Abstract
Myeloperoxidase (MPO)-generated halogenating molecules, such as hypochlorous acid and hypobromous acid (HOBr), in inflammatory regions are postulated to contribute to disease progression. In this study, we showed that ergothioneine (EGT), derived from an edible mushroom, inhibited MPO activity as well as the formation of 8-bromo-2′-deoxyguanosine in vitro. The HOBr scavenging effect of EGT is higher than those of ascorbic acid and glutathione. We initially observed that the administration of Coprinus comatus, an edible mushroom containing a high amount of EGT, inhibited the UV-B-induced inflammatory responses and DNA halogenation, suggesting that EGT is a promising anti-inflammatory agent from mushrooms.
Graphical abstract
The ergothioneine-rich Coprinus extract inhibited both MPO activity and HOBr-induced DNA damage in addition to inhibition of UV-B-induced inflammatory responses.
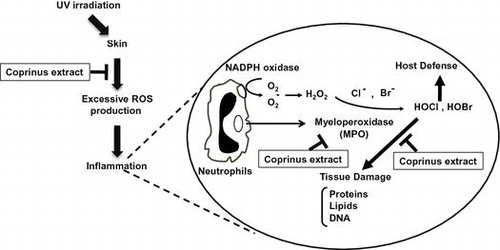
Myeloperoxidase (MPO), a heme protein secreted by activated leukocytes, plays an important role in the host defense at the sites of inflammation by the production of halogenating molecules such as hypochlorous acid (HOCl) and hypobromous acid (HOBr).Citation1,2) However, excessive halogenating species also modify the host’s biomacromolecules including DNA, proteins, and lipids.Citation3) Although halogenated adducts in DNA and proteins were detected in the liver of lipopolysaccharide (LPS)-treated mice, we have recently identified the halogenated adducts of nucleotides (8-halogenated dGs) as earlier inflammatory biomarkers.Citation4) Thus, the high concentrations of MPO-related halogenating species observed under inflammatory conditions are postulated to result in damage to the host tissue and contribute to the progression of inflammatory-related diseases including cancer, kidney disease, and atherosclerosis.Citation5)
Excessive exposure to the ultraviolet (UV) rays in sunlight is responsible for the oxidative damages to skin, resulting in sunburn, photoaging, and skin cancer.Citation6) Although UV-B is a minor component of UV (~4%), it directly causes inflammation by producing reactive oxygen species (ROS) and reducing antioxidants in the skin.Citation7) In fact, uncontrolled release of ROS by UV-B irradiation, which causes oxidative DNA base damage, such as 8-hydroxy-2′-deoxyguanosine (8-OHdG) formation,Citation8) is involved in the pathogenesis of human skin disorders. The use of anti-inflammatory agents is considered to be a reasonable approach to prevent UV-B-induced carcinogenesis.
Coprinus comatus, known as a shaggy ink cap, is an edible mushroom commonly found all over the world. It also contains a high amount of ergothioneine (EGT), a natural water-soluble amino acid that human cannot synthesize. EGT has been reported to show several physiological activities, including anti-cancer, anti-oxidant, immunomodulatory, and hypoglycemic activities.Citation9) In human, EGT is abundantly accumulated in the erythrocytes, bone marrow, liver, kidney, seminal fluid, and especially highly distributed in the small intestine.Citation9) However, the modulatory effects of EGT on inflammation are currently unclear.
In this study, to explore the possibility of EGT as an anti-inflammatory food material, we examined the protective effect of the EGT-containing mushroom, C. comatus, on DNA halogenation in both in vitro and in vivo. Our results show the possibility that EGT is one of the effective food factors to protect humans from UV-B-induced inflammatory damage.
Materials and methods
Materials
2′-Deoxyguanosine and 3,3′,5,5′-tetramethylbenzidine were obtained from Sigma-Aldrich (St. Louis, MO, USA). EGT, glutathione (GSH), ascorbic acid, l-histidine, l-tyrosine, and sodium hypochlorite (NaOCl) were obtained from Wako Pure Chemicals Industries (Osaka, Japan). MPO was purchased from Elastin Products Co., Inc. (Owensville, MO, USA). Anti-MPO polyclonal antibody was obtained from DAKO (Glostrup, Denmark). Horseradish peroxidase-labeled goat anti-mouse IgG was obtained from Cappel (Santa Ana, CA, USA). HOBr was prepared from an equimolar solution of NaOCl and KBr as previously reported.Citation10) 8-BrdG-BSA conjugate and anti-8-halogenated dG antibody (mAb8B3) were prepared as previously described.Citation4)
Extraction of mushrooms and measurement of EGT
Mushroom powders (C. comatus, Pleurotus comucopiae, Lentinula edodes and Grifola frondosa) were provided from Oryza Oil & Fat Chemical Co. Ltd. (Aichi, Japan). Mushroom powders (2.5 g) were extracted two times with 50 mL distilled water for 1 h at 80 °C with intermediate centrifugation (3000 rpm, 10 min). After concentrating the collected aqueous supernatants to 100 mL, a precipitation was vacuum freeze-dried. Measurement of EGT in the mushroom extract was performed using a high-pressure liquid chromatography (HPLC) connected to a UV detector with Develosil RPAQUEOUS (4.6 × 250 mm) column. The detection of EGT was done by measuring UV absorbance at 258 nm.
Measurement of MPO activity and 8-haloganated dGs
The formation of dityrosine from the MPO/H2O2/l-tyrosine system was used as an indicator of MPO activity as previously described.Citation11) Inhibition of the 8-halogenated dGs formation was evaluated by a competitive ELISA using the reaction mixture, which was previously reported.Citation4)
Animal experiments and immunohistochemistry
Six-week-old male HR-1 hairless mice (Hoshino Laboratory Animals Inc., Saitama, Japan) were used for UV-B irradiated inflammation model. After one week of acclimatization, 18 hairless mice were assigned to three groups: (1) control group (n = 6), (2) UV-B-irradiated group (n = 6), and (3) UV-B-irradiated and the Coprinus extract treatment group (n = 6). The Coprinus extract dissolved in 5% gum arabic were administrated by the gastric tubing introduction every day (200 mg/kg/day). Within 3 h after the Coprinus administration, mice were daily exposed to UV-B irradiation (120 mJ/cm2) for 3 min using a UV-B irradiation system (Solar Simulator; Ushio Inc., Tokyo, Japan). After 90 days of these treatments, dorsal skin samples were collected and fixed in 4% paraformaldehyde for immunohistochemical detection assay. The experiment was performed in accordance with the Guidelines for the Proper Conduct of Animal Experiments (Special Council of Japan, June 1 2006). The experiment was approved by the ethics committee of the research and development section in Oryza Oil & Fat Chemical Co. on July 1 2010. The immunohistochemical detection of 8-BrdG was done as previously reported.Citation8)
Statistical analysis
Statistical significance of the intergroup differences of means for multiple groups were determined using the Student Newman-Keuls multiple comparisons test after one-way ANOVA to determine variations among the group means, followed by Bartlett’s test to determine the homogeneity of variance.
Results
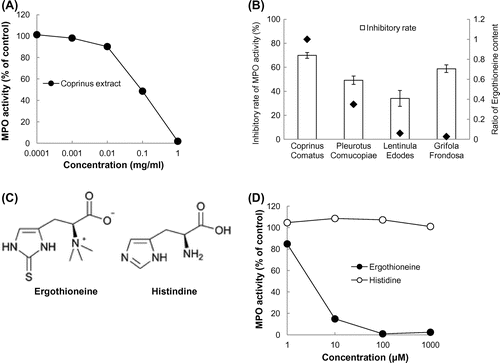
Since an early inflammation marker, 8-BrdG, was produced through the reaction of dG and HOBr generated from the MPO-H2O2-Cl−/Br− system,Citation2) inhibition of the 8-BrdG formation could result from either inhibition of MPO activity or the scavenging of HOBr. Thus, we examined the effect of the Coprinus (C. comatus) extract on MPO activity, measured as the amount of dityrosine derived from the reaction of MPO-H2O2-l-tyrosine system.Citation11) As shown in Fig. (A), the Coprinus extract dose-dependently inhibited MPO-dependent formation of dityrosine. Among the four mushroom extracts (C. comatus, P. comucopiae, L. edodes, and G. frondosa), Coprinus was the most effective mushroom extract to inhibit MPO activity (Fig. (B)). Since the Coprinus extract contains the highest amount of EGT compared to the other tested mushrooms, the inhibitory effect of EGT was examined and compared to that of l-histidine having a structure similar to EGT. As shown in Fig. (C) and (D), EGT significantly inhibited MPO activity even at the concentration of 1 µM. The inhibitory effect of EGT was dose-dependent, whereas l-histidine showed no inhibition.
Fig. 1. Mushroom extract inhibited MPO activity.
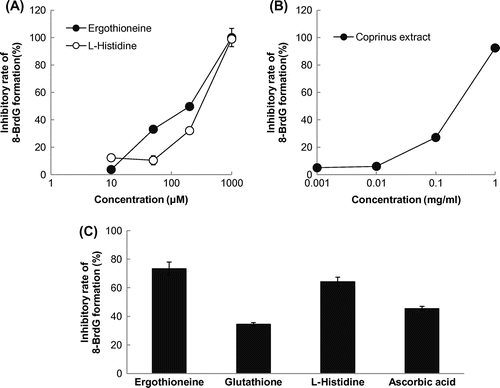
To examine the effect of EGT on the HOBr-dependent 8-BrdG formation, we performed a competitive ELISA using an 8-halogenated dG-specific antibody (mAb8B3). As shown in Fig. (A) and (B), not only the Coprinus extract, but also EGT inhibited the halogenation of dG in a dose-dependent manner. Histidine also showed a significant inhibition against the HOBr-dependent 8-BrdG formation (Fig. (A)), which was similar to that of EGT. The efficacy of EGT as a scavenger of HOBr was evaluated by comparison with the major antioxidants such as GSH and ascorbic acid. The inhibitory effect of EGT at 0.5 mM was 2.1-fold higher than that of GSH, and 1.6-fold greater than that of ascorbic acid under the same conditions (Fig. (C)).
Fig. 2. Inhibitory effect of the Coprinus extract in 8-BrdG formation.
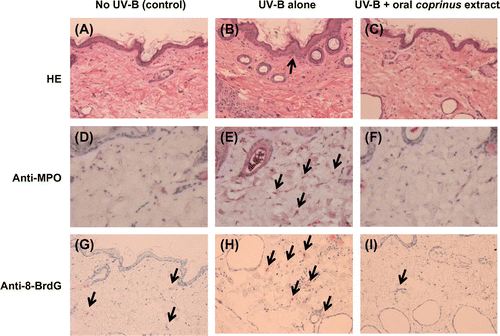
To evaluate the in vivo anti-inflammatory potencies of the Coprinus extract, the UV-B-irradiated mouse skin model was initially used. After UV-B irradiation for 90 days, the histopathological changes, such as the increase in the thickness of the epidermis and accumulation of neutrophils, were observed in the dorsal skin (Fig. (A) and (B)) and found consistent with previous reports.Citation12) As shown in Fig. (C), the oral administration of the Coprinus extract during irradiation inhibited these changes to the control level (Fig. (A)). To confirm the potential of the Coprinus extract as an inhibitor of the HOBr-induced DNA damage in vivo, an immunohistochemical analysis using the 8-halogenated dG-specific antibody (mAb8B3) was performed. As shown in Fig. (D) and (E), an increase in the MPO-positive immunostaining was observed in the skin tissue after UV-B irradiation compared to the non-irradiated control group, which is closely correlated to the infiltration of neutrophils (Fig. (A) and (B)). The generation of 8-BrdG was also obviously observed in the dermis of the UV-B-irradiated mice (Fig. (H)). The administration of the Coprinus extract resulted in the reduction of both the MPO and 8-BrdG staining in the dermis to the control level (Fig. (F) and (I)).
Fig. 3. Oral administration of the Coprinus extract reduced UV-B irradiation-induced 8-halogenated dG formation in dorsal skin.
Discussion
In this study, we confirmed the inhibitory effects of EGT and the EGT-containing mushroom extract on the MPO- or HOBr-induced halogenation in vitro. EGT is a natural sulfur-containing amino acid, synthesized by microorganisms in the soil and is absorbed into foods such as mushrooms.Citation13) Particularly, high levels of EGT are detected in some foods including not only certain species of mushrooms, beans, and grains, but also animal meat.Citation13) In addition, EGT has been used as an ingredient in foods, supplements, and cosmetics as well as a pharmaceutical additive due to its high safety; for example, EGT showed no toxicity even at 1500 mg/kg in the in vivo mouse mammalian erythrocyte micronucleus test.Citation14) Among the four representative extracts of mushrooms, the Coprinus extract showed the strongest MPO inhibitory activity correlated with its highest amount of EGT. The amount of EGT contained in the Coprinus extract was 1% of the extract (Fig. (B)), which accounts for 90% of the total inhibitory activity of the Coprinus extract against MPO activity (Fig. (A) and (D)). Taken together, EGT and the EGT-containing Coprinus extract could be applied as potential inhibitors against inflammation-derived DNA damage in vivo, in addition to the antioxidant function.
The in vitro data suggest that the possible inhibitory mechanisms of EGT are both the direct inhibition of MPO (Fig. ) and the scavenging of HOBr (Fig. ). Some flavonoids, such as quercetin, inhibit the MPO activity, possibly through the binding to MPO as substrates and their oxidization by MPO.Citation15) Kawai et al. also reported that tea catechins inhibited DNA halogenation by the scavenging of HOCl, resulting in the formation of chlorinated catechins.Citation16) In this study, EGT showed a stronger inhibitory activity against the 8-BrdG formation than GSH, one of the popular antioxidants with a sulfur atom. EGT exists as a tautomer between its thiol and thione forms at physiological condition,Citation9) which may contribute to its stability in contrast to GSH that is easily auto-oxidized. Therefore, EGT could act as a more stable cytoprotectant inhibiting DNA halogenation than GSH. Hypohalous acids react readily with amine group to form haloamines.Citation17) Chloramines of free α-amino acids readily eliminate HCl and then form an imine, an unstable intermediate subsequently hydrolyzed to the aldehyde.Citation17) Hypohalous acids also react with thiol group to form disulfide.Citation17) The fact that EGT has a trimethylammonium group supports the assumption that a plausible target for HOBr in EGT might be a tautomeric thiol group.
In addition to an in vitro study, we initially observed the possible inhibitory effects of an extract from a mushroom, C. comatus, on the UV-B induced halogenation of dG in vivo. In humans, EGT has been reported to distribute in cells and various organs such as the erythrocytes, bone marrow, liver, kidney, and eyes.Citation9) Although EGT is impermeable to plasma membranes, EGT is accumulated in organs and condensed up to a few millimolar through the EGT-specific transporter, carnitine/organic cation transporter 1 (OCTN1).Citation18) In addition, we observed that the treatment of the Coprinus extract inhibited the UV-B-induced halogenation of DNA and histopathological changes in vivo. Since the concentration of EGT in organs has been reported to reach up to 500 µM,Citation19) the direct effects of EGT on the inflammatory halogenation are quite feasible in vivo. On the other hand, anti-inflammatory effect of EGT or other constituents in the Coprinus extract could not be ruled out in the mechanism underlying inhibition of the DNA halogenation in mouse skin. Indeed, EGT was reported to inhibit acute lung inflammation in cytokine insufflated rats.Citation20) Therefore, further study is needed to clarify how the Coprinus extract modulates UV-B-induced neutrophil infiltration and how DNA halogenation is involved in the chronic inflammation development.
In summary, this study indicated the possibility that EGT in the C. comatus extract protects UV-B-induced DNA damage by inhibiting MPO activity and scavenging halogenous species. EGT at physiological concentrations is possible for clinical applications as an anti-inflammatory agent for not only UV-B-induced damage, but also chronic inflammatory diseases. Future efforts will be concerned with the in vivo significance of the inhibitory effect of EGT on several rodent inflammation models.
Authors contributions
Takashi Asahi, Tomomi Kanno, Yoji Kato, Yoshimasa Nakamura, and Toshihiko Osawa planned the experiments. Takashi Asahi, Xiaohong Wu, Hiroshi Shimoda, Shinsuke Hisaka, Tomomi Kanno and Yoji Kato performed the experiments. Takashi Asahi and Xiaohong Wu analyzed the data. Hiroshi Shimoda and Etsuko Harada contributed reagents or other essential material. Takashi Asahi, Yoji Kato, and Yoshimasa Nakamura wrote the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was partly supported by MEXT KAKENHI [grant number 25292073] (YN).
Notes
Abbreviations: EGT, ergothioneine; MPO, myeloperoxidase; HOCl, hypochlorous acid; HOBr, hypobromous acid; dG, 2′-deoxyguanosine; N4,5-diCldC, N4,5-dichloro-deoxycytidine; dG, 2′-deoxyguanosine; 8-CldG, 8-chloro-2′-deoxyguanosine; 8-BrdG, 8-bromo-2′-deoxyguanosine; 5-BrdC; 5-bromo-2′-deoxycytidine, 8-BrdA; 8-bromo-2′-deoxyadenosine, LPS, lipopolysaccharide; 8-OxodG, 8-hydroxy-2′-deoxyguanosine; TMB, 3,3′,5,5′-tetramethylbenzidine; BSA, bovine serum albumin; NaOCl, sodium hypochlorite; PBS, phosphate-buffered saline; ELISA, enzyme-linked immunosorbent assay; 3-ClTyr, 3-chlorotyrosine; 3-BrTyr, 3-bromotyrosine, OCTN1; carnitine/organic cation transporter 1.
References
- Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J. Biol. Chem. 1976;251:1371–1374.
- Gaut JP, Yeh GC, Tran HD, et al. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc. Natl. Acad. Sci. USA. 2001;98:11961–11966.10.1073/pnas.211190298
- Henderson JP, Byun J, Heinecke JW. Molecular chlorine generated by the myeloperoxidase-hydrogen peroxide-chloride system of phagocytes produces 5-chlorocytosine in bacterial RNA. J. Biol. Chem. 1999;274:33440–33448.10.1074/jbc.274.47.33440
- Asahi T, Kondo H, Masuda M, et al. Chemical and immunochemical detection of 8-halogenated deoxyguanosines at early stage inflammation. J. Biol. Chem. 2010;285:9282–9291.10.1074/jbc.M109.054213
- Takeshita J, Byun J, Nhan TQ, et al. Myeloperoxidase generates 5-chlorouracil in human atherosclerotic tissue: a potential pathway for somatic mutagenesis by macrophages. J. Biol. Chem. 2006;281:3096–3104.10.1074/jbc.M509236200
- Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339.10.1038/379335a0
- Bruner SD, Norman DP, Verdine GL. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature. 2000;403:859–866.
- Yin Y, Li W, Son YO, et al. Quercitrin protects skin from UVB-induced oxidative damage. Toxicol. Appl. Pharmacol. 2013;269:89–99.10.1016/j.taap.2013.03.015
- Irwin KC, Barry H. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta. 2012;1822:784–793.
- Henderson JP, Byun J, Williams MV, et al. Bromination of deoxycytidine by eosinophil peroxidase: a mechanism for mutagenesis by oxidative damage of nucleotide precursors. Proc. Natl. Acad. Sci. USA. 2001;98:1631–1636.10.1073/pnas.98.4.1631
- Kato Y, Nagao A, Terao J, et al. Inhibition of myeloperoxidase-catalyzed tyrosylation by phenolic antioxidants in vitro. Biosci. Biotechnol. Biochem. 2003;67:1136–1139.10.1271/bbb.67.1136
- Ishitsuka Y, Maniwa F, Koide C, et al. Detection of modified tyrosines as an inflammation marker in a photo-aged skin model. Photochem. Photobiol. 2007;83:698–705.10.1562/2006-07-24-RA-978
- Ey J, Schömig E, Taubert D. Dietary sources and antioxidant effects of ergothioneine. J. Agric. Food Chem. 2007;55:6466–6474.10.1021/jf071328f
- Schauss AG, Beres E, Vertesi A, et al. The effect of ergothioneine on clastogenic potential and mutagenic activity: genotoxicity evaluation. Int. J. Toxicol. 2011;30:405–409.10.1177/1091581811405856
- Shiba Y, Kinoshita T, Chuman H, et al. Flavonoids as substrates and inhibitors of myeloperoxidase: molecular actions of aglycone and metabolites. Chem. Res. Toxicol. 2008;21:1600–1609.10.1021/tx8000835
- Kawai Y, Matsui Y, Kondo H, et al. Galloylated catechins as potent inhibitors of hypochlorous acid-induced DNA damage. Chem. Res. Toxicol. 2008;21:1407–1414.10.1021/tx800069e
- Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274.10.1007/s00726-003-0016-x
- Grundemann D, Harlfinger S, Golz S, et al. Discovery of the ergothioneine transporter. Proc. Natl. Acad. Sci. USA. 2005;102:5256–5261.10.1073/pnas.0408624102
- Kato Y, Kubo Y, Iwata D, et al. Gene knockout and metabolome analysis of carnitine/organic cation transporter OCTN1. Pharm. Res. 2010;27:832–840.10.1007/s11095-010-0076-z
- Repine JE, Elkins ND. Effect of ergothioneine on acute lung injury and inflammation in cytokine insufflated rats. Prev Med. 2012;54:S79–S82.10.1016/j.ypmed.2011.12.006
