Abstract
Isoflavone-metabolizing bacteria, Adlercreutzia equolifaciens, Asaccharobacter celatus, Slackia equolifaciens, and Slackia isoflavoniconvertens catalyzed C-ring cleavage of (–)-epicatechin and (+)-catechin, (+)-epicatechin, and (–)-catechin in varying degrees. The cleaving abilities of (–)-epicatechin and (+)-catechin were enhanced by hydrogen, except (+)-catechin cleavage by S. equolifaciens, which was not accelerated. (−)-Catechin cleavage by Ad. equolifaciens was remarkably accelerated by hydrogen.
Recent reports have shown catechins to be poorly absorbed in the body, whereas catechin metabolites degraded by intestinal bacteria were found to be easily absorbed.Citation1–3) Accordingly, studies on the catabolism of catechins by intestinal bacteria are receiving a considerable amount of attention as they may aid in the understanding of health-promoting effects of catechins such as hypotensive, hypoglycemic, and chemopreventive activities.Citation4,5) Previously, we reported on the catabolism of (+)-catechin (+C) and (–)-epicatechin (–EC) by rat intestinal microbiota and proposed possible metabolic pathways of these two catechins.Citation6) We further isolated and identified rat intestinal bacteria, Adlercreutzia equolifaciens MT4s-5 and Flavonifractor plautii MT42 as (–)-epigallocatechin (EGC)-metabolizing bacteria and reported on their degradation abilities.Citation7) Ad. equolifaciens MT4s-5 was reported to convert an isoflavone, daidzein, into equol.Citation8) Most recently, we reported on the degradation abilities of EGC and (–)-gallocatechin (GC) by several isoflavone-metabolizing bacteria.Citation9) However, the metabolizing abilities of these bacteria on catechol-type catechins still remain to be investigated. In this report, we describe the degradation ability on –EC, (+)-epicatechin (+EC), (–)-catechin (–C), and +C by four isoflavone-metabolizing bacteria which are commercially available.
-EC, +EC, –C, and +C were obtained from Sigma-Aldrich Japan (Tokyo). Metabolites, (2S)-1-(3, 4-dihydroxyphenyl)-3-(2, 4, 6-trihydroxyphenyl)propan-2-ol (1S), (2R)-1-(3, 4-dihydroxyphenyl)-3-(2, 4, 6-trihydroxyphenyl)propan-2-ol (1R) were prepared according to the methods previously reportedCitation6) and were used as reference standards. General anaerobic medium (GAM) was obtained from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan). Adlercreutzia equolifaciens JCM 14793Citation10), Asaccharobacter celatus JCM 14811Citation11,12), Slackia equolifaciens JCM 16059Citation13,14), and Slackia isoflavoniconvertens JCM 16137Citation15) were purchased from RIKEN BioResource Center (Ibaraki, Japan). All other chemicals were available products of analytical or HPLC grade. All cultures in this study were carried out under anaerobic condition with an Anaero Pack (anaerobic cultivation) system (Mitsubishi Gas Chemical, Tokyo, Japan) unless otherwise stated.
Four isoflavone-metabolizing bacteria (Ad. equolifaciens JCM 14793, As. celatus JCM 14811, S. equolifaciens JCM 16059, and S. isoflavoniconvertens JCM 16137) were separately cultured in GAM broth (5 mL) containing 1 mM –EC, +EC, –C or +C at 37 °C under anaerobic condition. For cultivation in the presence of hydrogen, hydrogen gas was aseptically bubbled into the above incubation mixture for 10 s at a flow rate of about 50 mL/min and the resulting culture in test tube was packed in an Anaero Pouch (800 mL), to which hydrogen gas was injected for 30 s at a flow rate of 800 mL/min after removal of the air roughly by hand.Citation9) In this experiment, the hydrogen concentration in the incubation mixture was not determined but the concentration could be around 1.4 ppm (0.7 mM) of hydrogen gas, judging from the solubility of hydrogen in water. Sample collection and preparation for the analyses,Citation9) and LC/MSCitation6) and LC/MS/MSCitation7) analyses were done according to the method described in our previous paper.
We investigated whether or not the four isoflavone-metabolizing bacteria possessed degradation ability on –EC, +EC, –C and +C. All the isoflavone-metabolizing bacteria catalyzed C-ring cleavage of –EC to produce (2S)-1-(3, 4-dihydroxyphenyl)-3-(2, 4, 6-trihydroxyphenyl)propan-2-ol (1S), and the formation was accelerated in the presence of hydrogen (Fig. ). The bacterial strains also showed ring-cleaving ability on +C to yield (2R)-1-(3, 4-dihydroxyphenyl)-3-(2, 4, 6-trihydroxyphenyl)propan-2-ol (1R), and this reaction was enhanced by the presence of hydrogen, with the exception of S. equolifaciens JCM 16059 as indicated in Fig. . All of the bacteria showed only a slight ability to cleave C-ring of –C without hydrogen. However, only the ability of Ad. equolifaciens JCM 14793 was drastically enhanced in the presence of hydrogen (Fig. ). In the case of +EC, all the bacterial strains had a slight cleaving ability regardless of the presence of hydrogen (Fig. ).
Fig. 1. Biotransformation of –EC and +C by isoflavone-metabolizing bacteria.
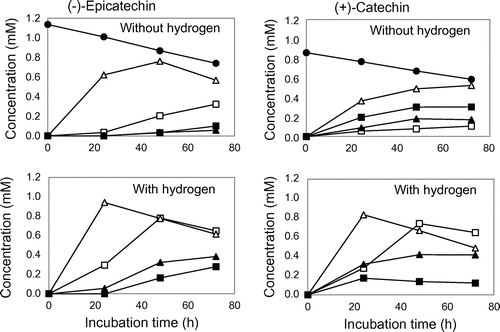
Fig. 2. Biotransformation of –C and +EC by isoflavone-metabolizing bacteria.
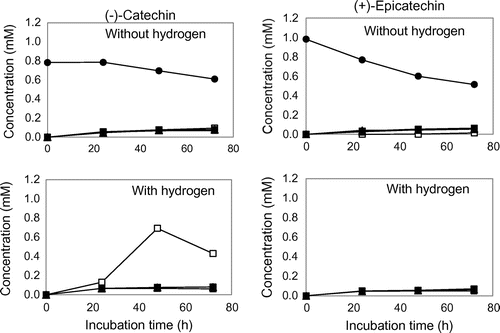
The ability of the isoflavone-metabolizing bacteria to cleave the C-ring of –EC, +EC, –C, and +C is summarized in Fig. . These bacteria, except S. isoflavoniconvertens, have been shown to biotransform (–)-epigallocatechin (EGC) and (–)-gallocatechin (GC).Citation9) In this study, all the isoflavone-metabolizing bacteria were found to biotransform –EC and + C. However, since these bacteria had lesser ability to cleave +EC and –C than –EC and +C, it is considered that their cleaving abilities depend largely on the steric configuration of catechins. In addition, we have found that EGC-metabolizing bacteria, Ad. equolifaciens MT4s-5 and Eggerthella lenta JCM 9979, facilitated C-ring cleavage of EGC and GC and subsequent dehydroxylation at the 4′ position in their B ring in the presence of hydrogen, which was supplied by syntrophic bacteria such as Escherichia coli and Butyricimonas sp. strains.Citation7) Ad. equolifaciens JCM 14793 and As. celatus JCM 14811 used in this study also showed the stimulation of C-ring cleavage and dehydroxylation of EGC and GC with hydrogen.Citation9) Accordingly, we examined the effects of hydrogen on the degradation of four catechol-type catechins. The ring-cleaving ability of –EC and +C was found to be enhanced by hydrogen while 4′-dehydroxylation in the B ring did not take place. The results suggested that the dehydroxylation abilities of at least two bacteria, Ad. equolifaciens JCM 14793 and As. celatus JCM 14811, were dependent on the number of hydroxyl groups in the B ring of catechins.
Fig. 3. Bioconversion scheme of –EC, –C, +C, and +EC by four isoflavone-metabolizing bacteria.
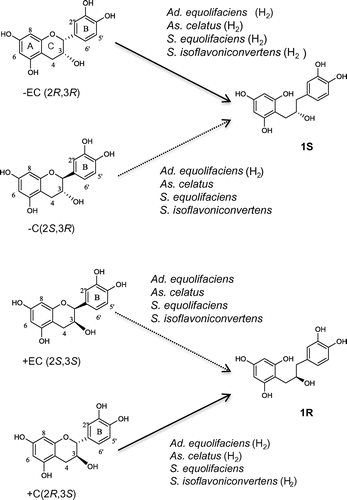
In this study, we demonstrated that isoflavone-metabolizing bacteria are involved in the degradation of catechol-type catechins in addition to pyrogallol-type catechinsCitation9) such as EGC and GC. The results imply that these bacteria may be responsible not only for isoflavone metabolism but also for catechin metabolism in the gut tract. Furthermore, the bacterial strains accelerated the cleavage of the catechins in the presence of hydrogen. In relation to this, Decroos et al. reported that equol production from isoflavone, daidzein, by a bacterial consortium of four bacteria (EPC4) partially isolated from human feces, was stimulated by hydrogen gas.Citation16) Recently, Bolca and Verstraete found that the EPC4 in the presence of daidzein significantly suppressed methane and hydrogen sulfide formation, which are negatively associated with health, through the consumption of fermentative hydrogen by reductive conversion of daidzein into equol.Citation17) Since these observations seem to be equally applicable to catechin, it may be expected that in the presence of catechins isoflavone-metabolizing bacteria also reduce methane or hydrogen sulfide. In addition, there is a possibility that oral intake of catechins may increase isoflavone-metabolizing bacterial population in the large intestine and in turn increase the production of equol as a bioactive substance.
Author contributions
AT and FN designed the study. AT performed the experiments. FN drafted the manuscript. All authors reviewed and approved the final manuscript.
Acknowledgments
We acknowledge the assistance of Andrea K. Suzuki (Mitsui Norin Co., Ltd.) in the proofreading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kohri T, Matsumoto N, Yamakawa M, et al. Metabolic fate of (–)-[4-3H]epigallocatechin gallate in rats after oral administration. J. Agric. Food Chem. 2001;49:4102–4112.10.1021/jf001491+
- Kohri T, Suzuki M, Nanjo F. Identification of metabolites of (−)-epicatechin gallate and their metabolic fate in the rat. J. Agric. Food Chem. 2003;51:5561–5566.10.1021/jf034450x
- Clifford MN, van der Hooft JJJ, Crozier A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am. J. Clin. Nutr. 2013;98:1619S–1630S.10.3945/ajcn.113.058958
- Hara Y. Green tea: health benefits and applications. New York, (NY): CRC Press; 2001.10.1201/CRCFOOSCITEC
- Yang CS, Lambert JD, Hou Z, Ju J, Lu G, Hao X. Molecular targets for the cancer preventive activity of tea polyphenol. Mol. Carcinog. 2006;45:431–435.10.1002/(ISSN)1098-2744
- Takagaki A, Nanjo F. Catabolism of (+)-catechin and (−)-epicatechin by rat intestinal microbiota. J. Agric. Food Chem. 2013;61:4927–4935.10.1021/jf304431v
- Takagaki A, Kato Y, Nanjo F. Isolation and characterization of rat intestinal bacteria involved in biotransformation of (–)-epigallocatechin. Arch. Microbiol. 2014;196:681–695.10.1007/s00203-014-1006-y
- Ito K, Ishida H, Takagaki A, Nanjo F, Maruo T, Ito C. Metabolism of isoflavone from soy bean by Eggerthella sp. MT4s-5, Part 1 Metabolism of daidzein, glycitein, and genistein. Poster session presented at: The Pharmaceutical Society of Japan. 128th Annual Meeting Abstract 2. P. 87; 2008 Mar 26–28; Yokohama, Japan.
- Takagaki A, Nanjo F. Biotransformation of (−)-epigallocatechin and (−)-gallocatechin by intestinal bacteria involved in isoflavone metabolism. Biol. Pharm. Bull. 2015;38:325–330.10.1248/bpb.b14-00646
- Maruo T, Sakamoto M, Ito C, Toda T, Benno Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int. J. Syst. Evol. Microbiol. 2008;58:1221–1227.10.1099/ijs.0.65404-0
- Minamida K, Tanaka M, Abe A, et al. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J. Biosci. Bioeng. 2006;102:247–250.10.1263/jbb.102.247
- Minamida K, Ota K, Nishimukai M, et al. Asaccharobacter celatus gen. nov., sp. nov., isolated from rat caecum. Int. J. Syst. Evol. Microbiol. 2008;58:1238–1240.10.1099/ijs.0.64894-0
- Jin J-S, Nishihata T, Kakiuchi N, Hattori M. Biotransformation of C-glucosylisoflavone puerarin to estrogenic (3S)-equol in co-culture of two human intestinal bacteria. Biol. Pharm. Bull. 2008;31:1621–1625.10.1248/bpb.31.1621
- Jin J-S, Kitahara M, Sakamoto M, Hattori M, Benno Y. Slackia equolifaciens sp. nov., a human intestinal bacterium capable of producing equol. Int. J. Syst. Evol. Microbiol. 2010;60:1721–1724.10.1099/ijs.0.016774-0
- Matthies A, Blaut M, Braune A. Isolation of a human intestinal bacterium capable of daidzein and genistein conversion. Appl. Environ. Microbiol. 2009;75:1740–1744.10.1128/AEM.01795-08
- Decroos K, Vanhemmens S, Cattoir S, Boon N, Verstraete W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 2005;183:45–55.10.1007/s00203-004-0747-4
- Bolca S, Verstraete W. Microbial equol production attenuates colonic methanogenesis and sulphidogenesis in vitro. Anaerobe. 2010;16:247–252.10.1016/j.anaerobe.2010.03.002
