Abstract
Effective utilization of microbes often requires complex genetic modification using multiple antibiotic resistance markers. Because a few markers have been used in Geobacillus spp., the present study was designed to identify a new marker for these thermophiles. We explored antibiotic resistance genes functional in Geobacillus kaustophilus HTA426 and identified a thiostrepton resistance gene (tsr) effective at 50 °C. The tsr gene was further used to generate the mutant tsrH258Y functional at 55 °C. Higher functional temperature of the mutant was attributable to the increase in thermostability of the gene product because recombinant protein produced from tsrH258Y was more thermostable than that from tsr. In fact, the tsrH258Y gene served as a selectable marker for plasmid transformation of G. kaustophilus. This new marker could facilitate complex genetic modification of G. kaustophilus and potentially other Geobacillus spp.
Graphical abstract
A thiostrepton resistance gene (tsr) and its mutant were identified as new selectable markers for Geobacillus kaustophilus HTA426.
The members of the genus Geobacillus include aerobic or facultatively anaerobic, gram-positive, and Bacillus-related thermophiles.Citation1) These organisms have been isolated from a wide range of environmentsCitation2) and usually exhibit optimum growth at temperatures ranging from 55 to 60 °C. Because of their thermophilic properties, the members of this genus can be used in microbial bioprocesses performed at high temperature. High-temperature bioprocesses have considerable advantages over ordinary-temperature bioprocesses, as exemplified by the growth inhibition of animal pathogens, low energy expenditure for agitation and cooling, and easy removal of volatile products.Citation3) In addition, several Geobacillus spp. have exhibited remarkable properties useful in microbial bioprocesses. For instance, G. thermoglucosidasius exhibits ethanol tolerance and has been studied for high-temperature ethanol and isobutanol production.Citation4–7) Another strain can produce acetoin and 2,3-butanediol.Citation8) Some others have the ability to utilize celluloseCitation9) or hemicelluloses,Citation10) to accumulate arsenateCitation11) or toxic metal ionsCitation12) and to degrade hydrocarbons,Citation13) long-chain alkanes,Citation14,15) herbicides,Citation2) or polyvinyl alcohol.Citation16) These properties make Geobacillus spp. attractive for use in high-temperature bioprocesses intended to bioproduction, biodegradation, biomineralization, and bioremediation.
In general, effective utilization of microbes requires genetic modification such as gene inactivation and forced gene expression to enhance the bioactivity and/or alter the metabolic flux of the microbes. Genetic modification is commonly performed using antibiotic resistance genes as selectable markers. In addition, the combined use of multiple markers enables more complex microbial modifications, including the stable maintenance of multiple plasmids and inactivation of multiple genes. However, fewer antibiotic resistance genes are used in thermophiles relative to mesophiles. In Geobacillus spp., only kanamycin, chloramphenicol, and tetracycline resistance genes have been demonstrated to serve as selectable markers.Citation5,17–23) Therefore, there is room to expand new markers for more effective utilization of Geobacillus spp. through complex genetic modifications.
Geobacillus kaustophilus HTA426 is an aerobic strain that had been isolated from deepest sea mud of the Mariana Trench.Citation24,25) We have studied this strain as a model for biotechnological applications of Geobacillus spp., because basal genetic tools have been established for this strainCitation18,26–28) and a whole genome sequence is also available.Citation29) In this study, we explored antibiotic resistance genes functional in G. kaustophilus HTA426 to expand selectable markers for Geobacillus spp. Here, we report a new marker, tsr, which is a thiostrepton resistance gene from Streptomyces azureus.Citation30) Thiostrepton inhibits protein translation by firmly binding to the complex formed by 23S rRNA and ribosomal protein L11 in bacterial ribosomes.Citation31) The tsr gene encodes an RNA methyltransferase that prevents thiostrepton from binding to ribosomes by 23S rRNA methylation.Citation32) We also generated tsr mutants and characterized their availability as selectable markers.
Materials and methods
Bacterial strains, media, and plasmids
Table lists G. kaustophilus strains used. An error-prone strain, MK480, was used for thermoadaptation-directed evolution of tsr gene. Strain MK242 was used for gene characterization because it is genetically stable. These strains were grown at 60 °C in Luria–Bertani (LB) medium. However, G. kaustophilus [pGKE75 derivative], where square brackets denote the plasmid-carrier state, was grown in LB medium supplemented with 5 mg/L of kanamycin (LK5). Escherichia coli DH5α (Takara Bio, Otsu, Shiga, Japan) and E. coli BL21-CodonPlus(DE3)-RIPL (Agilent Technologies, Santa Clara, USA) were used for DNA manipulation and recombinant protein production, respectively. E. coli strains were grown at 37 °C in LB medium. Ampicillin (50 mg/L), kanamycin (50 mg/L), and chloramphenicol (13 mg/L) were added when necessary. The plasmid pGKE75 was constructed previously.Citation33) pUC19 and pET-16b were purchased from Takara Bio and Merck KGaA (Darmstadt, Germany), respectively.
Table 1. G. kaustophilus strains used in this study.
Construction of pGKE75-tsr
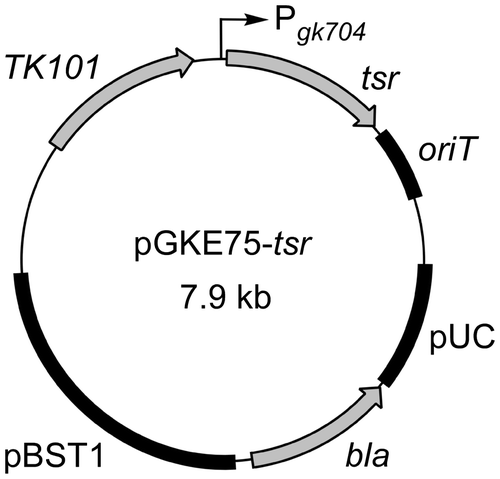
The tsr gene was amplified from plasmid pIJ4123Citation34) using primers 5′-GCCGCATGCCTGAGTTGGACACCATC-3′ (SphI site underlined; start codon italicized) and 5′-GCCGGATCCTTATCGGTTGGCCGCGAGATTC-3′ (BamHI site underlined; stop codon italicized). The amplified fragment was trimmed with SphI and BamHI and subcloned between the SphI and BamHI sites of pGKE75 to generate pGKE75-tsr (Fig. ).
Fig. 1. pGKE75-tsr structure.
Plasmid transformation of G. kaustophilus
The derivatives of pGKE75 were introduced into G. kaustophilus by conjugative DNA transfer from E. coli.Citation27) E. coli [pGKE75-tsr derivative] and E. coli [pUB307] were used as the DNA donor and conjugation helper, respectively. G. kaustophilus MK242 was used as the DNA recipient. These strains were cultured until the optical density at 600 nm reached 0.3. Cultures (E. coli, 10 mL; G. kaustophilus, 100 mL) were then mixed and concentrated by centrifugation. Cell mixture was spotted on LB plates and incubated for 4 h at 37 °C to achieve conjugation. When kanamycin was used for transformant selection, cells were recovered from LB plates and incubated at 60 °C on LK5 plates. When thiostrepton was used, cells were recovered following further preincubation at 52 °C for 4 h and subsequently incubated on LB plates supplemented with thiostrepton (1 mg/L) at 52 °C to isolate transformants.
Thiostrepton resistance assay
G. kaustophilus MK242 [pGKE75-tsr derivative] was precultured at 50 °C and then incubated at 50, 55, and 60 °C on LK5 plates with and without thiostrepton (0.5 mg/L). The colonies were counted to calculate the thiostrepton resistance efficiency, which was defined as the ratio of thiostrepton-resistant cells (grown on LK5 with thiostrepton) to the total number of cells (grown on LK5 without thiostrepton).
Generation of the tsrH258Y mutant using thermoadaptation-directed evolution
G. kaustophilus MK480 [pGKE75-tsr] was incubated on LK5 plates containing thiostrepton (0.1 mg/L) at 45 °C for 2 days. The cells were collected and further incubated on LK5 plates containing thiostrepton (0.5 mg/L) at 50 °C for 1 day. Similar successive cultivations were repeated on LK5 plates with thiostrepton (0.5 mg/L) at 55 °C for 3 days, 55 °C for 1 day, and 60 °C for 1 day. In addition, apparent colonies were regrown on LK5 plates with thiostrepton (0.5 mg/L) at 60 °C. pGKE75-tsr was isolated from the clones that showed evident thiostrepton resistance, and the tsr sequence was analyzed.
Site-directed mutagenesis
The tsr gene was excised from pGKE75-tsr with SphI and BamHI and subcloned between the SphI and BamHI sites of pUC19. The resulting plasmid was used as a template to amplify tsr partial fragments (361 bp) with the primers 5′-GGGGATCATCCTGGTAGACAGTGACATCACC-3′ and 5′-CCTGTCGATCCTCTCxxxCAGCGCGATTCCGAG-3′ (xxx: CGC for tsrH258A; GCA for tsrH258C; GTC for tsrH258D; TTC for tsrH258E; AAA for tsrH258F; GCC for tsrH258G; GAT for tsrH258I; TTT for tsrH258K; CAA for tsrH258L; CAT for tsrH258M; GTT for tsrH258N; CGG for tsrH258P; TTG for tsrH258Q; GCG for tsrH258R; CGA for tsrH258S; CGT for tsrH258T; GAC for tsrH258V; CCA for tsrH258W). Using the fragments as mutagenic primers, pUC19 harboring tsr was replicated in vitro to generate plasmids carrying the tsr mutants. The template plasmid was digested with DpnI treatment. The resulting DNA was subsequently propagated in E. coli. The tsr mutants were excised from the obtained plasmids and subcloned between the SphI and BamHI sites of pGKE75 to generate pGKE75-tsr derivatives, including pGKE75-tsrH258W and pGKE75-tsrH258F.
Preparation of recombinant proteins
The tsr, tsrH258Y, tsrH258W, and tsrH258F genes were amplified from appropriate pGKE75-tsr derivatives using the primers 5′-GCCCATATGCCTGAGTTGGACACCATC-3′ (NdeI site underlined; start codon italicized) and 5′-GCCGGATCCTTATCGGTTGGCCGCGAGATTC-3′ (BamHI site underlined; stop codon italicized) and subcloned between the NdeI and BamHI sites of pET-16b. The resulting plasmid was introduced into E. coli BL21-CodonPlus(DE3)-RIPL. The transformant was cultured at 20 °C for 20 h in LB medium containing 1% lactose. Cells were harvested by centrifugation (5000 × g for 5 min) and homogenized in buffer (20 mM sodium phosphate, pH 7.4, 0.5 M NaCl) by ultrasonication. The lysate was clarified by centrifugation (18,000 × g for 10 min at 4 °C) and then applied to a Talon column (metal ion, Co2+; bed volume, 0.2 mL; Takara Bio) equilibrated with buffer. The column was washed with buffers containing 20 mM imidazole (2 mL) and then 50 mM imidazole (0.5 mL). Subsequently, recombinant proteins were eluted with buffer containing 200 mM imidazole. Purified proteins were dialyzed against 10 mM sodium phosphate (pH 7.0) containing 0.5 M NaCl. The purity of proteins was analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein concentration was determined by the Bradford method using bovine serum albumin as a standard.
Thermostability assay of Tsr proteins
A protein solution (20 µL; 0.4 g/L protein) was incubated at 40–55 °C for 1 h in a gradient temperature heater (Thermal Cycler Dice Gradient; Takara Bio). Aggregated proteins were removed by centrifugation (18,000 × g for 10 min at 4 °C). Soluble proteins retained in the supernatant were analyzed by SDS-PAGE and the Bradford method. The temperature at which the residual protein = 50% (T1/2 value) was calculated by the linear regression of thermal aggregation curves (Tsr, 44.3–48.3 °C; TsrH258Y, 48.3–51.1 °C; TsrH258W, 48.3–51.1 °C; TsrH258F, 44.3–48.3 °C).
Gel filtration column chromatography
Gel filtration was performed using a Superdex 75 10/300 GL column (GE Healthcare Japan, Tokyo, Japan) and an ÄKTA pure 25 system (GE Healthcare Japan) by isocratic elution in 20 mM sodium phosphate (pH 7.4) containing 0.5 M NaCl at a flow rate of 0.5 mL/min. Conalbumin (75 kDa), bovine serum albumin (67 kDa), ovalbumin (43 kDa), chymotrypsinogen (25 kDa), and ribonuclease A (14 kDa) were used as molecular weight standards.
Thermostability assay of thiostrepton
LB plates supplemented with thiostrepton (0.5 mg/L) were preincubated at 50, 55, and 60 °C for 24, 48, and 72 h. These plates were then used for incubation of G. kaustophilus MK242 (109 cells) at 50 °C to assess effective thiostrepton remained.
Results
The tsr gene confers thiostrepton resistance on G. kaustophilus
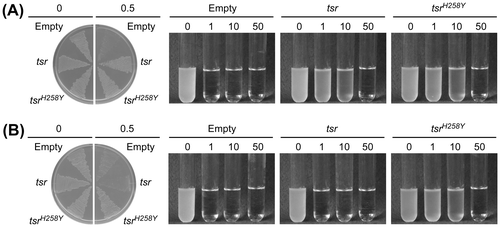
Thiostrepton, ampicillin, and spectinomycin resistance genes had been retained in our laboratory; therefore, we tentatively examined the utility of these genes as selectable markers for G. kaustophilus. Spectinomycin (100 mg/L) was ineffective against G. kaustophilus. Ampicillin was effective but the ampicillin resistance gene (bla) from pUC19 could not confer resistance to ampicillin (0.1 mg/L) on G. kaustophilus even at 45 °C. However, although G. kaustophilus was intrinsically sensitive to thiostrepton at 45–70 °C (IC50 = 10–50 µg/L), G. kaustophilus MK242 [pGKE75-tsr] could grow in LK5 media supplemented with thiostrepton (0.5 mg/L) at 50 °C following preculture at 50 °C (Fig. (A)). Preculture was essential for thiostrepton resistance presumably because of sufficient rRNA methylation prior to thiostrepton exposure. It was likely that Tsr protein undergoes thermal denaturation at 55 °C, because MK242 [pGKE75-tsr] could not grow at 55 °C (Fig. (B)) and cells that were precultured at 55 °C were sensitive to thiostrepton even at 50 °C (data not shown).
Fig. 2. Thiostrepton resistance conferred by tsr and tsrH258Y genes.
Thermostability of thiostrepton
LB plates supplemented with thiostrepton completely inhibited G. kaustophilus growth even after preincubation at 50–55 °C for 48 h (Table ). However, a few cells grew on the plates preincubated at 55 °C for 72 h. When the plate was preincubated at 60 °C, thiostrepton was obviously inactivated by 72-h incubation. These results suggest that thiostrepton (0.5 mg/L) is effective at temperatures below 55 °C for 48 h but may be inactivated by longer or higher temperature incubations.
Table 2. The frequency of cells growing on LB plates that contained thiostrepton but were preincubated at high temperatures.
Plasmid transformation of G. kaustophilus using tsr as a selectable marker
To determine whether tsr actually serves as a selectable marker in G. kaustophilus, we introduced pGKE75-tsr into strain MK242 and selected transformants using thiostrepton selection. Plasmid introduction was performed using conjugative DNA transfer from E. coli, because it was an only practical method for plasmid introduction into G. kaustophilus. In this method, plasmid was transferred from E. coli [pGKE75-tsr] to G. kaustophilus with the mediation of E. coli [pUB307] during incubation of their cell mixture at 37 °C. The mixture was then preincubated at 52 °C for intracellular Tsr production and 23S rRNA methylation. Subsequently, an aliquot of cells was incubated at 52 °C on LB plates supplemented with thiostrepton. This process successfully provided 78 colonies after 3-day incubation, although an equal aliquot provided 188 colonies on LK5 plates within 24 h. Repeated analysis indicated that the colonies obtained using thiostrepton accounted for approximately 42% of those obtained using kanamycin (Table ). Several transformants obtained using thiostrepton were sensitive to kanamycin. These false positives can be attributed to thermal inactivation of thiostrepton by 3-day incubation (Table ). When preincubation and incubation were performed at temperatures above 53 °C, few transformants grew probably because of Tsr denaturation and subsequent insufficient 23S rRNA methylation.
Table 3. Plasmid transformation of G. kaustophilus with pGKE75-tsr derivatives using thiostrepton.
Generation of the tsrH258Y mutant by thermoadaptation-directed evolution
We previously constructed an error-prone thermophile, G. kaustophilus MK480, and demonstrated that this strain can generate mutant genes encoding more thermostable variants than the parent enzymes during periods of cell growth at high temperatures.Citation33,35) The results suggested the possibility that this approach, thermoadaptation-directed evolution, might generate tsr mutants that confer substantial thiostrepton resistance on G. kaustophilus at temperatures above 50 °C. Thus, we examined thermoadaptation-directed tsr evolution by serially culturing G. kaustophilus MK480 [pGKE75-tsr] under thiostrepton selection pressures. This approach generated clones that were resistant to thiostrepton at 60 °C. pGKE75-tsr was isolated from eight clones and analyzed for the tsr sequence. All eight plasmids carried the tsrH258Y mutant that has C772T mutation and encodes H258Y protein variant (TsrH258Y).
Thiostrepton resistance conferred by tsr mutants
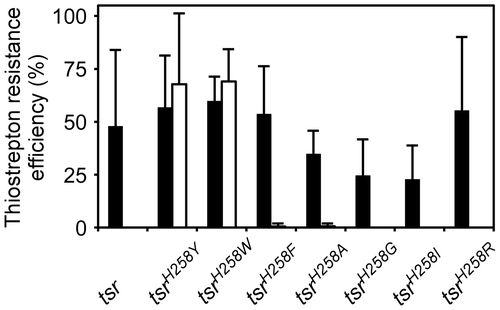
In addition to tsrH258Y, we generated additional tsr mutants (tsrH258A, tsrH258C, tsrH258D, tsrH258E, tsrH258F, tsrH258G, tsrH258I, tsrH258K, tsrH258L, tsrH258M, tsrH258N, tsrH258P, tsrH258Q, tsrH258R, tsrH258S, tsrH258T, tsrH258V, and tsrH258W) by in vitro mutagenesis approach. These saturation mutants regarding the 258th codon were subcloned in pGKE75 and introduced into G. kaustophilus MK242 to analyze the thiostrepton resistance conferred by the genes. As expected, pGKE75-tsrH258Y conferred the thiostrepton resistance at 55 °C (Fig. (B)). Quantitative analysis showed that approximately 68% of MK242 [pGKE75-tsrH258Y] cells were resistant to thiostrepton (Fig. ). pGKE75-tsrH258W also conferred substantial resistance at 55 °C. The tsrH258F, tsrH258A, tsrH258G, tsrH258I, and tsrH258R mutants were functional at 50 °C but not 55 °C. Other mutants were not functional even at 50 °C.
Fig. 3. Comparison of thiostrepton resistance conferred by tsr mutants.
Plasmid transformation of G. kaustophilus using tsrH258Y and tsrH258W as selectable markers
When G. kaustophilus was transformed with pGKE75-tsr using thiostrepton selection, it was necessary for 3 days to identify transformant colonies (see above). However, pGKE75-tsrH258Y provided transformants within 48 h even when preincubation and incubation were performed at 53 °C or 54 °C (Table ). Moreover, the selection was achieved with higher efficiency relative to tsr and produced no false positives. pGKE75-tsrH258W also provided transformants, but these contained false positives and the selection efficiency was lower than that of pGKE75-tsrH258Y (Table ). Thus, tsrH258Y served as the most efficient marker among tsr and its mutants examined.
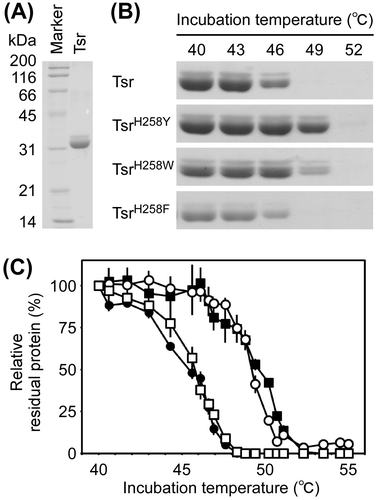
Thermostability of Tsr, TsrH258Y, TsrH258W, and TsrH258F proteins
Tsr, TsrH258Y, TsrH258W, and TsrH258F proteins were heterologously produced as soluble proteins fused with an N-terminal octahistidine-tag and were purified using immobilized metal ion affinity chromatography (Fig. (A)). Gel filtration analysis of the purified proteins revealed single protein peaks corresponding to 64–68 kDa. These observations indicated that Tsr forms a homodimer, as suggested by X-ray crystallographic analysis,Citation36) and that the homodimeric structure is not disrupted by H258Y, H258W, and H258F replacements. Because it is difficult to quantify Tsr activity in vitro, we analyzed thermostability of recombinant proteins using protein aggregation assay (Fig. (B) and (C)). Tsr protein was completely aggregated by incubation at approximately 48 °C. A similar profile was observed for TsrH258F. However, complete aggregation of TsrH258Y and TsrH258W was observed at temperatures above 51 °C. The T1/2 values of Tsr, TsrH258Y, TsrH258W, and TsrH258F were 45.3, 49.5, 49.3, and 45.7 °C, respectively. These data indicated that TsrH258Y and TsrH258W are more thermostable than Tsr and TsrH258F.
Fig. 4. Thermostability of Tsr, TsrH258Y, TsrH258W, and TsrH258F proteins.
Discussion
While the tsr gene has been used as a selectable marker exclusively in Streptomyces spp.,Citation37) we found that tsr is functional at 50 °C in G. kaustophilus HTA426 even though the performance as a selectable marker was unsatisfactory. To improve the performance, we examined thermoadaptation-directed evolution of tsr and identified a more efficient marker, tsrH258Y, which conferred thiostrepton resistance at 55 °C. The enhanced functional temperature is attributable to the enhanced thermostability of the gene product, as the recombinant TsrH258Y protein was more thermostable than Tsr. The T1/2 enhancement by the H258Y replacement was only 4.2 °C, but tsrH258Y efficiently selected transformants within 48 h at temperatures higher than 52 °C. The thiostrepton concentration required for transformant selection was only 1 mg/L. Moreover, the selection produced no false positives. This is probably due to short periods required for transformant selection because thiostrepton was substantially effective at 50–55 °C within 48 h although not over the time. tsrH258Y marker may be unsuitable for stable plasmid maintenance at temperatures above 55 °C but is available for complex genome modification, such as inactivation and integration of targeted genes, along with other proven selectable markers. Thus, tsrH258Y and thiostrepton constitute a practical marker system for G. kaustophilus.
Thiostrepton resistance assay indicated that MK242 [pGKE75-tsrH258Y] cells resistant to thiostrepton were less than those resistant to kanamycin (Fig. ). In G. kaustophilus transformation, transformants selected by tsrH258Y with thiostrepton did not outreach transformants selected by the kanamycin resistance gene with kanamycin (Table ). Preincubation was also required to select transformants using thiostrepton. These observations can be explained by inefficient expression of tsrH258Y due to codon bias, because tsrH258Y gene has a high GC content (61.4%) in contrast to a moderate content in G. kaustophilus (52.1%).Citation29) This idea is supported by negligible production of TsrH258Y protein in E. coli BL21(DE3), which has no extra copies of rare tRNA genes unlike E. coli BL21-CodonPlus(DE3)-RIPL. Therefore, more efficient tsrH258Y marker may be generated by codon optimization of the gene for G. kausotphilus.
In the course of tsrH258Y generation, G. kaustophilus MK480 [pGKE75-tsr] cells had adapted to thiostrepton at 60 °C, whereas MK242 cells that had been freshly transformed with pGKE75-tsrH258Y were sensitive to thiostrepton at 60 °C. This difference may arise from a factor to enhance thiostrepton resistance during thiostrepton-exposed cultivations, such as ribosomal gene mutation to prevent thiostrepton from binding to ribosomes,Citation38) activation of drug efflux pumps to eliminate thiostrepton to the extracellular space, and induction of protein chaperones (or chaperone-like factors) to thermostabilize the Tsr protein in vivo. Because of such adaptation, our attempts to generate other variants from tsrH258Y have not yielded mutations, even though MK480 [pGKE75-tsrH258Y] cells were found to adapt to thiostrepton at 70 °C. Therefore, we conducted saturation mutagenesis regarding the H258 codon to generate a tsr mutant that confers thiostrepton resistance more efficiently than does tsrH258Y. Unfortunately, this approach did not provide a mutant that functions at 60 °C but determined that tsrH258W functions at 55 °C as with tsrH258Y. The enhanced functional temperature is also attributable to the enhanced thermostability of the gene product, as suggested by thermostability assay of TsrH258W protein (Fig. ).
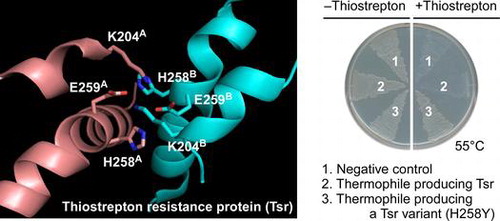
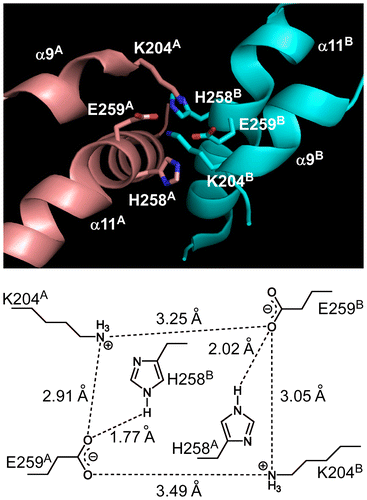
In a homodimeric structure of the Tsr protein,Citation36) two pairs of K204 and E259 residues interact with tetragonal ionic networks at the contact surface between the two subunits (Fig. ). The two H258 residues are located on the same side of the tetragonal ionic network, in which the residue of each subunit interacts to reciprocally with the E259 residue of the other subunit. Thus, the H258 residues appear to optimize the ionic networks formed between K204 and E259 residues. This observation implies that replacement of histidine at residue 258 to tyrosine or tryptophan, which is aromatic and bulky amino acid, may optimize the ionic networks and thereby render the structure more robust. However, TsrH258F protein showed the thermostability comparable to Tsr protein despite an aromatic side chain at residue 258 as with TsrH258Y and TsrH258W. Moreover, there was no clear association between the side chain structures of residue 258 in the Tsr variants and their functionality at 55 °C. Therefore, it is likely that the H258 replacements affected the catalytic activity and/or protein thermostability through complex molecular mechanisms.
Fig. 5. Three-dimensional structure of Tsr protein around the H258 residues.
In summary, we generated tsrH258Y as a selectable marker available for G. kaustophilus. This new marker extends the genetic toolbox of G. kaustophilus and potentially other Geobacillus spp., providing new opportunities for the effective utilization of these thermophiles through complex genetic modifications.
Author contributions
H.S. designed the study. K.W., J.K., M.F., and H.S. performed the experiments. K.W., K.D, T.O., and H.S. analyzed the data. K.W. and H.S. wrote the manuscript. K.D. and T.O. contributed to the critical revision of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Programme for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry, Japan, under Grant: The Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry, Japan [grant number 26033A]; JSPS KAKENHI [grant number 25450105]; The Institute for Fermentation, Osaka, Japan.
References
- Nazina TN, Tourova TP, Poltaraus AB, et al. Taxonomic study of aerobic thermophilic bacilli: descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermoglucosidasius and Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans, G. kaustophilus, G. thermoglucosidasius and G. thermodenitrificans. Int. J. Syst. Evol. Microbiol. 2001;51:433–446.
- McMullan G, Christie JM, Rahman TJ, Banat IM, Ternan NG, Marchant R. Habitat, applications and genomics of the aerobic, thermophilic genus Geobacillus. Biochem. Soc. Trans. 2004;32:214–217.10.1042/BST0320214
- Wiegel J, Ljungdahl LG. The importance of thermophilic bacteria in biotechnology. Crit. Rev. Biotechnol. 1986;3:39–108.
- Cripps RE, Eley K, Leak DJ, et al. Metabolic engineering of Geobacillus thermoglucosidasius for high yield ethanol production. Metab. Eng. 2009;11:398–408.10.1016/j.ymben.2009.08.005
- Taylor MP, Esteban CD, Leak DJ. Development of a versatile shuttle vector for gene expression in Geobacillus spp. Plasmid. 2008;60:45–52.10.1016/j.plasmid.2008.04.001
- Taylor MP, Eley KL, Martin S, Tuffin MI, Burton SG, Cowan DA. Thermophilic ethanologenesis: future prospects for second-generation bioethanol production. Trends Biotechnol. 2009;27:398–405.10.1016/j.tibtech.2009.03.006
- Lin PP, Rabe KS, Takasumi JL, Kadisch M, Arnold FH, Liao JC. Isobutanol production at elevated temperatures in thermophilic Geobacillus thermoglucosidasius. Metab. Eng. 2014;24:1–8.10.1016/j.ymben.2014.03.006
- Xiao ZJ, Wang XM, Huang YL, et al. Thermophilic fermentation of acetoin and 2,3-butanediol by a novel Geobacillus strain. Biotechnol. Biofuels. 2012;5:88.10.1186/1754-6834-5-88
- Assareh R, Shahbani Zahiri H, Akbari Noghabi K, Aminzadeh S, Bakhshi Khaniki G. Characterization of the newly isolated Geobacillus sp. T1, the efficient cellulase-producer on untreated barley and wheat straws. Bioresour. Technol. 2012;120:99–105.10.1016/j.biortech.2012.06.027
- Tabachnikov O, Shoham Y. Functional characterization of the galactan utilization system of Geobacillus stearothermophilus. FEBS J. 2013;280:950–964.
- Cuebas M, Sannino D, Bini E. Isolation and characterization of arsenic resistant Geobacillus kaustophilus strain from geothermal soils. J. Basic Microbiol. 2011;51:364–371.10.1002/jobm.v51.4
- Özdemir S, Kilinc E, Poli A, Nicolaus B, Güven K. Cd, Cu, Ni, Mn and Zn resistance and bioaccumulation by thermophilic bacteria, Geobacillus toebii subsp. decanicus and Geobacillus thermoleovorans subsp. stromboliensis. World J. Microbiol. Biotechnol. 2012;28:155–163.10.1007/s11274-011-0804-5
- Zheng C, He J, Wang Y, Wang M, Huang Z. Hydrocarbon degradation and bioemulsifier production by thermophilic Geobacillus pallidus strains. Bioresour. Technol. 2011;102:9155–9161.10.1016/j.biortech.2011.06.074
- Wang L, Tang Y, Wang S, et al. Isolation and characterization of a novel thermophilic Bacillus strain degrading long-chain n-alkanes. Extremophiles. 2006;10:347–356.10.1007/s00792-006-0505-4
- Kato T, Miyanaga A, Kanaya S, Morikawa M. Gene cloning and characterization of an aldehyde dehydrogenase from long-chain alkane-degrading Geobacillus thermoleovorans B23. Extremophiles. 2010;14:33–39.10.1007/s00792-009-0285-8
- Kim MN, Yoon MG. Isolation of strains degrading poly(Vinyl alcohol) at high temperatures and their biodegradation ability. Polym. Degrad. Stab. 2010;95:89–93.10.1016/j.polymdegradstab.2009.09.014
- Narumi I, Nakayama N, Nakamoto S, Kimura T, Yanagisawa T, Kihara H. Construction of a new shuttle vector pSTE33 and its stabilities in Bacillus stearothermophilus, Bacillus subtilis, and Escherichia coli. Biotechnol. Lett. 1993;15:815–820.
- Suzuki H, Yoshida K. Genetic transformation of Geobacillus kaustophilus HTA426 by conjugative transfer of host-mimicking plasmids. J. Microbiol. Biotechnol. 2012;22:1279–1287.10.4014/jmb
- Liao H, McKenzie T, Hageman R. Isolation of a thermostable enzyme variant by cloning and selection in a thermophile. Proc. Nat. Acad. Sci. USA. 1986;83:576–580.10.1073/pnas.83.3.576
- Imanaka T, Fujii M, Aramori I, Aiba S. Transformation of Bacillus stearothermophilus with plasmid DNA and characterization of shuttle vector plasmids between Bacillus stearothermophilus and Bacillus subtilis. J. Bacteriol. 1982;149:824–830.
- Wu LJ, Welker NE. Protoplast transformation of Bacillus stearothermophilus NUB36 by plasmid DNA. J. Gen. Microbiol. 1989;135:1315–1324.
- Min Z, Nakai H, Imanaka T. Useful host-vector systems in Bacillus stearothermophilus. Appl. Environ. Microbiol. 1988;54:3162–3164.
- De Rossi E, Brigidi P, Welker NE, Riccardi G, Matteuzzi D. New shuttle vector for cloning in Bacillus stearothermophilus. Res. Microbiol. 1994;145:579–583.10.1016/0923-2508(94)90074-4
- Takami H, Inoue A, Fuji F, Horikoshi K. Microbial flora in the deepest sea mud of the Mariana Trench. FEMS Microbiol. Lett. 1997;152:279–285.10.1111/j.1574-6968.1997.tb10440.x
- Takami H, Nishi S, Lu J, Shinamura S, Takaki Y. Genomic characterization of thermophilic Geobacillus species isolated from the deepest sea mud of the Mariana Trench. Extremophiles. 2004;8:351–356.10.1007/s00792-004-0394-3
- Suzuki H, Murakami A, Yoshida K. Counterselection system for Geobacillus kaustophilus HTA426 through disruption of pyrF and pyrR. Appl. Environ. Microbiol. 2012;78:7376–7383.10.1128/AEM.01669-12
- Suzuki H, Wada K, Furukawa M, Doi K, Ohshima T. A ternary conjugation system for the construction of DNA libraries for Geobacillus kaustophilus HTA426. Biosci. Biotechnol. Biochem. 2013;77:2316–2318.10.1271/bbb.130492
- Suzuki H, Yoshida K, Ohshima T. Polysaccharide-degrading thermophiles generated by heterologous gene expression in Geobacillus kaustophilus HTA426. Appl. Environ. Microbiol. 2013;79:5151–5158.10.1128/AEM.01506-13
- Takami H, Takaki Y, Chee GJ, et al. Thermoadaptation trait revealed by the genome sequence of thermophilic Geobacillus kaustophilus. Nucleic Acids Res. 2004;32:6292–6303.10.1093/nar/gkh970
- Cundliffe E. Mechanism of resistance to thiostrepton in the producing-organism Streptomyces azureus. Nature. 1978;272:792–795.10.1038/272792a0
- Thompson J, Cundliffe E, Stark M. Binding of thiostrepton to a complex of 23-S rRNA with ribosomal protein L11. Eur. J. Biochem. 1979;98:261–265.10.1111/ejb.1979.98.issue-1
- Thompson J, Schmidt F, Cundliffe E. Site of action of a ribosomal RNA methylase conferring resistance to thiostrepton. J. Biol. Chem. 1982;257:7915–7917.
- Kobayashi J, Furukawa M, Ohshiro T, Suzuki H. Thermoadaptation-directed evolution of chloramphenicol acetyltransferase in an error-prone thermophile using improved procedures. Appl. Microbiol. Biotechnol. 2015;99:5563–5572.10.1007/s00253-015-6522-4
- Takano E, White J, Thompson CJ, Bibb MJ. Construction of thiostrepton-inducible, high-copy-number expression vectors for use in Streptomyces spp. Gene. 1995;166:133–137.10.1016/0378-1119(95)00545-2
- Suzuki H, Kobayashi J, Wada K, Furukawa M, Doi K. Thermoadaptation-directed enzyme evolution in an error-prone thermophile derived from Geobacillus kaustophilus HTA426. Appl. Environ. Microbiol. 2015;81:149–158.10.1128/AEM.02577-14
- Dunstan MS, Hang PC, Zelinskaya NV, Honek JF, Conn GL. Structure of the thiostrepton resistance methyltransferase·S-adenosyl-L-methionine complex and its interaction with ribosomal RNA. J. Biol. Chem. 2009;284:17013–17020.10.1074/jbc.M901618200
- Hopwood DA, Bibb MJ, Chater KF, et al. Genetic manipulation in Streptomyces: a laboratory manual. Norwich: The John Innes Foundation; 1985.
- Cameron DM, Thompson J, Gregory ST, March PE, Dahlberg AE. Thiostrepton-resistant mutants of Thermus thermophilus. Nucleic Acids Res. 2004;32:3220–3227.10.1093/nar/gkh644
