Abstract
Factors that increase protein thermostability are of considerable interest in both scientific and industrial fields. Disulfide bonds are one of such factors that increase thermostability, but are rarely found in intracellular proteins because of the reducing environment of the cytosol. Here, we report the first example of an intermolecular disulfide bond between heteromeric subunits of a novel-type phosphoserine phosphatase from a thermophilic bacterium Hydrogenobacter thermophilus, which contributes to the protein thermostability at the physiological temperature. Comparison of remaining soluble proteins between wild-type and cysteine-deleted mutant using SDS-PAGE revealed that the disulfide bond increases the thermostability of the whole protein by tightly connecting a subunit with low solubility to the partner with higher solubility. Furthermore, it was strongly suggested that the disulfide bond is formed and contributes to the stability in vivo. This finding will open new avenues for the design of proteins with increased thermostability.
Graphical abstract
Intermolecular disulfide bond was found in a heterodimeric protein from a thermophilic bacterium. It is essential for the protein thermostability.
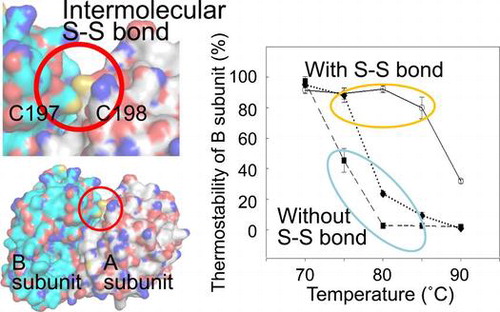
Despite considerable research efforts over the last few decades in both scientific and industrial sectors to identify factors that contribute to the thermostability of proteins,Citation1–4) no single or universal factor responsible for protein thermostability has been identified.Citation5–8) However, comparisons of protein homologs between mesophilic and (hyper-) thermophilic organisms, and the mutagenic screening of thermostable proteins have revealed that electrostatic surface interactions, hydrogen bonding, compact protein packing, intrinsic secondary structure propensity, and disulfide bond formation all contribute to thermostability.Citation2,9–12)
The formation of intracellular disulfide bonds is considered to be extremely rare because of the reducing environment of the cytoplasm.Citation13–15) However, crystal structure analyses have revealed that several intracellular proteins from thermophilic organisms contain disulfide bonds within or between subunits that contribute to thermostability.Citation7,13,16) In addition, thermophilic microorganisms, particularly hyperthermophiles, are reported to have a higher ratio of intracellular disulfide bonds compared to mesophiles.Citation16,17) For this reason, a number of researchers have attempted to create thermostable proteins for industrial applications by artificially introducing disulfide bonds.Citation18–21) However, the disulfide bonds found in crystal structures or those that have been introduced manually are limited to intrasubunit bonds or those between two identical subunits.
Novel-type serine-synthesizing enzymes, termed metal-independent phosphoserine phosphatases (iPSPs; EC 3.1.3.3), were recently identified and characterized from the thermophilic bacterium Hydrogenobacter thermophilus, which grows optimally at 70–75 °C.Citation22–24) H. thermophilus has two types of iPSPs, iPSP1 and iPSP2. The former is a homodimer of PspA subunits, and the latter is a heterodimer of PspA and PspB subunits. Although PspA and PspB share 35% amino acid sequence identity and contain a conserved catalytic domain of the histidine phosphatase superfamily, only the PspA subunit shows substantial PSP activity.Citation22,25) Km values of iPSP1 and iPSP2 for phosphoserine are comparable while Vmax of iPSP2 is almost the half of iPSP1,Citation22) suggesting that monomeric PspA is the minimum unit for the activity but dimerization stabilizes the whole structure of iPSPs. Although homodimers of PspBs have not been detected in H. thermophilus, this type of PSP enzyme is not likely formed, because co-expression of PspA and PspB is essential for PspB accumulation in the cytosol of Escherichia coli. In addition, no member of this superfamily protein appears to function as a chaperone.Citation22)
Crystal structure analysis of iPSP1 revealed that this protein forms an intermolecular disulfide bond between the two C198 residues at the interface of the PspA subunits.Citation25) As the C198 residue of PspA is conserved in PspB as C197, it is expected that iPSP2 can also form an intermolecular disulfide bond between PspA and PspB. We therefore hypothesized that these intermolecular disulfide bonds are necessary for the thermostability of iPSP1 and iPSP2. To confirm this hypothesis, here, the existence of a disulfide bond in iPSP2, both in purified soluble protein and under in vivo conditions, was investigated, and the contribution of this bond to the thermostability of iPSP2 was then examined.
Materials and methods
Construction of plasmids for site-directed mutants
The genes encoding the PspA (HTH_0103) and PspB (HTH_0183) subunits of H. thermophilus TK-6 (IAM 12695, DSM 6534) were previously cloned into the expression vectors pCDFDuet-1 and pET21c (Novagen, Darmstadt, Germany), respectively.Citation25) The constructed plasmids were then mutated to express C198S and C197S mutants of the PspA and PspB subunits, in which the 198th and 197th cysteine residues, respectively, were converted to serine. The mutated plasmids were constructed using the primer pairs 5′-ATAACCAGCCATCTGGGAGAGTTT-3′ and 5′-AGATGGCTGGTTATGTTAAGCTTTAG-3′ (for PspA), and 5′-AAACTTTCCCACACAAGACAGCTTAC-3′ and 5′-TGTGTGGGAAAGTTTGTTTAGATAAACC-3′ (for PspB), and Prime STAR Mutagenesis Basal Kit (Takara Bio, Otsu, Japan) according to the manufacturer’s instructions.
Heterologous protein expression and purification
iPSP1 (A-A), iPSP2 (A-B), and the corresponding dimeric proteins formed with the PspA C198S and PspB C197S mutated subunits were expressed in E. coli BL21-Codon Plus (DE3)-RIL and then purified using the protocol described previously, with a minor modification.Citation22) Here, the heat treatment of cell lysate at 80 °C was omitted, as this study was focused on protein thermostability. Instead, the cell lysate was applied to a Q-Sepharose Fast-flow column (GE Healthcare) equilibrated with buffer containing 20 mM Tris–HCl (pH 8.0) and was then eluted with a gradient of NaCl from 0 to 1 M in the same buffer. The fraction containing iPSPs were further purified using Butyl-Toyopearl and MonoQ columns, as described previously.Citation22) For performing the elution from the Butyl-Toyopearl column, the first ammonium sulfate concentration was decreased to 20% saturation.
Reductive and non-reductive SDS-PAGE
Reductive and non-reductive SDS-PAGE Citation26) were conducted using a 5% stacking and 10% separating gel with and without DTT in the loading buffer, respectively. Samples to be analyzed by reductive SDS-PAGE were mixed with loading buffer (4 mM DTT, final concentration) and incubated at 95 °C for 10 min prior to separation. After SDS-PAGE, the separated proteins were stained with CBB, and Image J software was used to quantify the band intensity of stained proteins.
Enzyme assays
PSP activity was assayed by measuring the production of inorganic phosphate, as described previously with minor modifications.Citation22) Briefly, the reaction mixture contained 200 mM HEPES-NaOH (pH 8.0 at room temperature), 10 mM L-phosphoserine, 1.0 mM EDTA (pH 8.0), and enzyme solution (total volume = 50 μL). The reaction mixture was incubated for 7 min at 70 °C for iPSPs proteins. One unit of PSP activity was defined as the amount of enzyme producing 1 μmol of inorganic phosphate per min.
Thermostability analysis
One mL of 20 mM Tris–HCl (pH 8.0) with 1 mM EDTA containing 400 μg of purified proteins were incubated at 70, 75, 80, 85, and 90 °C for 10 min, and were then placed into ice-water. After 30 min, the precipitants were removed by centrifugation at 20,000×g for 30 min. Ten microliter of the supernatants were subjected to SDS-PAGE analysis to confirm the residual proteins in the soluble fraction. Additionally, the supernatants diluted 20 times were subjected to enzyme assays to measure the residual enzyme activity per volume of the sample.
Western blotting
Rabbit antisera for PspA and PspB were prepared by Eurofins Operon, Japan, using synthesized peptides (67AEAKNLEVIKED78 for PspA and 83MSFGEYEGKH92 for PspB) as antigens. For Western blotting (WB), proteins separated on SDS-PAGE gels were transferred to PVDF membranes, which were then blocked for at least 4 h at room temperature using TBST buffer (50 mM Tris–HCl [pH 7.5], 150 mM NaCl, and 0.1% Tween 20) containing 5% (w/v) skim milk. Blocked membranes were probed overnight at 4 °C with PspA or PspB antiserum (1/1000 and 1/250 dilutions, respectively) in TBST containing skim milk. After washing the membranes three times in TBST, they were probed with goat anti-rabbit IgG (pAb, HRP conjugate; Enzo) in TBST (1/1000 dilution). After washing the membranes twice in TBST, once in TBST without Tween 20, and once in distilled water, the immunopositive spots were visualized using a POD Immunostain Set (Wako) as directed by the manufacturer.
Protein assay
Protein concentrations were measured using the Bradford protein assay (Bio-Rad) with bovine serum albumin as the standard.
Fluorescent labeling of cysteines involved in disulfide bonds
A slightly modified method of Boutz et al.Citation16) was used to fluorescently label the cysteines that formed disulfide bonds. Briefly, H. thermophilus or E. coli cell pellets corresponding to 650 μg protein were suspended in 0.1 mL lysate buffer (20 mM Tris–HCl pH 8.0, 10 mM NaCl, 1 mM EDTA, and 20 mM iodoacetamide [IAA]) and centrifuged at 20,000×g for 5 min. The washed cell pellets were resuspended in 0.1 mL lysate buffer, lysed on ice by sonication, and then centrifuged at 20,000×g for 10 min. SDS and lysate buffer were added to the supernatant to yield 500 μL sample containing 1% SDS (final concentration). The protein samples were denatured by heating at 95 °C (2 min for E. coli, 4 min for H. thermophilus) and then mixed with 26.3 μL of 400 mM IAA solution to block free cysteine thiols. After a 30-min incubation in the dark at room temperature, IAA was diluted approximately 1000-fold by adding excess amounts of lysate buffer containing 0.1% SDS, but without IAA, and the sample was then concentrated using ultrafiltration spin columns (Vivaspin 5,000 MWCO; Sartorius Stedim). Samples were reduced with 10 mM tris(2-carboxyethyl)phosphine (TCEP; final concentration; adjusted to pH 7.0 with NaOH) during a 30-min dark incubation at room temperature. Following disulfide bond cleavage, samples were reacted within 50 μM 7-diethylamino-3-(4′-maleimidylphenyl)-4-methylcoumarin (CPM) in the dark at room temperature for 30 min for the fluorescent labeling of free thiols. Proteins were then separated by non-reducing SDS-PAGE on a 12% acrylamide gel, and CPM-labeled protein bands were visualized by excitation at a wavelength of 365 nm. Precision Plus Protein™ Dual Color Standards (Bio-Rad) were used as protein molecular weight markers.
Results
Construction of mutant proteins
Mutated iPSP1 and iPSP2 proteins were constructed to confirm the presence of intermolecular disulfide bonds between the PspA and PspB subunits in soluble form. C198 of PspA and C197 of PspB were changed to serine, because serine appears to effectively suppress sulfur chemistry without influencing protein structure.Citation27) Hereafter, wild-type iPSP1 and iPSP2 are referred to as A-A and A-B, respectively, and the mutant forms of each recombinant protein are called As-As and As-Bs, respectively.
The wild-type and mutant proteins were heterologously expressed using the same procedure in E. coli. The elution patterns of the mutants during the purification by column chromatography exhibited similar profiles as the respective wild-type proteins, suggesting that the overall structure was not changed by the mutations. The homogeneity of the purified proteins was confirmed by SDS-PAGE and CBB staining. It was also confirmed that the mutations did not affect the Km and Vmax values.
Detection of intermolecular disulfide bonds by non-reducing SDS-PAGE
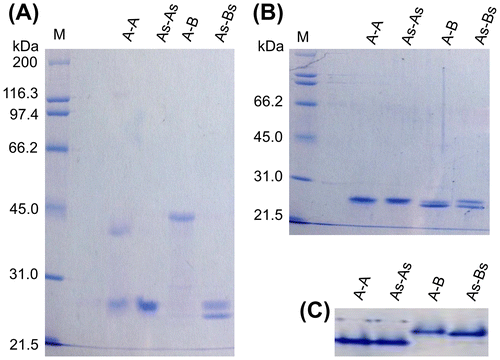
To determine whether intermolecular disulfide bonds are present not only in the crystal of A-A, but also in the soluble form of A-A and A-B, SDS-PAGE analysis of A-A, A-B, and the generated mutants were performed under non-reducing conditions. Two distinct bands of 24.0 and 38.0 kDa were detected when A-A was subjected to non-reduced SDS-PAGE (Fig. (A)), whereas only the 24.0-kDa band, which was consistent with the predicted molecular weight of the PspA subunit (24.6 kDa), was detected from As-As, as expected. In contrast, a single major protein band of 45.0 kDa was observed when A-B was subjected to non-reducing SDS-PAGE (Fig. (A)), whereas 23.5- and 24.5-kDa bands, corresponding to PspB (estimated molecular mass of 23.5 kDa) and PspA, respectively, were detected when reduced A-B or non-reduced As-Bs were analyzed by SDS-PAGE (Fig. (A) and (B)). The two monomeric size bands were also detected when As-B and A-Bs were subjected to non-reduced SDS-PAGE (data not shown). In addition, a single major protein band was observed when A-A, As-As, A-B, or As-Bs were subjected to native-PAGE (Fig. (C)), and a single peak corresponding to the dimeric form of each protein was observed by size exclusion chromatographies (data not shown). Therefore, the 38.0- and 45.0-kDa proteins detected in the non-reduced SDS-PAGE analyses were A-A and A-B dimers, respectively. These results clearly indicated that heterologously expressed and purified A-A and A-B have intermolecular disulfide bonds between C198 of PspA and between C198 of PspA and C197 of PspB, respectively, in the soluble form. From the CBB-stained band intensities in the non-reduced SDS-PAGE gels, the ratio of proteins containing an intermolecular disulfide bond was estimated to be 35% for A-A and 97% for A-B.
Fig. 1. Detection of monomeric and dimeric iPSPs by SDS-PAGE.
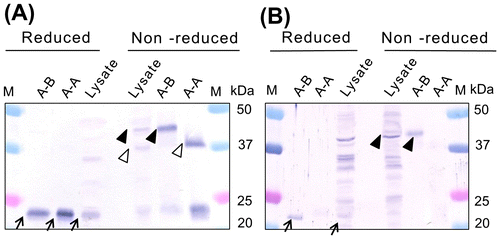
To determine whether the intermolecular disulfide bonds between the PspA and PspB subunits also exist in A-A and A-B obtained from H. thermophilus lysate, WB was performed using anti-PspA or PspB antiserum. The specificity of anti-PspA and PspB antisera to each subunit was confirmed using purified A-A and A-B (Fig. ). When anti-PspA antiserum was reacted with reduced H. thermophilus lysate, a distinct band was observed at 24.5 kDa, confirming the presence of monomeric PspA subunits (Fig. (A)). However, when the anti-PspA antibody was reacted with non-reduced lysate, the 24.5-kDa band had markedly reduced intensity and additional bands of 38.0 and 43.0 kDa were also observed (Fig. (A)). These two bands most likely corresponded to A-A and A-B protein dimers that contained an intermolecular disulfide bonds. Although greater cross-reactivity with proteins in the H. thermophilus lysate was observed with the anti-PspB antiserum, a 23.5-kDa band corresponding to monomeric PspB was detected in reduced lysate (Fig. (B)). Moreover, a 43.0-kDa band was present in the non-reduced lysate sample, also suggesting that PspB forms a heterodimer with PspA, and that the two subunits are interconnected by a disulfide bond.
Fig. 2. Detection of iPSPs from H. thermophilus cell lysate by WB using anti-PspA (A) and anti-PspB (B) antisera.
Intermolecular disulfide bond enhances protein thermostability
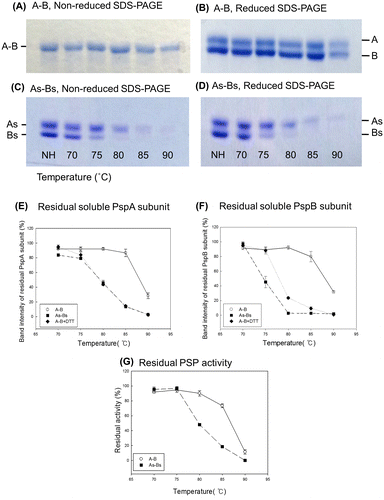
To confirm the function of the intermolecular disulfide bond identified between the PspA and PspB subunits, the thermostabilities of purified, electrophoretically homogeneous wild-type and mutant A-B enzymes were analyzed. A-B was targeted in this experiment as nearly all of the purified A-B heterodimers had intermolecular disulfide bonds. Because the PspB subunit does not have clear enzymatic activity but both PspA and PspB subunits are required for the existence of iPSP2 (=A-B), thermostability was defined as the ability of both the subunits to maintain solubility after heat treatment (if only PspB precipitated, PspA may still be able to stay in the soluble fraction as A-A but it is not the thermostability as A-B but A-A). When purified A-B was incubated at 90 °C, approximately 30% of the PspA and PspB subunits remained in the soluble fraction (Fig. (A), (B), (E), and (F)). In contrast, only 3% of PspA and almost no PspB retained solubility when As-Bs, which cannot form an intermolecular disulfide bond, was heat-treated at 90 °C (Fig. (C)–(F)). The ratio of residual soluble PspA and B subunits from As-Bs was similar to that of A-B incubated with DTT (Fig. (E) and (F)), supporting the speculation that the observed difference in thermostability between A-B and As-Bs is attributable to the presence of an intermolecular disulfide bond. Notably, the PspB subunit from As-Bs was precipitated at lower temperature than PspA, whereas the wild-type PspA and B subunits were precipitated at almost the same conditions (Fig. (A)–(D)). In the case of As-Bs, 55 and 100% of PspB subunits were precipitated at 75 and 80 °C, respectively, whereas only 21% and 55% of PspA subunits were precipitated at those respective temperatures (Fig. (E) and (F)). This indicates that the thermostability of As-Bs can be defined as the ratio of residual soluble PspB subunit. Residual PSP activity (Fig. (G)) showed the similar trend with the ratio of residual soluble PspA rather than that of PspB. In case of A-B, almost all the activity and both subunits were retained after incubation at 80 °C. On the other hand, in case of As-Bs, about 50% of PspA subunit and PSP activity was retained while almost all the PspB subunits were disappeared from the supernatant after incubation at 80 °C. Therefore, we concluded that the intermolecular disulfide bond of the heterodimer enhanced thermostability of the whole protein, especially of PspB subunit, by increasing the solubility at high temperature.
Fig. 3. Thermostability of A-B and a mutated form (As-Bs) that cannot form an intermolecular disulfide bond.
Detection of disulfide bonds by fluorescent labeling
To determine whether the intermolecular disulfide bonds detected in the A-A and A-B proteins were formed in vivo or after cell lysis, thiols from disulfide bonds were labeled with the thiol-reactive fluorescent reagent CPM (16). For the analysis, free thiols were blocked before cell lysis by adding the alkylation reagent IAA, disulfide bonds were then reduced by treatment with TCEP, and the cleaved thiols were labeled with CPM. The proper blocking of free thiol groups by IAA and thiol labeling by CPM were confirmed by including control samples without added TCEP and IAA, respectively (Supplementary Fig. 1).
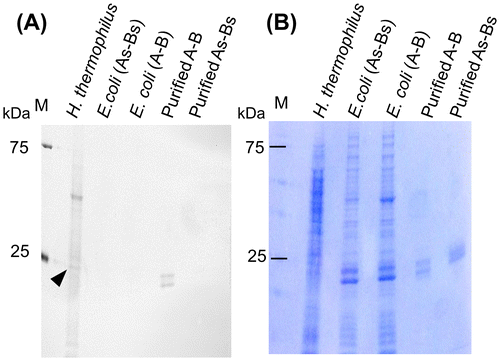
Two monomeric bands corresponding to PspA and PspB were detected from the positive control sample, purified A-B, but not from the negative control, purified As-Bs, confirming that this assay system was able to detect intermolecular disulfide bonds (Fig. (A)). In contrast, no additional bands were observed in whole cell lysates of E. coli cells expressing A-B compared with lysates from cells expressing As-Bs, indicating that the disulfide bond between PspA and PspB was not formed in E. coli. When the same amount of cell lysate from H. thermophilus and E. coli was analyzed, more bands were clearly observed in the cell lysate from H. thermophilus compared to E. coli, indicating that various proteins within H. thermophilus contain disulfide bonds. In addition, a relatively strong band was observed around 24.5 kDa, which is the same size as PspA, suggesting that PspA in H. thermophilus has a disulfide bond.
Fig. 4. Detection of intracellular proteins containing disulfide bonds.
Conservation of cysteine residues able to form intermolecular disulfide bonds
The distribution of cysteine residues with the potential to form intermolecular disulfide bonds was examined among species of the order Aquificales with sequenced genomes. Our previous studies suggested that the ancestor of PspA and PspB divided into PspA and PspB after the family Desulfurobacteriaceae arose, but before the division of Aquificaceae and Hydrogenothermaceae.Citation22) Multiple sequence alignments of iPSP homologs from these three families using the CLUSTALW programCitation28) showed that the cysteine residues that correspond to C198 and C197 of H. thermophilus PspA and PspB, respectively, were conserved in all homologs from Aquificaceae, except one of the two PspAs (ZP_02179977) from Hydrogenivirga, but not in those from Hydrogenothermaceae or Desulfurobacteriaceae (Table ). The PspA of Hydrogenivirga without the cysteine residue was acquired by lateral gene transfer from Hydrogenothermaceae.Citation22) In contrast, another PspA of Hydrogenivirga (ZP_02178481), which was acquired by vertical inheritance, conserved the cysteine residues. Therefore, iPSP2 (A-B) from Hydrogenivirga can also form intermolecular disulfide bond.
Table 1 Growth temperature range and optimal temperature of selected members of Aquificaceae, Hydrogenothermaceae, and Desulfurobacteriaceae.
Discussion
This study presents the first example of a heterodimeric protein from a thermophilic bacterium with an intermolecular disulfide bond that contributes to protein thermostability. The soluble forms of both heterologously expressed and purified iPSP1, a homodimer of PspA (A-A), and iPSP2, a heterodimer of PspA and PspB (A-B), were shown to be connected by disulfide bonds formed between the 198th and 197th cysteine residues of PspA and PspB, respectively (Fig. (A)). Nearly 100% of A-B dimers were connected by a disulfide bond. Comparison of the thermostabilities between wild-type A-B, A-B under reducing conditions, and the cysteine mutant of A-B clearly showed that the disulfide bonds increase thermostability (Fig. (B) and (D)–(F)). These findings are consistent with studies reporting that tight interfacial connections between subunits mediated by hydrogen bonding,Citation29) hydrophobic interactions,Citation30) or disulfide bondsCitation31) increase protein thermostability. In addition, the importance of interactions between subunits for increasing multimeric protein solubility has already been reportedCitation32); however, these studies were limited to homomultimeric proteins. To our knowledge, the findings presented here are the first example of an intracellular protein that contains an intermolecular disulfide bond between heteromeric subunits that contributes to thermostability, and the contribution to thermostability was unique to heteromeric nature.
Interestingly, PspB subunits that were not connected to PspA by a disulfide bond were precipitated at lower temperature than PspA, whereas both subunits, when they were connected with an intermolecular disulfide bond, were precipitated under the same conditions, namely both subunits start to precipitate around 85 °C and about 70% of them precipitated at 90 °C (Fig. (B) and (D)–(F)). This observation likely indicates that attachment to PspA is required for PspB to exist in the soluble fraction. Our speculation concerning this point is as follows: PspA and PspB can stably form heterodimers without a disulfide bond at 70 °C or lower, and therefore the intermolecular disulfide bond is not essential below the optimal growth temperature of H. thermophilus. However, the intermolecular disulfide bond between PspA and PspB is necessary for the solubility of PspB at 75 °C or higher because molecular motion is markedly increased at these high temperatures and the probability of detachment of the subunits is also increased. If PspB detaches from PspA, it may immediately precipitate and disappear from the soluble phase while PspA can remain in the soluble fraction as a monomer for a very short time and then find other PspA monomer to make stable homodimer, A-A immediately. It is also supported by the result that residual PSP activity of A-B and As-Bs after heat treatment well agrees with the ratio of residual soluble PspA subunit (Fig. (E) and (G)). Therefore, the strong connection of PspB to PspA through the disulfide bond may prevent the precipitation of PspB. This speculation well agrees with the following observations from the present and past studies: (1) PspB does not remain in the soluble fraction when expressed without PspA in E. coliCitation22); (2) the elution pattern of A-A and A-B from a hydrophobic column suggests that the surface of PspB has higher hydrophobicity than that of PspA; and (3) the surface charge of modeled PspB structure calculated by PyMOL was 0.0, whereas that of PspA was −4.0, suggesting that the surface electron charge of PspB is very low (data not shown). We therefore propose that intermolecular disulfide bonds between subunits with low solubility and those with higher solubility can increase the thermostability of multimeric proteins.
It is noteworthy that the intermolecular disulfide bond between PspA-PspB is essential for the PspB subunit to exist in the soluble fraction at 75 °C, which is the upper limit of the optimal growth temperature of H. thermophiles.Citation24) Thus, the intermolecular disulfide bond appears to be physiologically important for this protein to maintain solubility in H. thermophilus. However, due to the reducing environment of the cytosol, disulfide bonds are not typically found in cytosolic proteins. In eukaryotes, disulfide bonds are formed in the lumen of the endoplasmic reticulum in reactions catalyzed by protein disulfide isomerase.Citation33) As the intracellular redox potential of E. coli, a mesophilic prokaryote, is around −200 to −300 mV, recombinant proteins with disulfide bonds may not fold properly.Citation17,34) Therefore, we examined whether the disulfide bonds found in heterologously expressed and purified proteins also exist in vivo. The results of a CPM assay showed that A-B does not form intermolecular S–S bonds in E. coli (Fig. ), a finding that does not conflict with the above information indicating that the intracellular environment of E. coil is reduced. In contrast, numerous disulfide bonds were detected in total protein samples from H. thermophilus, in addition to the relatively strong band around 24.5 kDa that may be derived from PspA (Fig. ). Although a band of 23.5 kDa corresponding to PspB was not clearly observed, A-B may still form an intermolecular disulfide bond in H. thermophilus, because PspB is estimated to have a lower molecular number than that of PspACitation22) and therefore more difficult to be detected. This speculation does not conflict with the WB data that H. thermophilus lysate contained both A-A and A-B dimers with disulfide bonds (Fig. (A) and (B)).
The physiological importance of the intermolecular disulfide bond identified in iPSP1 and iPSP2 is also supported by the strict conservation of the cysteine residues corresponding to the 197th or 198th cysteines among homologs of these proteins in Aquificacea (Table ). Although the cysteine residues are not conserved in PspA or PspB from Hydrogenothermaceae, it is unclear whether PspB subunits from this family are unable to remain in the soluble phase at physiological temperature. In addition, the growth temperature of many members of Hydrogenothermaceae is lower than that of several Aquificaceae species (Table ). We speculate that the evolution of iPSP in Aquificales occurred as follows. When a single iPSP gene was duplicated to generate PspA and PspB in the ancestor of Aquificaceae and Hydrogenothermaceae, both proteins had iPSP activity and were soluble as homo- and hetero-dimers. Subsequently, PspA maintained PSP activity and solubility, whereas PspB lost PSP activity and became less soluble, but may have acquired other functions. During the evolution of PspB, the solubility of this protein might have been reduced to the point that B-B became insoluble. However, PspB retained its ability to form heterodimers with PspA, and therefore can exist in soluble form as a heterodimer. Concurrent with the evolution of PspB in Aquificaceae, PspB inherited cysteine residues from an ancestor of Aquificaceae that allowed for the formation of a disulfide bond between PspA and PspB.
CPM assay revealed that various intracellular proteins of H. thermophilus contain disulfide bonds (Fig. (A)). This observation is consistent with several recent reports that several thermophilic eukaryotes have numerous intracellular proteins with disulfide bonds.Citation16,17,35) As such proteins are rare in mesophiles, it appears that the formation of intramolecular and intermolecular disulfide bonds within proteins is a common strategy for thermophiles to increase protein thermostability and allow adaptation to high temperatures. However, it remains unclear how disulfide bonds are formed in intracellular environments.Citation17) As H. thermophilus utilizes the reductive tricarboxylic acid cycle, which is used to fix CO2 in reducing environments, it seems highly unlikely that the disulfide bond between PspA and PspB would spontaneously form in cells. Therefore, it is more likely that a specific system selectively forms disulfide bonds in thermophiles. H. thermophilus has several genes that are predicted to encode protein disulfide isomerases and thioredoxins, which may catalyze the formation of disulfide bonds.
In this study, we demonstrated that an intermolecular disulfide bond contributes to the thermostability of a heterodimeric protein from a thermophilic bacterium. The disulfide bond increases the thermostability of the whole protein by specifically increasing the solubility of a single subunit at high temperature connecting it to the partner. This finding provides new insight into the evolution of proteins with high thermostability and is expected to contribute to the development of new strategies for increasing the thermostability of target proteins.
Author contributions
Y.C. and M.I. designed this study. K.T.K and Y.C. performed the experiment. K.T.K and H.A. analyzed the data. K.T.K, Y.C., and M.I. wrote the manuscript. All the authors reviewed the results and approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported in part by a Grant-in-aid for Scientific Research (A) 21248010 from the Japan Society for the Promotion of Science.
Supplemental materials
Supplemental material for this article can be accessed at http://dx.doi.org/10.1080/09168451.2015.1079476.
Supplementary_figure_with_legend.pdf
Download PDF (2.4 MB)Acknowledgments
The authors gratefully acknowledge Shoichiro Horita for discussion, and the help of Makoto Ato, Suhee Cho, and Masaru Ishizaki, who provided technical assistance with several molecular biology techniques.
References
- Imanaka T, Shibazaki M, Takagi M. A new way of enhancing the thermostability of proteases. Nature. 1986;324:695–697.10.1038/324695a0
- Chakravarty S, Varadarajan R. Elucidation of determinants of protein stability through genome sequence analysis. FEBS Lett. 2000;470:65–69.10.1016/S0014-5793(00)01267-9
- Dominy BN, Minoux H, Brooks CL 3rd. An electrostatic basis for the stability of thermophilic proteins. Proteins. 2004;57:128–141.10.1002/prot.20190
- Missimer JH, Steinmetz MO, Baron R, Winkler FK, Kammerer RA, Daura X, van Gunsteren WF. Configurational entropy elucidates the role of salt-bridge networks in protein thermostability. Protein Sci. 2007;16:1349–1359.10.1110/ps.062542907
- Kumar S, Tsai CJ, Nussinov R. Factors enhancing protein thermostability. Protein Eng. 2000;13:179–191.10.1093/protein/13.3.179
- Yano JK, Poulos TL. New understandings of thermostable and peizostable enzymes. Curr. Opin. Biotechnol. 2003;14:360–365.10.1016/S0958-1669(03)00075-2
- Karlström M, Stokke R, Helene Steen I, Birkeland N-K, Ladenstein R. Isocitrate dehydrogenase from the hyperthermophile Aeropyrum pernix: X-ray structure analysis of a ternary enzyme-substrate complex and thermal stability. J. Mol. Biol. 2005;345:559–577.10.1016/j.jmb.2004.10.025
- Trivedi S, Gehlot HS, Rao SR. Protein thermostability in Archaea and Eubacteria. Genet. Mol. Res.: GMR. 2006;5:816–827.
- DeDecker BS, O'Brien R, Fleming PJ, Geiger JH, Jackson SP, Sigler PB. The crystal structure of a hyperthermophilic archaeal TATA-box binding protein. J. Mol. Biol. 1996;264:1072–1084.10.1006/jmbi.1996.0697
- Roca M, Liu H, Messer B, Warshel A. On the relationship between thermal stability and catalytic power of enzymes. Biochemistry. 2007;46:15076–15088.10.1021/bi701732a
- Basu S, Sen S. Do homologous. Thermophilic-mesophilic proteins exhibit similar structures and dynamics at optimal growth temperatures? A molecular dynamics simulation study. J. Chem. Inf. Model. 2013;53:423–434.10.1021/ci300474h
- McCully ME, Beck DAC, Daggett V. Promiscuous contacts and heightened dynamics increase thermostability in an engineered variant of the engrailed homeodomain. Protein Eng. Des. Sel. 2013;26:35–45.10.1093/protein/gzs063
- Toth EA, Worby C, Dixon JE, Goedken ER, Marqusee S, Yeates TO. The crystal structure of adenylosuccinate lyase from Pyrobaculum aerophilum reveals an intracellular protein with three disulfide bonds. J. Mol. Biol. 2000;301:433–450.10.1006/jmbi.2000.3970
- Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Adv. Enzymol. Relat. Areas Mol. Biol. 1990;63:69–172.
- Bessette P, Aslund F, Beckwith J, Georgiou G. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13703–13708.10.1073/pnas.96.24.13703
- Boutz DR, Cascio D, Whitelegge J, Perry LJ, Yeates TO. Discovery of a thermophilic protein complex stabilized by topologically interlinked chains. J. Mol. Biol. 2007;368:1332–1344.10.1016/j.jmb.2007.02.078
- Mallick P, Boutz DR, Eisenberg D, Yeates TO. Genomic evidence that the intracellular proteins of archaeal microbes contain disulfide bonds. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9679–9684.10.1073/pnas.142310499
- Van den Akker F, Feil IK, Roach C, Platas AA, Merritt EA, Hol WG. Crystal structure of heat-labile enterotoxin from Escherichia coli with increased thermostability introduced by an engineered disulfide bond in the A subunit. Protein Sci. 1997;6:2644–2649.
- Takagi H, Takahashi T, Momose H, Inouye M, Maeda Y, Matsuzawa H, Ohta T. Enhancement of the thermostability of subtilisin E by introduction of a disulfide bond engineered on the basis of structural comparison with a thermophilic serine protease. J. Biol. Chem. 1990;265:6874–6878.
- Ko JH, Jang WH, Kim EK, Lee HB, Park KD, Chung JH, Yoo OJ. Enhancement of thermostability and catalytic efficiency of AprP, an alkaline protease from Pseudomonas sp., by the introduction of a disulfide bond. Biochem. Biophys. Res. Commun. 1996;221:631–635.10.1006/bbrc.1996.0647
- Liu L, Deng Z, Yang H, Li J, Shin HD, Chen RR, Du G, Chen J. In silico rational design and systems engineering of disulfide bridges in the catalytic domain of an alkaline alpha-amylase from Alkalimonas amylolytica to improve thermostability. Appl. Environ. Microbiol. 2014;80:798–807.10.1128/AEM.03045-13
- Chiba Y, Oshima K, Arai H, Ishii M, Igarashi Y. Discovery and analysis of cofactor-dependent phosphoglycerate mutase homologs as novel phosphoserine phosphatases in hydrogenobacter thermophilus. J. Biol. Chem. 2012;287:11934–11941.10.1074/jbc.M111.330621
- Kawasumi T, Igarashi Y, Kodama T, Minoda Y. Isolation of strictly thermophilic and obligately autotrophic hydrogen bacteria. Agric. Biol. Chem. 1980;44:1985–1986.10.1271/bbb1961.44.1985
- Kawasumi T, Igarashi Y, Kodama T, Minoda Y. Hydrogenobacter thermophilus gen. nov., sp. nov., an extremely thermophilic, aerobic, hydrogen-oxidizing bacterium. Int. J. Syst. Bacteriol. 1984;34:5–10.10.1099/00207713-34-1-5
- Chiba Y, Horita S, Ohtsuka J, Arai H, Nagata K, Igarashi Y, Tanokura M, Ishii M. Structural units important for activity of a novel-type phosphoserine phosphatase from Hydrogenobacter thermophilus TK-6 revealed by crystal structure analysis. J. Biol. Chem. 2013;288:11448–11458.10.1074/jbc.M112.449561
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.10.1038/227680a0
- Marino SM, Gladyshev VN. Cysteine function governs its conservation and degeneration and restricts its utilization on protein surfaces. J. Mol. Biol. 2010;404:902–916.10.1016/j.jmb.2010.09.027
- Thompson JD, Higgins DG, Gibson TJ. Improved sensitivity of profile searches through the use of sequence weights and gap excision. Comput. Appl. Biosci. 1994;10:19–29.
- Williams JC, Zeelen JP, Neubauer G, Vriend G, Backmann J, Michels PAM, Lambeir A-M, Wierenga RK. Structural and mutagenesis studies of leishmania triosephosphate isomerase: a point mutation can convert a mesophilic enzyme into a superstable enzyme without losing catalytic power. Protein Eng. Des. Sel. 1999;12:243–250.10.1093/protein/12.3.243
- Kirino H, Aoki M, Aoshima M, Hayashi Y, Ohba M, Yamagishi A, Wakagi T, Oshima T. Hydrophobic interaction at the subunit interface contributes to the thermostability of 3-isopropylmalate dehydrogenase from an extreme thermophile, Thermus thermophilus. Eur. J. Biochem. 1994;220:275–281.10.1111/ejb.1994.220.issue-1
- Guelorget A, Roovers M, Guerineau V, Barbey C, Li X, Golinelli-Pimpaneau B. Insights into the hyperthermostability and unusual region-specificity of archaeal Pyrococcus abyssi tRNA m1A57/58 methyltransferase. Nucleic. Acids Res. 2010;38:6206–6218.10.1093/nar/gkq381
- Sørensen HP, Mortensen KK. Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microb. Cell Fact. 2005;4:1.10.1186/1475-2859-4-1
- Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim. Biophys. Acta. 2004;1699:35–44.10.1016/S1570-9639(04)00063-9
- Hwang C, Sinskey A, Lodish H. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502.10.1126/science.1523409
- Dutton RJ, Boyd D, Berkmen M, Beckwith J. Bacterial species exhibit diversity in their mechanisms and capacity for protein disulfide bond formation. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11933–11938.10.1073/pnas.0804621105
- Eder W, Huber R. New isolates and physiological properties of the Aquificales and description of Thermocrinis albus sp. nov. Extremophiles. 2002;6:309–318.10.1007/s00792-001-0259-y
- Deckert G, Warren PV, Gaasterland T, Young WG, Lenox AL, Graham DE, Overbeek R, Snead MA, Keller M, Aujay M, Huber R, Feldman RA, Short JM, Olsen GJ, Swanson RV. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature. 1998;392:353–358.
- Freedman Z, Zhu C, Barkay T. Mercury resistance and mercuric reductase activities and expression among chemotrophic thermophilic aquificae. Appl. Environ. Microbiol. 2012;78:6568–6575.10.1128/AEM.01060-12
- Nakagawa S, Shtaih Z, Banta A, Beveridge TJ, Sako Y, Reysenbach AL Sulfurihydrogenibium yellowstonense sp. nov., an extremely thermophilic, facultatively heterotrophic, sulfur-oxidizing bacterium from Yellowstone National Park, and emended descriptions of the genus Sulfurihydrogenibium, Sulfurihydrogenibium subterraneum and Sulfurihydrogenibium azorense. Int. J. Syst. Evol. Microbiol. 2005;55:2263–2268.10.1099/ijs.0.63708-0
- Gotz D, Banta A, Beveridge TJ, Rushdi AI, Simoneit BRT, Reysenbach AL. Persephonella marina gen. nov., sp. nov. and Persephonella guaymasensis sp. nov., two novel, thermophilic, hydrogen-oxidizing microaerophiles from deep-sea hydrothermal vents. Int. J. Syst. Evol. Microbiol. 2002;52:1349–1359.10.1099/ijs.0.02126-0
- L'Haridon S, Cilia V, Messner P, Raguénès G, Gambacorta A, Sleytr UB, Prieur D, Jeanthon C. Desulfurobacterium thermolithotrophum gen. nov., sp. nov., a novel autotrophic, sulphur-reducing bacterium isolated from a deep-sea hydrothermal vent. Int. J. Syst. Bacteriol. 1998;48:701–711.10.1099/00207713-48-3-701
- Vetriani C, Speck MD, Ellor SV, Lutz RA, Starovoytov V. Thermovibrio ammonificans sp. nov., a thermophilic, chemolithotrophic, nitrate-ammonifying bacterium from deep-sea hydrothermal vents. Int. J. Syst. Evol. Microbiol. 2004;54:175–181.10.1099/ijs.0.02781-0
