Abstract
Leucine is known to increase mTOR-mediated phosphorylation of 4EBP. In this study, leucine was administered to skeletal muscle-PGC-1α knockout mice. We observed attenuated 4EBP phosphorylation in the skeletal muscle, but not in the liver, of the PGC-1α knockout mice. These data suggest that skeletal muscle-PGC-1α is important for leucine-mediated mTOR activation and protein biosynthesis.
Branched chain amino acids (BCAAs) such as valine, leucine, and isoleucine are essential amino acids and components of proteins which are known to stimulate protein biosynthesis.Citation1) Among the BCAAs, leucine is known to be a signaling molecule that stimulates protein biosynthesis in tissues such as the skeletal muscle and liver.Citation2) The primary regulator of protein biosynthesis is mammalian target of rapamycin (mTOR), which is an evolutionally conserved serine/threonine kinase.Citation3) Eukaryotic initiation factor 4E-binding protein (4EBP) is a suppressor of protein translation and a substrate for mTOR. The phosphorylation of 4EBP by mTOR prevents the suppressor activity of 4EBP and, thus, increases protein biosynthesis.Citation1) The phosphorylation status of 4EBP determines its binding to eukaryotic translation initiation factor 4E (eIF4E), a rate-limiting component of the eukaryotic translation apparatus, and suppressing protein translation; γ phosphorylation form, but not α, β phosphorylation forms, of 4EBP does not bind to eIF4E, and does not inhibit translation.Citation4) Further, it has been reported that the oral administration of leucine in rodents increases the phosphorylation of 4EBP (γ form) and stimulates protein synthesis.Citation5,6)
Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a co-activator of transcription factors, including nuclear receptors, and is known to increase mitochondrial biogenesis and mitochondria-rich type-I fiber formation in skeletal muscle.Citation7,8) D’Antona et al. reported that the administration of BCAAs in aged mice caused an increase in PGC-1α levels in their skeletal muscle.Citation9) PGC-1α is reported to associate with mTORCitation10) and may play a role in mTOR-mediated protein biosynthesis. However, the relationship between BCAAs (particularly leucine), mTOR, and PGC-1α has not yet been investigated. Thus, in this study, we examined the role of PGC-1α in leucine-activated mTOR (4EBP) signaling using PGC-1α-knockout (KO) mice in skeletal muscle.
To control the ablation of PGC-1α, we generated a conditional KO version of the PGC-1α gene using the Cre–loxP recombination system. Exons 3–5 of the PGC-1α gene were flanked by loxP sites in the target construct.Citation11) Mice with the conditional allele of PGC-1α were crossed with transgenic mice expressing the Cre recombinase in skeletal muscle driven by the human α-actin promoter.Citation12) Homozygous PGC-1α lox allele mice were crossed with heterozygous Cre transgenic mice, and the offsprings were used for experiments. The genotypes of offspring were PGC-1α flox/flox with Cre (KO) and PGC-1α flox/flox without Cre (wildtype, WT). Mice were maintained in a 12-h light/dark cycle at 24 °C and were fed a normal chow diet ad libitum (CRF-1; Oriental Yeast, Tokyo, Japan). Mice were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and our institutional guidelines. All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee of Kyoto Prefectural University (No. KPU260407).
Total RNA was prepared using TRIzol (Life Technologies, Carlsbad, CA, USA). cDNA was synthesized from 500 ng of total RNA using the ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan). Gene expression levels were measured with ABI PRISM 7000 using Thunderberd SYBR qPCR Mix (Toyobo, Osaka, Japan) designed to detect cDNAs. The following primers were used as follows: PGC-1α Fw, 5′-CGGAAATCATATCCAACCAG-3′; PGC-1α Rv, 5′-TGAGGACCGCTAGCAAGTTTG-3′ and 36B4 Fw, 5′-GGCCCTGCACTCTCGCTTTC-3′; 36B4 Rv, 5′-TGCCAGGACGCGCTTGT-3′.
The enzyme activity of citrate synthase was measured by spectrophotometric analysis. The citrate synthase assay was performed at 412 nm following the reduction of 5,5′-dithiobis (2-nitrobenzoic acid) as previously described.Citation13)
The samples of the tibialis anterior muscle from WT and PGC-1α KO mice at 12 weeks of age were frozen in liquid nitrogen-cooled isopentane, and transverse sections were analyzed by enzyme histochemistry to evaluate succinate dehydrogenase activities.Citation14)
After fasting for 24 h, leucine (1.35 mg/g body weight) or vehicle was administered to the experimental mice. Thirty minutes later, the samples of skeletal muscle and liver were obtained. Western blot analysis was performed as previously described.Citation15) The following primary antibodies were used as follows: anti-phospho 4EBP (#2855; Cell Signaling Technology Japan, Tokyo, Japan) and anti-GAPDH (C14C10; Cell Signaling Technology Japan, Tokyo, Japan). Western blot signals were calculated using densitometry (LAS 1000; Fuji Film, Tokyo, Japan).
Data were evaluated by Student’s t-test or one-way analysis of variance followed by Tukey’s honestly post hoc test. P values below 0.05 were considered statistically significant.
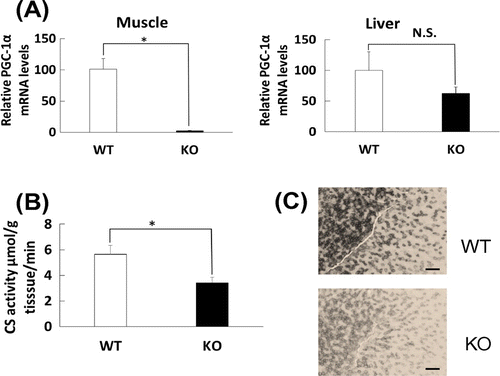
Fig. (A) shows that PGC-1α mRNA level was markedly decreased in the skeletal muscle of PGC-1α KO mice compared with that of WT mice, but not in the liver of WT and PGC-1α KO mice. To assess functionality of the decreased PGC-1αmRNA in KO mice, we examined mitochondrial marker levels in the skeletal muscle of the PGC-1α KO mice, as PGC-1α is a regulator of mitochondrial biogenesis. Fig. (B) shows that a decrease in the activity of citrate synthase, a mitochondrial enzyme of the TCA cycle,Citation16) was observed. The histological staining of succinate dehydrogenase, another mitochondrial enzyme of the TCA cycle, also showed decreased signal in the transverse sections of the skeletal muscle of PGC-1α KO mice (Fig. (C)). Thus, in the PGC-1α KO mice, mitochondrial activity is decreased, suggesting that PGC-1α is functionally knocked out in the skeletal muscle.
Fig. 1. PGC-1α mRNA level in skeletal muscle of WT and PGC-1α knockout mice and mitochondrial enzyme activities in skeletal muscle of PGC-1α knockout mice.
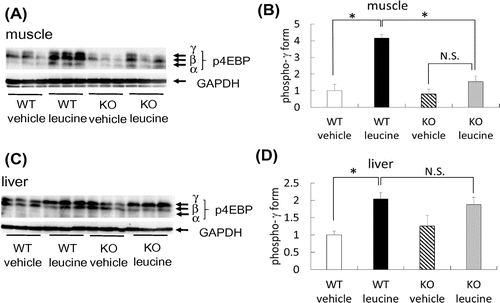
We orally administered leucine to WT and PGC-1α KO mice, after which the phosphorylation of 4EBP in the skeletal muscle and liver was examined. In WT mice, consistent with the previous reports,Citation2,5,6) the phosphorylation of 4EBP, including the γ form, was increased in the skeletal muscle (Fig. (A) and (B)). In contrast, phospho-4EBP (γ form) level was markedly reduced in the skeletal muscle of PGC-1α KO mice (Fig. (A) and (B)). In the liver, leucine administration increased phospho-4EBP level (γ form) in both WT and PGC-1α KO mice (Fig. (C) and (D)). Thus, PGC-1α is involved in leucine-mediated mTOR activation and possibly in protein biosynthesis.
Fig. 2. Phospho-4EBP levels in the skeletal muscle and liver of mice following leucine administration.
Author contributions
R.Y., K.M., S.M., and Y.K. designed the research; R.Y. and K.M. performed the research; R.Y., K.M., S.M., and Y.K. wrote the manuscript; J.M. and N.S. participated in critical animal experiments; All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Council for Science, Technology and Innovation (CSTI), Cross-ministerial Strategic Innovation Promotion Program (SIP), and “Technologies for creating next-generation agriculture, forestry and fisheries” (funding agency: Bio-oriented Technology Research Advancement Institution, NARO). This work was also supported by grants-in-aid for scientific research (KAKENHI) from the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT, Tokyo), and The Tojuro Iijima Foundation for Food Science and Technology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
Histological microscopic image analysis was, in part, performed at the Medical Research Support Center, Graduate School of Medicine, Kyoto University.
References
- Yoshizawa F. Regulation of protein synthesis by branched-chain amino acids in vivo. Biochem. Biophys. Res. Commun. 2004;313:417–422.
- Yoshizawa F, Mochizuki S, Sugahara K. Differential dose response of mTOR signaling to oral administration of leucine in skeletal muscle and liver of rats. Biosci. Biotechnol. Biochem. 2013;77:839–842.
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484.
- Yanagiya A, Suyama E, Adachi H, et al. Translational homeostasis via the mRNA cap-binding protein, eIF4E. Mol. Cell. 2012;46:847–858.
- Anthony JC, Yoshizawa F, Anthony TG, et al. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000;130:2413–2419.
- Anthony JC, Reiter AK, Anthony TG, et al. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes. 2002;51:928–936.
- Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801.
- Tadaishi M, Miura S, Kai Y, et al. Skeletal muscle-specific expression of PGC-1α-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS ONE. 2011;6:e28290.
- D'Antona G, Ragni M, Cardile A, et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12:362–372.
- Cunningham JT, Rodgers JT, Arlow DH, et al. mTOR controls mitochondrial oxidative function through a YY1–PGC-1α transcriptional complex. Nature. 2007;450:736–740.
- Sawada N, Jiang A, Takizawa F, et al. Endothelial PGC-1α mediates vascular dysfunction in diabetes. Cell Metab. 2014;19:246–258.
- Brennan KJ, Hardeman EC. Quantitative analysis of the human alpha-skeletal actin gene in transgenic mice. J. Biol. Chem. 1993;268:719–725.
- Trounce IA, Kim YL, Jun AS, et al. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509.
- Miura S, Tomitsuka E, Kamei Y, et al. Overexpression of peroxisome proliferator-activated receptor γ co-activator-1α leads to muscle atrophy with depletion of ATP. Am. J. Pathol. 2006;169:1129–1139.
- Kamei Y, Hattori M, Hatazawa Y, et al. FOXO1 activates glutamine synthetase gene in mouse skeletal muscles through a region downstream of 3′-UTR: possible contribution to ammonia detoxification. Am. J. Physiol. Endocrinol. Metab. 2014;307:E485–E493.
- Crumbley C, Wang Y, Banerjee S, et al. Regulation of expression of citrate synthase by the retinoic acid receptor-related orphan receptor α (RORα). PLoS ONE. 2012;7:e33804.
