hydrate, as previously described.Citation20) For quantification of cell death in planta, the number of appressoria beneath which the epidermal cells exhibit granulation was counted.
For pathogen assays using suspension-cultured cells, cell death was evaluated by Evan’s blue staining.Citation21) In brief, suspension cells (ca. 100 mg) were sub-cultured for 36 h in fresh medium and stained with 0.05% Evans blue in 500 mM HEPES-KOH (pH 7.2) for 20 min, followed by four washes with 500 mM HEPES-KOH (pH 7.2). The Evan’s blue dye was eluted by N,N-dimethylformamide and the spectral absorbance at 595 nm was measured.
Construction of binary vectors and rice transformation
All PCR products for the construction of plasmids were generated using gene-specific primers (Table S1) and the resultant products were verified by DNA sequencing. The cDNA fragment encoding the ORF of CIGR2 (1712 bp) was amplified by PCR using gene-specific primers (Table S1) and cloned into pGEM-T Easy (Promega, Madison, WI, USA). For in plant expression of CIGR2, the resultant pGEM-CIGR2 plasmid was digested with SpeI and the resultant fragment was ligated into the SpeI site of pTA7001.
For the generation of CIGR2-RNAi rice lines, the 956 bp loop sequence of GUS was amplified by PCR using the primers shown in Table S1 and cloned into the XbaI/SacI site of pBI333, to yield pBI333/GUS-Loop. The trigger region (sense- and antisense-strand) of CIGR2 was amplified by PCR (496 bp) and cloned into pBI333/GUS-Loop at the XbaI/KpnI (antisense-strand) and SalI/SacI (sense-strand). For the construction of OsHsf23-RNAi, the trigger sequence was amplified by PCR (256 bp) and cloned into pANDA,Citation22) using the Gateway system (Invitrogen). The resultant vector was introduced into rice via R. radiobacter strain EHA105-mediated transformation, as previously described.Citation19)
Reporter assay for transcriptional activation by CIGR2
A DNA fragment containing ORF of CIGR2 was amplified by PCR. The coding sequence for the GAL4 DNA binding domain (GAL4-BD) was fused with the CIGR2 ORF-coding sequence, as amplified by PCR, noted above. The resultant chimeric gene was cloned into pBI221 and used as an effector. The reporter plasmid was kindly supplied by Dr M. Ohme-Takagi of The National Institute of Advanced Industrial Science and Technology (Japan). Plasmid pPTRL, which contains a Renilla luciferase gene under the control of the CaMV 35S promoter, was used as an internal control. In the transient assays, plasmids of reporter (2 μg), effector (2 μg), and internal control (0.04 μg) were mixed and introduced into suspension-cultured rice cells at four days after sub-culture by particle bombardment method using the PDS-1000 He biolistic particle delivery system (Bio-Rad, Hercules, CA, USA). Transformed rice cells were further incubated for 24 h at 24 °C in the dark. LUC assay was performed with the dual-luciferase reporter assay system (Promega) according to the manufacturer's instructions. Luminescence was measured using a TD20/20 luminometer (Turner Designs, Sunnyvale, CA, USA).
Transactivation of OsHsf23 by CIGR2
The open reading frame of GUS in pBI221 was replaced with the DNA fragment, including entire ORF and 3′-UTR, of CIGR2 (1946 bp) amplified by PCR. A DNA fragment containing entire ORF of EL2 was prepared by digestion of the previously isolated cDNA cloneCitation23) with restriction enzymes and substituted for GUS in pBI221. These two plasmids were used as effectors. For construction of reporter plasmid, 35S promoter of pBI221 was replaced with the 5′-upstream sequence of OsHsf23 (2086 bp) amplified by PCR. Transient assays in cultured rice cells by particle bombardment were performed as described above. GUS activity was measured as previously reported.Citation24)
RNA isolation and RT-PCR
Total RNA was extracted from the suspension-cultured cells or leaves of rice using Sepasol RNA I Super (Nacalai Tesque, Kyoto, Japan), and 1 µg was used for first-strand cDNA synthesis using the PrimeScript RT Reagent Kit (Takara Co., Ltd.). PCR analysis was performed as follows: denaturation a 98 °C (2 min), followed by 28 cycles of 98 °C (30 s), 55 °C (30 s), 72 °C (30 s), with a final extension of 72 °C (2 min). In some experiments, the relative levels of mRNAs were quantified by quantitative PCR using MX3000P (Stratagene), with OsUBI1 (Os06g0681400) as an internal standard. The sequences of the PCR primers are listed in Table S1.
Microarray and northern blot analysis
Rice plants transformed with pTA7001/CIGR2 or vector were grown as described above and sprayed with dexamethasone (DEX) in ethanol at a final concentration of 30 μM. At 24 and 30 h after treatment, total RNAs were isolated, mixed at 1:1, and subjected to microarray analysis.Citation25) In brief, total RNAs from the pTA7001/CIGR2- and vector control-line were labeled with cy5 and cy3, respectively, and probed with rice 22k oligoarray (Agilent technologies, G4138A), according to the manufacturer’s protocol. Signal data were log transformed and analyzed by two-way analysis of variance using Subio platform software (Subio Inc. Japan, http://www.subio.jp/). Experiments were carried out twice and clones with a fold change of 2< in average were scored as up-regulated genes.
Results
CIGR2 is a transcriptional activator
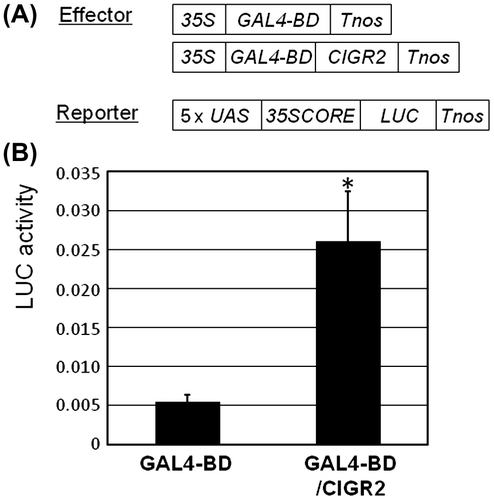
We previously demonstrated that CIGR2 is localized within the nucleus of onion epidermal cells,Citation8) supporting our hypothesis that it functions as a transcription factor. To further test this, we constructed an effector plasmid encoding a fusion protein of CIGR2 with the DNA binding domain of yeast GAL4 under the control of a constitutive 35S promoter, and co-introduced this fusion with a reporter plasmid containing a GAL4-binding sequence (UAS) into cultured cells of rice by particle bombardment. As shown in Fig. , we observed an approximate 4.5-fold induction in LUC activity induced by the effector plasmid, compared to the induction by our control plasmid in cultured cells at 48 h after injection. Based on this, we conclude that CIGR2 possesses a high likelihood to function as a transcriptional activator.
Fig. 1. Transactivation of 5xGAL4-UAS::LUC reporter gene by GAL4-BD/CIGR2 in suspension-cultured rice cells.
The heat shock transcription factor OsHsf23 is regulated by CIGR2
Based on our observation of the transcriptional activity of CIGR2, we hypothesized that we could use this function to identify genes that were regulated in response to the chitin-inducible expression of CIGR2. To this end, the full-length cDNA of CIGR2 was cloned into a DEX-inducible binary vector, pTA7001Citation26) and introduced into rice for the generation of stable homozygous expression lines. In total, three independent transgenic lines of rice were generated, and our analysis (data not shown) showed that one line (i.e. Line #7) showed an induction of CIGR2 following treatment with DEX, while the additional two lines did not. The accumulation of the CIGR2 mRNA was visible as detected by northern blot hybridization at 4–72 h after treatment. In contrast, when “vector only”-control rice plants (vector control-line) were treated with DEX, no such accumulation of CIGR2 mRNA was observed (data not shown).
To identify CIGR2-regulated genes, total RNA isolated at 24–30 h after treatment with DEX were pooled and probed with the rice 22k oligoarray; three biological replicates for pTA7001/CIGR2-transformed, as well as vector control-line, were used as oligoarray substrates. Using this approach, we identified 153 genes whose mRNA levels were at least twofold higher in pTA7001/CIGR2-expressing rice than those in vector control-line (Table S2). However, it was considered that the genes responsive to DEX both in pTA7001/CIGR2- and vector control-line were also included in these candidates. In fact, RT-PCR analysis of randomly selected five genes indicated that four genes were weakly activated in vector control-line (data not shown), and one for OsHsf23 was specifically activated in pTA7001/CIGR2-line treated with DEX (Fig. (A)).
Fig. 2. Activation of OsHsf23 by CIGR2.
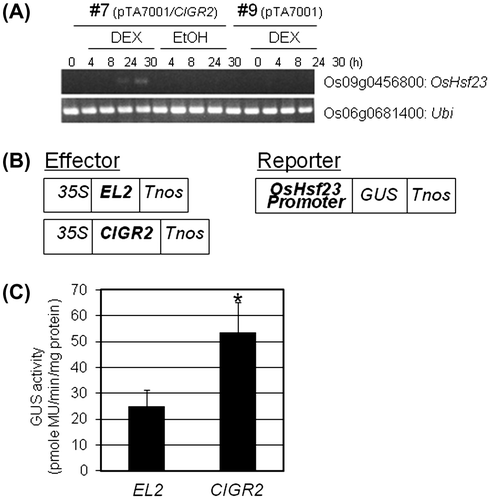
To further confirm the induced expression of OsHsf23 by CIGR2, we carried out an effector/reporter assay whereby the full-length cDNA of CIGR2 under the control of 35S promoter (effector), and 5′-upstream region of OsHsf23 (2086 bp) fused with GUS (reporter), were co-introduced into cultured rice cells by particle bombardment. GUS activity was measured in order to represent the activity of CIGR2 as transcriptional factor. As a control, EL2, previously isolated as an elicitor-responsive gene,Citation23) was substituted for CIGR2 (Fig. (B)). As shown in Fig. (C), OsHsf23 was significantly activated by CIGR2 compared to EL2, indicating that OsHsf23 is regulated by CIGR2.
Construction of the knock-down lines of CIGR2 and OsHsf23
To define the biological function(s) of CIGR2 and OsHsf23, we constructed RNAi-based binary vectors to suppress the expression of these two genes, and constructed transgenic rice plants expressing the CIGR2- and OsHsf23-RNAi derivatives. As shown in Fig. S1, qRT-PCR analysis demonstrated that we successfully isolated transformed rice plants in which CIGR2 and OsHsf23 expression was suppressed in their respective stable lines. In the experiments described below, we used transformed lines of rice in which the expression of CIGR2 or OsHsf23 was not suppressed (i.e. negative control).
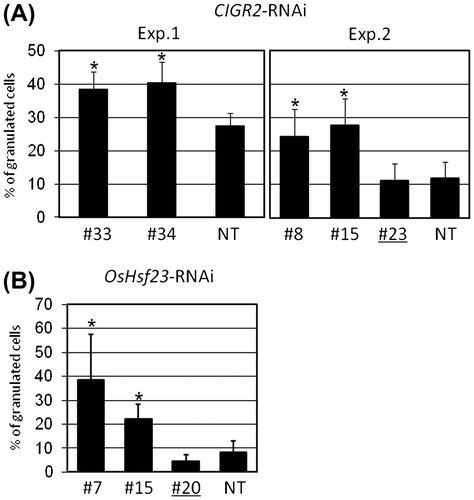
Suppression of CIGR2 and OsHsf23 leads to increased level of granulated cells during an incompatible interaction with M. oryzae
During incompatible rice-M. oryzae interactions, hypersensitive cell death (HCD) is considered to occur in the primarily invaded epidermal cells of the leaf sheath as they exhibit cytoplasmic granulation accompanied by the loss of plasmolysis.Citation16) The observation that the level of cell death evaluated by Evans blue staining increases in the suspension-cultured cells in the CIGR2- and OsHsf23-RNAi knock-down lines (Fig. S2) prompted us to examine the ratio of granulated in the leaf sheath of the RNAi lines inoculated with an avirulent isolate of M. oryzae, P91-15B. As shown in Fig. S3, we could detect the cytoplasmic granulation in epidermal cells from rice leaf sheath cells following pathogen inoculation. By microscopic observations, we found that the suppression of CIGR2 or OsHsf23 significantly increased the ratio granulated cells (Fig. ). In these experiments, responses by the null RNAi lines were indistinguishable from those by non-transgenic rice.
Fig. 3. Ratio of granulated cells in the epidermis of leaf sheath of CIGR2-RNAi and OsHsf23-RNAi lines inoculated with an avirulent isolate of M. oryzae, P91-15B.
Unchanged resistance of the CIGR2-RNAi lines to M. oryzae
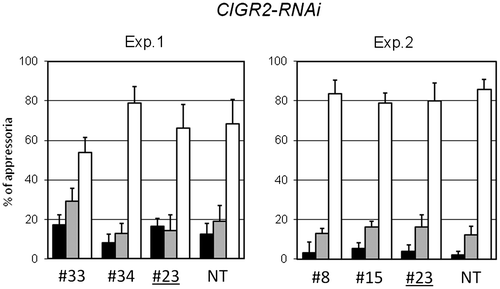
HCD is generally considered to be a key component of host resistance in rice in response to M. oryzae infection. To define the function of CIGR2 and OsHsf23-RNAi lines to M. oryzae infection, we examined the response during infection. As shown in Figs. and S4, following spray-inoculation of the RNAi lines with avirulent M. oryzae, lesion development in the RNAi lines tended to be larger than those in non-transformed plants, or in null RNAi lines, implying a compromise in resistance. To extend this, we next assessed the hyphal growth of M. oryzae in the RNAi-lines. As shown in Fig. , contrary to our prediction, we did not observe a significant difference in the extent of hyphal invasion of P91-15B in the leaf sheath of CIGR2-RNAi lines. Interestingly, these lines also exhibited similar susceptibility to a virulent isolate, Ai79-142, when spot-inoculated on the leaf (Fig. S5). These observations suggest that the increase in the levels of HCD found in CIGR2-RNAi is not tightly coupled with host resistance.
Fig. 4. Length of lesions formed on the leaves of CIGR2-RNAi lines.
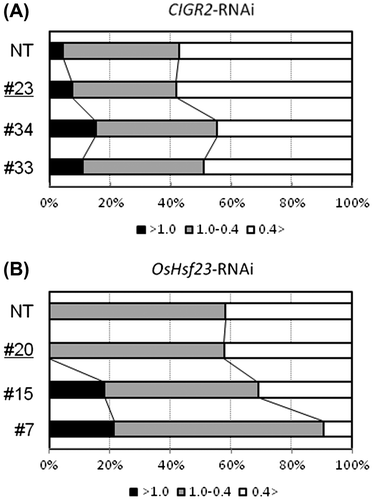
Fig. 5. Leaf sheath assay of CIGR2-RNAi lines.
Discussion
To the best of our knowledge, this is the first report demonstrating that a GRAS family protein functions as a transcriptional activator during plant immune signaling. Among the GRAS family of proteins, NSP1 of M. truncatula has been shown to directly bind its target gene, ENOD11, through the formation of heterodimers with another GRAS family protein, NSP2.Citation14) This co-factor binding function is similar to the DELLA family of proteins, which are required for GA signaling.Citation27) It remains to be determined if CIGR2 functions through co-factor binding to activate the transcription of target genes, including the activation of OsHsf23, through which the molecular mode of activation of target genes by CIGR2 will be clarified.
In the present study, we showed that suppression of either CIGR2 or OsHsf23 by RNAi resulted in increased cell death in rice following pathogen infection (Fig. ), suggesting that the activity of these two genes may function as suppressors of cell death. This is significant, as both CIGR2 and OsHsf23 are expressed and responsive to GNn in suspension-cultured rice cells,Citation6,7) and in general, oligosaccharide elicitors, including GNn, are not potent inducers of cell death.Citation28) Based on our observations, coupled with the elicitor activity of GNn, we posit that the activity of CIGR2 and OsHsf23 may function, in part, by suppressing the excessive cell death activity during pathogen infection. Indeed, this hypothesis is supported by our observation that in CIGR2-RNAi lines, we did not observe enhanced resistance to virulent or avirulent isolates of M. oryzae (Figs. and S5). In fact, in leaves sprayed with an avirulent isolate, lesions appeared as brown-colored spots, and in general, were larger than those in control plants (Fig. ). These data are in favor of a mechanism through which the development of larger lesions is mediated through the induction of cell death in the cells surrounding the pathogen-invaded cells. Similar, confirmatory, results were obtained using the OsHsf23-RNAi lines. In total, based on these observations, we conclude that CIGR2 functions in the suppression of excessive cell death triggered by the activation of R-gene mediated resistance. Additionally, as shown, this activity is mediated in part by the expression and activity of OsHsf23. We previously reported that suppression of CEBiP or OsCERK1, involved in GNn recognition in rice, results in the enhanced susceptibility of rice to M. oryzae, indicating that low level of GNn detectable by these receptors is generated during rice-M. oryzae interactions.Citation29,30) It is, therefore, tempting to speculate that the locally produced GNn induces CIGR2 and OsHsf23, leading to the suppression of excessive cell death during incompatible interactions.
In plants, HSTs are divided into three primary classes: A, B, and C.Citation31) The HST characterized herein, OsHsf23, encodes a B-type HST, identifiable by the absence of a trans-activation domain localized within the C-terminal region of the protein. To date, two genes encoding for B-type HSTs from Arabidopsis, AtHsfB1, and AtHsfB2b, have been shown to encode transcriptional repressors of a suite of genes associated with response to heat shock.Citation32) By analogy, we posit that OsHsf23 plays a role as a repressor of transcription of genes associated with HCD. To this end, the work described in the present study represents the first report demonstrating the role of a B-type HST in cell death. In Arabidopsis, AtHsfA4a, encoding an A-type HST, is a prominent factor in the tolerance to oxidative stress, likely through the regulation of the gene for ascorbate peroxidase, a key enzyme in the quenching system of reactive oxygen species.Citation33) The orthologue of AtHsfA4a in rice, Spl7, which was identified as a causal gene of lesion-mimic phenotype of spl7, is the only HST reported to be involved in cell death.Citation34) In this study, an analysis of the nucleotide coding sequence revealed that spl7 encodes a dominant negative form which lacks DNA-binding activity. Thus, the target gene of Spl7 is considered to be involved in cell death signaling via the regulation of reactive oxygen species. Because OsHsf23-RNAi lines did not exhibit any growth retardation or lesion-mimic phenotype under normal growth conditions (data not shown), we posit that the mode of action of OsHsf23 is distinct from that of Spl7.
Author contributions
E. Minami, N. Shibuya, R.B. Day, and Y. Nishizawa designed the research. S. Tanabe, N. Hara, H. Onodera, S. Toki, N. Ishii-Minami, T. Hagio, Y. Fujisawa, and Y. Nishizawa performed experiments. E. Minami, N. Shibuya, Y. Nishizawa, and B. Day wrote the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2015.1075866.
Funding
This work was supported by the Ministry of Agriculture, Forestry and Fisheries of Japan under Rice Genome Project [grant number IP-4003].
Table_S2._Tanabe_et_al._2015_accepted.xlsx
Download MS Excel (24 KB)Table_S1._Tanabe_et_al._2015_accepted.xlsx
Download MS Excel (10.2 KB)Figure_S5._Tanabe_et_al._2015_accepted.tif
Download TIFF Image (182.5 KB)Figure_S4._Tanabe_et_al._2015_accepted.tif
Download TIFF Image (116 KB)Figure_S3._Tanabe_et_al._2015_accepted.tif
Download TIFF Image (98.9 KB)Figure_S2._Tanabe_et_al._2015_accepted.tif
Download TIFF Image (101.1 KB)Figure_S1._Tanabe_et_al._2015_accepted.tif
Download TIFF Image (100.5 KB)Acknowledgments
The authors are grateful to Dr Takashi Aoyama of Kyoto University (Japan) and Dr Nam-Hai Chua of Rockfeller University (USA) for providing us with a plasmid for pTA7001. We also thank Dr Masaru Ohme-Tanagi of The National Institute of Advanced Industrial Science and Technology (Japan) and late Dr Ko Shimamoto of The Nara Institute of Technology (Japan) for providing us with a plasmid for reporter assay and pANDA vector, respectively.
Notes
Abbreviations: SD, standard deviation; hpi, hs post-inoculation.
References
- Newman MA, Sundelin T, Nielsen JT, Erbs G. MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front Plant Sci. 2013;4:1–14.
- Kaku H, Nishizawa Y, Ishii-Minami N, et al. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc. Natl. Acad. Sci. USA. 2006;103:11086–11091.10.1073/pnas.0508882103
- Shimizu T, Nakano T, Takamizawa D, et al. Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J. 2010;64:204–214.10.1111/tpj.2010.64.issue-2
- Miya A, Albert P, Shinya T, et al. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2007;104:19613–19618.10.1073/pnas.0705147104
- Cao Y, Liang Y, Tanaka K, et al. The kinase LYK5 is a major chitin receptor in Arabidopsis and forms a chitin-induced complex with related kinase CERK1. eLife. 2014;3:e03766.
- Kouzai Y, Mochizuki S, Nakajima K, et al. Targeted gene disruption of OsCERK1 reveals its indispensable role in chitin perception and involvement in the peptidoglycan response and immunity in rice. Plant-Microbe Interact. 2014;27:975–982.10.1094/MPMI-03-14-0068-R
- Day RB, Akimoto-Tomiyama C, Yazaki J, et al. Large-scale identification of elicitor-responsive genes in suspension -cultured rice cells by DNA microarray. Plant Biotech. 2002;19:153–155.10.5511/plantbiotechnology.19.153
- Day RB, Shibuya N, Minami E. Identification and characterization of two new members of the GRAS gene family in rice responsive to N-acetylchitooligosaccharide elicitor. Biochim. Biophys. Acta. 2003;1625:261–268.10.1016/S0167-4781(02)00626-7
- Di Laurenzio L, Wysocka-Diller J, Malamy JE, et al. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;86:423–433.10.1016/S0092-8674(00)80115-4
- Bolle C. The role of GRAS proteins in plant signal transduction and development. Planta. 2004;218:683–692.10.1007/s00425-004-1203-z
- Hirsch S, Oldroyd GE. GRAS-domain transcription factors that regulate plant development. Plant Signal. Behav. 2009;4:698–700.10.4161/psb.4.8.9176
- Levesque MP, Vernoux T, Busch W, et al. Whole-genome analysis of the SHORT-ROOT developmental pathway in Arabidopsis. PLoS Biol. 2006;4:e143.10.1371/journal.pbio.0040143
- Zentella R, Zhang ZL, Park M, et al. Global analysis of DELLA direct targets in early gibberellin signaling in Arabidopsis. Plant Cell. 2007;19:3037–3057.10.1105/tpc.107.054999
- Hirsch S, Kim J, Muñoz A, Heckmann AB, Downie JA, Oldroyd GE. GRAS proteins form a DNA binding complex to induce gene expression during nodulation signaling in Medicago truncatula. Plant Cell. 2009;21:545–557.10.1105/tpc.108.064501
- Mayrose M, Ekengren SK, Melech-Bonfil S, Martin GB, Sessa G. A novel link between tomato GRAS genes, plant disease resistance and mechanical stress response. Mol. Plant Pathol. 2006;7:593–604.10.1111/mpp.2006.7.issue-6
- Koga H. Hypersensitive death, autofluorescence, and ultrastructural changes in cells of leaf sheaths of susceptible and resistant near-isogenic lines of rice (Pi-zt) in relation to penetration and growth of Pyricularia oryzae. Can. J. Bot. 1994;72:1463–1477.10.1139/b94-180
- Ando S, Tanabe S, Akimoto-Tomiyama C, Nishizawa Y, Minami E. The supernatant of a conidial suspension of Magnaporthe oryzae contains a factor that promotes the infection of rice plants. J. Phytopathol. 2009;157:420–426.10.1111/jph.2009.157.issue-7-8
- Tanabe S, Okada M, Jikumaru Y, et al. Induction of resistance against rice blast fungus in rice plants treated with a potent elicitor, N-acetylchitooligosaccharide. Biosci. Biotechnol. Biochem. 2006;70:1599–1605.10.1271/bbb.50677
- Toki S, Hara N, Ono K, et al. Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J. 2006;47:969–976.10.1111/tpj.2006.47.issue-6
- Choi D-S, Hwang B-K. Proteomics and functional analyses of pepper Abscisic Acid-Responsive 1 (ABR1), which is involved in cell death and defense signaling. Plant Cell. 2011;23:823–842.10.1105/tpc.110.082081
- He Z, Wang ZY, Li J, et al. Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science. 2000;288:2360–2363.10.1126/science.288.5475.2360
- Miki D, Shimamoto K. Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol. 2004;45:490–495.10.1093/pcp/pch048
- Minami E, Kuchitsu K, He D-Y, et al. Two novel genes rapidly and transiently activated in suspension-cultured rice cells by treatment with N-acetylchitoheptaose, a biotic elicitor for phytoalexin production. Plant Cell Physiol. 1996;37:563–567.10.1093/oxfordjournals.pcp.a028981
- Kosugi S, Ohashi Y, Nakajima K, Arai Y. An improved assay for β-glucuronidase in transformed cells: methanol almost completely suppresses a putative endogenous β-glucuronidase activity. Plant Sci. 1990;70:133–140.10.1016/0168-9452(90)90042-M
- Kato T, Tanabe S, Nishimura M, et al. Differential responses of rice to inoculation with wild-type and non-pathogenic mutants of Magnaporthe oryzae. Plant Mol. Biol. 2009;70:617–625.10.1007/s11103-009-9495-9
- Aoyama T, Chua NH. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 1997;11:605–612.10.1046/j.1365-313X.1997.11030605.x
- Yoshida H, Hirano K, Sato T, et al. DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. Proc. Natl. Acad. Sci. USA. 2014;111:7861–7866.
- Shibuya N, Minami E. Oligosaccharide signalling for defence responses in plant. Physiol. Mol. Plant Pathol. 2001;59:223–233.10.1006/pmpp.2001.0364
- Kishimoto K, Kouzai Y, Kaku H, Shibuya N, Minami E, Nishizawa Y. Perception of the chitin oligosaccharides contributes to disease resistance to blast fungus Magnaporthe oryzae in rice. Plant J. 2010;64:343–354.10.1111/tpj.2010.64.issue-2
- Kouzai Y, Mochizuki S, Nakajima K, et al. Targeted gene disruption of OsCERK1 reveals its indispensable role in chitin perception and involvement in the peptidoglycan response and immunity in rice. Mol Plant Microbe Interact. 2014;27:975–982.10.1094/MPMI-03-14-0068-R
- Miller G, Mittler R. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 2006;98:279–288.10.1093/aob/mcl107
- Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance. Plant Physiol. 2011;157:1243–1254.10.1104/pp.111.179036
- Davletova S, Rizhsky L, Liang H, et al. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell. 2005;17:268–281.10.1105/tpc.104.026971
- Yamanouchi, U, Yano, M, Lin, H, Ashikari, M, Yamada, K, A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc. Natl. Acad. Sci. USA. 2002;99:7530–7535.
