Abstract
Lactoferrin (LTF), a multifunctional glycoprotein of the transferrin family mainly found in exotic secretions in mammals, is an important defense molecule against not only microbial invasion but also tumors. It folds into two globular domains (N- and C-lobes) each containing an iron-binding site. The cationic antimicrobial peptide in N-lobe is known to exert anti-tumor effect via a non-receptor-mediated pathway. However, whether LTF C-lobe also contributes to its anti-tumor activity remains to be investigated. In this study, a human LTF fragment (amino acid residues 343–682) covering the C-lobe was expressed with a histidine tag in E. coli and the purified polypeptide refolded through a series of buffer changing procedure. The resultant recombinant protein caused significant growth arrest of breast carcinoma cells MDA-MB-231 in a dose- and time-dependent manner, evidently via induction of apoptosis of the cell. Our data suggest a positive role for the C-lobe of human LTF in controlling tumors in vitro.
Graphical abstract
Recombinant human lactoferrin C-lobe exhibited significant inhibitory effect on the growth of MDA-MB-231 cells and morphological changes in a dose and time-dependent manner.
Key words:
Lactoferrin (LTF), an 80 kDa single-chain iron-binding glycoprotein of the transferrin family, is found in milk and other biological fluids of mammals as well as in the secondary granules of neutrophils.Citation1) Since LTF by far did not show any toxicity and it is a major component of the mammalian innate immune system and possesses many biological functions (antimicrobial, anti-inflammatory, and anti-cancer activities), it is often used as a food additive in yogurt, infant formulas, and cosmetics.Citation2–6) Results from in vivo and in vitro studies indicate that the anti-tumor effect of LTF is attributed mostly to its ability to inhibit tumor cell proliferation,Citation7,8) enhance apoptosis or necrosis in cancer cells,Citation2,9–11) inhibit angiogenesis,Citation7) increase IL-18 production,Citation5) and regulate NK cell activity.Citation4) Strong protective effects of LTF against chemically induced carcinogenesis, tumor growth, and metastasis in animal experiments endorse its great potential therapeutic use in cancer disease prevention and treatments.Citation12–14)
LTF folds into 2 homologous lobes comprising amino acid residues 1–334 (N-lobe) and 348–684 (C-lobe) with a three-turn α-helix (amino acid residues 335–347) linkage, and each lobe contains a single Fe3+ ion-binding site in a deep cleft.Citation15,16) The two lobes share over 40% homology in amino acid sequence and adopt similar secondary and tertiary structures.Citation11) Much progress has been made on the biological function of the N-lobe in the previous decades, which has the ability to sequester iron required for bacterial growth and also cause direct bactericidal effect via lactoferricin (a cationic antimicrobial peptide, a loop of 18 amino acid residues formed by a disulfide bond between cysteine residues 20 and 37). Lactoferricin can also act against cancer cells via a non-receptor-mediated pathway, resulting in lysis of tumor cells.Citation17–19) Recent reports suggest that LTF C-lobe also exhibits multiple functions in vivo. For instances, the C-lobe of bovine LTF (boLTF) is protective against various disorders including gastropathy,Citation18) diabetes,Citation19) and corneal wounds and injuries.Citation20) BoLTF C-lobe is almost twice as effective as native LTF or the N-lobe in corneal epithelial wound closure, suggestive of a novel treatment for corneal lesions with delayed healing.Citation21) However, whether LTF C-lobe possesses any anti-tumor effect remains unclear. In this study, we expressed a recombinant fragment (amino acid residues 343–682) covering human LTF-C lobe (huLTF-C) in E. coli and tested its anti-tumor activity on a human breast cancer cell line MDA-MB-231. The results point to a potential role for this multifunctional primary defense domain in protection against cancer.
Materials and methods
Proteins, antibodies, and cell line
Rabbit polyclonal Abs specific for human LTF (L3262) and LTF from bovine and human milk were purchased from Sigma (St. Louis, Mo.). Caspase-3 and β-actin antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). HRP-conjugated goat anti-rabbit or mouse IgG were purchased from Southern Biotech (Birmingham, USA). Human leukocytes were collected from healthy volunteers and this study has been reviewed and approved by the ethics committee of Soochow University. Human breast carcinoma cells MDA-MB-231 were kindly provided by Dr Yunseng Li (Institute of Biology and Medical Sciences, Soochow University).
Construction of expression vectors
Total cellular RNA was extracted from human leukocytes (from the volunteer) using Trizol (TaKaRa, Japan) and then reverse transcribed using a TaKaRa reverse transcriptase kit according to the manufacturer’s instructions. The resultant cDNA was employed as template for amplification of the gene encoding human LTFC-lobe by PCR using forward primer 5′-AATAAGGATCCCGTGCGCGGGTCGTGTGGT-3′ and reverse primer 5′-CCCAAGCTTTTAGGGGGAGGTTGAGCACTTTTTC-3′. The DNA product, verified by sequencing, was inserted into a pET28a expression vector (Novagen, Darmstadt, Germany).
Recombinant protein production and purification
Freshly transformed Escherichia coli BL21 (DE3) cells harboring plasmid pET28a-huLTF-C were cultured in LB medium with 25 μg/mL kanamycin at 37 °C, and 0.1 mM isopropyl-β-D-thiogalactopyranoside (IPTG, Sigma) was added as the cell density reached 0.8–1.0 (OD600 nm). After additional 4-h culture at 37 °C, the bacteria were harvested through centrifuging and suspended in 100 mL buffer A (50 mM Tris–HCl, 1 mM EDTA, 10 mM NaCl, pH 7.0). After sonication, the lysed cells were spun down and resuspended in 25 mL buffer B (2.5 M NaCl, 0.5% Triton X-100). After centrifuging at 5000g for 10 min at 4 °C, the pellet was resuspended in 25 mL buffer C (50 mM Tris–HCl, 2 mM EDTA, 2 M urea, pH 8.7) and centrifuged again. The purified inclusion bodies were solubilized in defined amount buffer D (25 mM Tris–HCl, 10 mM EDTA, 6 M guanidine hydrochloride, pH 8.7) through overnight incubation at 4 °C. After centrifuging at 10,000 g for 10 min, the supernatant was added with 10 mM 2-ME (Sigma) and incubated at 4 °C for 1.5 hr, then buffer E (25 mM Tris–HCl, 10 mM EDTA, 3 M urea, 10 mM GST, 0.4 M L-arginine, pH 8.7) was added drop to drop with constant agitation overnight at 4 °C. The solution was dialyzed with Tris–NaCl buffer (pH 8.0) containing gradient concentration urea (3, 2.5, 2, 1.5, 0.75, 0.5, 0.25 M, exchanged sequentially every 2 h), and finally dialyzed in PBS. To purify the refolded protein, the supernatant was incubated with 2 mL Ni Sepharose (GE Healthcare, Sweden) at 4 °C for 1hr, and the Sepharose was poured into a column and washed with 100 mL wash buffer (500 mM NaCl, 20 mM Tris–HCl, 20 mM imidazole, pH 7.9), then the recombinant protein was eluted with elute buffer (500 mM NaCl, 20 mM Tris–HCl, 500 mM imidazole, pH 7.9). The final products were dialyzed with PBS (pH 7.2) and store at −20 °C before use.
Western blot analysis
The separated protein bands in SDS-PAGE gels were electroblotted onto polyvinylidene difluoride (PVDF) membranes using 25 mM Tris–HCl, 192 mM glycine (pH 8.3) buffer containing 20% methanol, for 3 h at 250 mA. The strips were blocked for 1 h at room temperature with PBS containing 5% defatted dry milk and 0.5% Tween 20 (blocking buffer), followed by an overnight incubation at 4 °C with constant agitation in a 1/1000 dilution of rabbit anti-human LTF polyclonal Abs L3262 (Sigma) or monoclonal mouse anti-His-tag antibody (Sigma) in blocking buffer. After four washes with TBS containing 0.05% Tween 20, strips were incubated for 1 h with HRP-conjugated goat anti-rabbit or anti-mouse IgG (Southern Biotech) and the Ag–Ab complexes were visualized using the ECL detection system.
ELISA
Polyvinyl ELISA plates were coated overnight at 4 °C with 0.05 μM rhuLTF-C, huLTF, bovine LTF (bLTF), or OVA in carbonate buffer (pH 9.6) and subsequently incubated with 2% BSA in PBS for 2 h at 37 °C. The wells were washed five times with PBS containing 0.05% Tween 20 (PBST) and then 100 μL of diluted rabbit anti-human LTF polyclonal Abs L3262 or immunized sera were added in duplicates and incubated for 2 h at 37 °C. After five washes with PBST, the plates were incubated with HRP-labeled goat anti-rabbit or mouse IgG Abs (Southern Biotech) for 1 h at 37 °C. The reaction was developed with 100μL of O-phenylenediamine (OPD) (Sigma) for 5 min and stopped with 50μL of 2 M H2SO4. OD was measured at 492 nm in an ELISA spectrophotometer (Synergy H4 Hybrid Reader, BioTek).
Immunization of animals
Female BALB/c mice of 6–8 weeks of age were purchased from the Model Animal Research Center, Nanjing, China. All animals were maintained under specified-pathogen-free conditions and animal usage was conducted according to protocols approved by the Soochow University Institutional Animal Care and Use Committee. The mice were s.c. immunized with rhuLTF-C, rhuLTF, or OVA (100 μg/mouse) in complete Freund’s adjuvant (CFA, Sigma) at the base of the tail. The booster immunization was given on day 7 and 14 with Ag mixed with incomplete Freund’s adjuvant (IFA). Mouse blood was collected by tail bleeding on day 0, 7, 14, 21, and the serum samples were kept at −80 °C until use.
Cell proliferation assay
Cell viability was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. MDA-MB-231 cells were seeded in 96-well plates at a final density of 5 × 103 cells per well, treated with different concentrations (0, 0.38, 0.75, 1.5, 3 μM) of rhuLTF-C or huLTF for 48 h, using rEGFP as a negative control, and then 20 μL of MTT was added to each well and incubated for 4 h at 37 °C. Absorbance values were measured at 492 nm with an automated plate reader (Synergy H4 Hybrid Reader, Biotech). We also examined the growth curves of MDA-MB-231 cells, following incremental increases in concentration of rhuLTF-C, huLTF, or rEGFP at 1.5 μM for 24, 48, and 72 h. The results of the cell viability assay in three independent experiments (3 wells per condition) were normalized to the medium control group and expressed as mean ± SD.
FITC labeling of proteins
Protein was labeled by FITC using FluoroTag™ FITC Conjugation Kit (Sigma, US) according to the manufacturer’s instructions. In brief, 1 mg of proteins including rhuLTF-C, huLTF, and OVA was dialyzed with 0.1 M Na2CO3 (pH 9.5) at 5 mg/mL and then was incubated with 200 μg/mL Fluorescence isothiocyanate (FITC, Sigma). After gently mixed for 2 h at room temperature, separation of labeled protein from unbound FITC was performed on a Sephadex G-25 column.
Flow cytometric analysis
In order to detect binding of rhuLTF-C to cells, MDA-MB-231 cells were incubated in the presence of FITC-rhuLTF-C, FITC-huLTF, or FITC-OVA (10 μg/mL) for 30 min at 4 °C, and then followed by flow cytometric analysis. For rhuLTF-C-induced apoptosis of MDA-MB-231 cells, MDA-MB-231 cells were collected after treatment with rhuLTF-C, huLTF, or rEGFP at 1.5 μM for 48 h. After washing with Annexin V binding buffer (eBioscience), the pellets (1 × 106cells/tube) were incubated for 15 min at room temperature away from light with 1 μL of PE-conjugated Annexin V and 2 μL of 7-AAD. And then the cells were subjected to analysis using a fluorescence-activated cell sorter (FACS cantoII; BD Biosciences).
Statistical analysis
All experiments were repeated at least three times, and the results are expressed as mean ± SD. Statistical analysis was performed using the independent samples t-test or two-sided, paired t-test between groups using the Graphpad Prism 5. Differences were considered statistically significant at p < 0.05.
Results
Expression, purification, and refolding of the rhuLTF-C fragment
An expression vector, pET28-huLTF-C, encoding huLTF C-lobe (343–682aa) with a histidine tag was constructed and transfected into E. coli strain BL21. After 4 h IPTG induction at 37 °C, over-expressed recombinant product was found mainly in inclusion bodies. The inclusion bodies were solubilized in 6 M guanidine hydrochloride and then refolded in the refolding solution and gradient dialysis procedure. Next the refolded protein was affinity purified using a Ni column. Samples of the bacterial lysates as well as the purification products were subjected to SDS-PAGE and Western blot analysis using mAb against His-tag or polyclonal rabbit antibodies against huLTF (L3262), which confirmed that a His-tag-containing and L3262-specific 39 kDa recombinant protein of up to 85% homogeneity was successfully prepared (Fig. (a)–(c)). This purification and refolding procedure allowed production of rhuLTF-C fragment in soluble form at 700 μg/mL.
Fig. 1. Expression and characterization of the rhuLTF-C fragment.
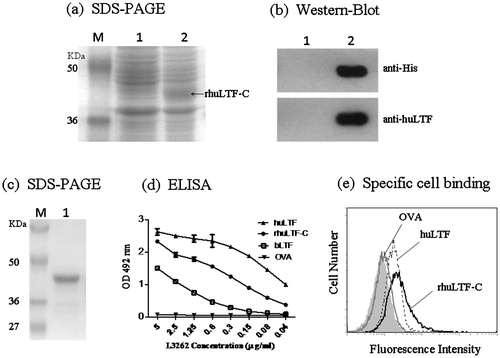
It is well established that the rabbit antibody L3262 recognize native (correctly folded), but not denatured (unfolded), huLTF. Fig. (d) clearly shows specific recognition of rhuLTF-C by L3262 in ELISAs, supporting the notion that rhuLTF-C adopted a three-dimensional structure similar to native protein. Also note that rhuLTF-C was better recognized than full-length native bovine LTF by L3262 in ELISAs (homology between human and bovine LTF is over 70%). Native huLTF can bind to cell surface via various LTF receptors.Citation22) To test whether rhuLTF-C was also able to do the same, huLTF, rhuLTF-C, and OVA were labeled with FITC using a FITC labeling kit and then employed to stain MDA-MB-231 cells for flow cytometric analysis. Like native huLTF, rhuLTF-C, but not OVA, bound to MDA-MB-231 cells with relatively high affinity (Fig. (e)). These results indicate that the refolded huLTF-C product can be used for functional assays.
Immunogenicity of the rhuLTF-C fragment
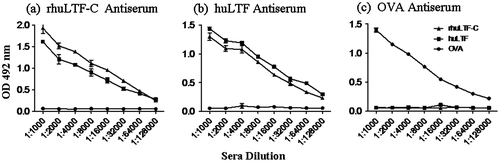
In order to assess the immunogenicity of rhuLTF-C, BALB/c mice were s.c. immunized with rhuLTF-C, or native huLTF, or OVA in CFA followed by a booster immunization with the immunogens in IFA. Serum samples were collected 7 days thereafter and analyzed for LTF-specific IgG Abs using ELISAs. Strong IgG responses against the immunizing antigens were induced by all the three protein preparations in mice. Moreover, IgG Abs elicited by rhuLTF-C and native huLTF effectively cross-recognized each other but not OVA. OVA-induced Abs recognized neither huLTF nor rhuLTF-C in parallel experiments (Fig. ).
Fig. 2. Antigenicity and immunogenicity of the rhuLTF-C fragment.
Inhibitory effect of rhuLTF-C on human breast carcinoma cells
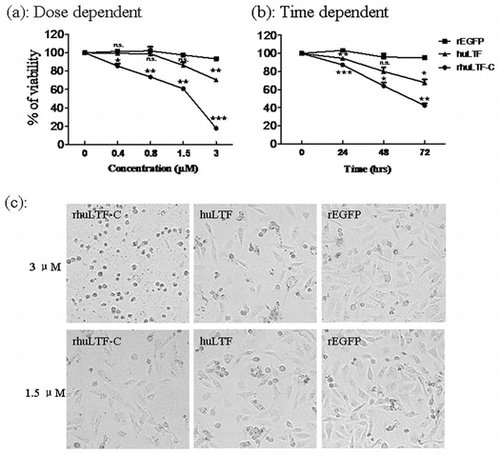
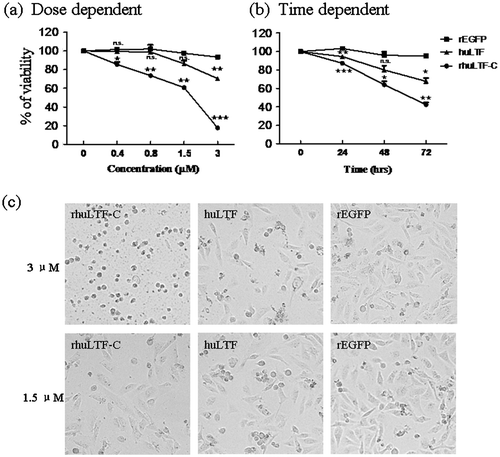
Native huLTF is known to be able to inhibit tumor cell growth both in vitro and in vivo.Citation2,23,24) We compared rhuLTF-C and native huLTF for ability to inhibit the growth of MDA-MB-231 cells in MTT assays, using soluble recombinant EGFP (rEGFP) as control. Fig. (a) shows that both huLTF and rhuLTF-C, but not rEGFP, exhibited significant inhibitory effect on the proliferation of MDA-MB-231 cells in a dose-dependent manner (up to 3 μM). Time course of LTF-mediated suppression on MDA-MB-231 cells, shown in Fig. (b), further endorses rhuLTF-C as an effective inhibitor of cancer cell growth in vitro. Note that rhuLTF-C was more effective than native huLTF in these experiments. The above observation is further confirmed by morphological changes (apparent shrinkage and cytoclasis) of MDA-MB-231 cells after a 48-h treatment with 3 μM rhuLTF-C (Fig. (c)). The cell cycle stages of the treated cells were evaluated with flow cytometry after 20 min staining with PI solution, and there was no significant difference among these groups (not shown).
Fig. 3. rhuLTF-C fragment-induced MDA-MB-231 cells growth inhibition in dose- and time-dependent manner.
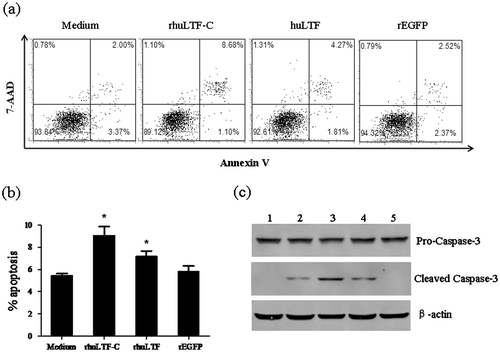
Apoptosis of MDA-MB-231 cells after rhuLTF-C treatment
One of the pathways for protein molecules to cause tumor cell damage is apoptosis induction via surface receptors. MDA-MB-231 cells that had been treated for 48 h with native huLTF, rhuLTF-C, or rEGFP were double stained with Annexin V and 7-AAD followed by FACS analysis. Fig. (a) and (b) show that percentage of Annexin V+ (7-AAD+ and 7-AAD−) subset (representing cells underwent programmed cell death) in the rhuLTF-C group (9.1 ± 0.8%) was significantly more than that in the huLTF (7.2 ± 0.5%) and rEGFP (5.5 ± 0.2%) groups. Moreover, the apoptotic subset was similarly targeted with both dyes, indicating that phosphatidylserine exposure was related to the loss of membrane integrity. The above data are further supported by dose-dependent acceleration of caspase-3 cleavage in MDA-MB-231 treated with rhuLTF-C or huLTF (Fig. (c)).
Fig. 4. The rhuLTF-C induced apoptosis in MDA-MB-231 cells.
Discussion
Preparation of correctly folded and soluble recombinant protein is of great importance for studying its biological function. In this study, we expressed a rhuLTF-C fragment in Escherichia coli, where it was mainly expressed in inclusion bodies. After purification and refolding procedures, rhuLTF-C with good solubility, correct folding, proper antigenicity/immunogenicity was obtained. Recombinant proteins produced in E. coli always contain bacterial-derived substances, especially lipopolysaccharide (LPS). It is well known that lactoferrin is a strong LPS-binding protein with two binding sites consisted of basic arginine- and lysine-rich sequences (2RRRR5 and 28RKVRGPP34), which are close and accessible at the molecular surface.Citation25,26) To exclude the possibility that rhuLTF-C had been contaminated with LPS, human monocytes from peripheral blood were cocultured with rhuLTF-C (30 μg/mL) for 48 h and TNF-α production was quantitated using ELISAs. Our results showed that rhuLTF-C could not induce monocytes activation (not shown), implying that LPS contamination in rhuLTF-C was negligible.
The main finding of this study is that rhuLTF-C exhibited potent tumoricidal effect, but without any significant inhibitory effect on primary human monocytes under similar conditions (data not shown). It inhibited MDA-MB-231 cell proliferation in a time- and dose-dependent manner, associated with induction of tumor cell apoptosis, as evidenced by increased Annexin V positive staining and cleaved caspase-3 expression (Fig. ), but not cell cycle arrest (data not shown). The more potent anti-tumor effect of rhuLTF-C compared to full-length native huLTF could be attributed to steric factors or greater substrate affinity, underlined by our observation that rhLTF-C gave better binding to MDA-MB-231 cells than huLTF in flow cytometry analysis (Fig. (e)), albeit further study is needed to (i) identify the rhuLTF-C-binding receptor molecules on the surface of MDA-MB-231 cells and (ii) delineate the actual affinity between rhuLTF-C and its receptors. Alternatively, attraction of the cationic arginines near the N-terminal of LTF to ubiquitous anionic substrates, such as glycosaminoglycans (GAGs),Citation27) might inhibit the LTF availability for the targeting sites on tumor cells. This hypothesis is supported by the fact that boLTF lacking N-terminal binds with higher affinity to a smaller number of sites on hepatocytes than native boLTF.Citation20,28)
It has been reported that Lactoferricin shows anti-tumor function via a non-receptor-mediated pathway.Citation17–19) The possibility that rhuLTF-C can also act against cancer cells in a similar fashion cannot be excluded. Although in this study rhuLTF-C was not tested on another cancer cell line, previous work showed that full-length lactoferrin was suppressive against various cancer lines such MCF-7 and EMT6 (breast cancer), OSCC (oral squamous cell carcinoma) and NPC (nasopharyngeal carcinoma),Citation2,29,30) implying a wide spectrum of cancer cells susceptible to rhuLTF-C suppression.
Taken together, our recombinant huLTF-C fragment provides a useful tool for further investigation on the molecular mechanisms of the anti-tumor protective effect of LTF, thereby allowing new insights in our understanding on the immunobiological functions of LTF. The role of rhuLTF-C toward tumors as well as the mechanisms involved necessitates in-depth studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the grants from National Foundation of Natural Science of China [grant number 31070781/30890142/31300738/31370871]; PCSIRT [grant number IRT1075].
Notes
Abbreviations: huLTF, human lactoferrin; rhuLTF-C, recombinant human lactoferrin C-lobe; rEGFP, recombinant enhanced GFP.
References
- Gonzalez-Chavez SA, Arevalo-Gallegos S, Rascon-Cruz Q. Lactoferrin: structure, function and applications. Int. J. Antimicrob. Agents. 2009;33:e301–e308.
- Wang J, Li Q, Ou Y, Han Z, et al. Inhibition of tumor growth by recombinant adenovirus containing human lactoferrin through inducing tumor cell apoptosis in mice bearing EMT6 breast cancer. Arch. Pharm. Res. 2011;34:987–995.
- Yamada Y, Sato R, Kobayashi S, et al. The antiproliferative effect of bovine lactoferrin on canine mammary gland tumor cells. J. Vet. Med. Sci. 2008;70:443–448.
- Kuhara T, Yamauchi K, Tamura Y, et al. Oral administration of lactoferrin increases nk cell activity in mice via increased production of IL-18 and type I IFN in the small intestine. J. Interferon. Cytokine Res. 2006;26:489–499.
- Kuhara T, Iigo M, Itoh T, et al. Orally administered lactoferrin exerts an antimetastatic effect and enhances production of IL-18 in the intestinal epithelium. Nutr. Cancer. 2000;38:192–199.
- Chandra Mohan KV, Kumaraguruparan R, Prathiba D, et al. Modulation of xenobiotic-metabolizing enzymes and redox status during chemoprevention of hamster buccal carcinogenesis by bovine lactoferrin. Nutrition. 2006;22:940–946.
- Shimamura M, Yamamoto Y, Ashino H, et al. Bovine lactoferrin inhibits tumor-induced angiogenesis. Int. J. Cancer. 2004;111:111–116.
- Lee SH, Park SW, Pyo CW, et al. Requirement of the JNK-associated Bcl-2 pathway for human lactoferrin-induced apoptosis in the Jurkat leukemia T cell line. Biochimie. 2009;91:102–108.
- Xiao Y, Monitto CL, Minhas KM, et al. Lactoferrin down-regulates G1 cyclin-dependent kinases during growth arrest of head and neck cancer cells. Clin. Cancer Res. 2004;10:8683–8686.
- Damiens E, El Yazidi I, Mazurier J, et al. Lactoferrin inhibits G1 cyclin-dependent kinases during growth arrest of human breast carcinoma cells. J. Cell Biochem. 1999;74:486–498.
- Vogel HJ. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. 2012;90:233–244.
- Sheth B, Stowell KM, Day CL, et al. Cloning and expression of the C-terminal lobe of human lactoferrin. Adv. Exp. Med. Biol. 1994;357:259–263.
- Lizzi AR, Carnicelli V, Clarkson MM, et al. Lactoferrin derived peptides: mechanisms of action and their perspectives as antimicrobial and antitumoral agents. Mini. Rev. Med. Chem. 2009;9:687–695.
- Puddu P, Latorre D, Valenti P, et al. Immunoregulatory role of lactoferrin-lipopolysaccharide interactions. BioMetals. 2010;23:387–397.
- Sinha M, Kaushik S, Kaur P, et al. Antimicrobial lactoferrin peptides: the hidden players in the protective function of a multifunctional protein. Int. J. Pept. 2013;2013: 390230.
- Takayama Y, Mizumachi K, Takezawa T. The bovine lactoferrin region responsible for promoting the collagen gel contractile activity of human fibroblasts. Biochem. Biophys. Res. Commun. 2002;299:813–817.
- Rahman MM, Kim WS, Ito T, et al. Growth promotion and cell binding ability of bovine lactoferrin to Bifidobacterium longum. Anaerobe. 2009;15:133–137.
- Mir R, Singh N, Vikram G, et al. The structural basis for the prevention of nonsteroidal antiinflammatory drug-induced gastrointestinal tract damage by the c-lobe of bovine colostrum lactoferrin. Biophys. J. 2009;97:3178–3186.
- Mir R, Kumar RP, Singh N, et al. Specific interactions of C-terminal half (C-lobe) of lactoferrin protein with edible sugars: binding and structural studies with implications on diabetes. Int. J. Biol. Macromol. 2010;47:50–59.
- Ashby B, Garrett Q, Willcox M. Bovine lactoferrin structures promoting corneal epithelial wound healing in vitro. Invest. Ophthalmol. Vis. Sci. 2011;52:2719–2726.
- Sharma S, Sinha M, Kaushik S, et al. C-lobe of lactoferrin: the whole story of the half-molecule. Biochem. Res. Int. 2013;2013: 271641.
- Legrand D, Elass E, Carpentier M, et al. Interactions of lactoferrin with cells involved in immune function. Biochem. Cell Biol. 2006;84:282–290.
- Artym J. Antitumor and chemopreventive activity of lactoferrin. Postepy. Hig. Med. Dosw. (Online). 2006;60:352–369.
- Zhang Y, Lima CF, Rodrigues LR. Anticancer effects of lactoferrin: underlying mechanisms and future trends in cancer therapy. Nutr. Rev. 2014;72:763–773.
- Elass-Rochard E, Roseanu A, Legrand D, et al. Lactoferrin-lipopolysaccharide interaction: involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem. J. 1995;312:839–845.
- Van Berkel PH, Geerts ME, Van Veen HA, et al. N-terminal stretch Arg2, Arg3, Arg4 and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem. J. 1997;328:145–151.
- Andersen JH, Jenssen H, Sandvik K, et al. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J. Med. Virol. 2004;74:262–271.
- Ziere GJ, Kruijt JK, Bijsterbosch MK, et al. Recognition of lactoferrin and aminopeptidase M-modified lactoferrin by the liver: involvement of proteoglycans and the remnant receptor. Biochem. J. 1996;313:289–295.
- Zhou Y, Zeng Z, Zhang W, Wu M, et al. Lactotransferrin: a candidate tumor suppressor-deficient expression in human nasopharyngeal carcinoma and inhibition of npc cell proliferation by modulating the mitogen-activated protein kinase pathway. Int. J. Cancer. 2008;123:2065–2072.
- McKeown ST, Lundy FT, Nelson J, et al. The cytotoxic effects of human neutrophil peptide-1 (HNP1) and lactoferrin on oral squamous cell carcinoma (OSCC) in vitro. Oral Oncol. 2006;42:685–690.
