Abstract
Members of the genus Bacillus are considered to be both, among the best studied and most commonly used bacteria as well as the most still unexplored and the most wide-applicable potent bacteria because novel Bacillus strains are continuously being isolated and used in various areas. Production of optically pure l-lactic acid (l-LA), a feedstock for bioplastic synthesis, from renewable resources has recently attracted attention as a valuable application of Bacillus strains. l-LA fermentation by other producers, including lactic acid bacteria and Rhizopus strains (fungi) has already been addressed in several reviews. However, despite the advantages of l-LA fermentation by Bacillus strains, including its high growth rate, utilization of various carbon sources, tolerance to high temperature, and growth in simple nutritional conditions, it has not been reviewed. This review article discusses new findings on LA-producing Bacillus strains and compares them to other producers. The future prospects for LA-producing Bacillus strains are also discussed.
Graphical abstract
New application of Bacillus strains for optically pure l-lactic acid production.
Genus Bacillus is Gram staining-variable (Gram-positive, Gram-positive in the early stages of growth, or Gram-negative), endospore-forming, aerobic or facultative anaerobic rods with a wide range of nutritional requirements (simple to complex), growth conditions (ranging from acidophilic to alkaliphilic, psychrophilic to thermophilic, and halotolerant), DNA base composition, and metabolic diversity.Citation1) Many Bacillus strains have been widely used as producers of fermented foods (e.g. natto in Japan),Citation2) enzymes (e.g. amylases, pullulanases, and proteases),Citation3) purine nucleotides,Citation4) biopesticides,Citation5) poly-(γ-glutamic acid),Citation6) l-lactic acid,Citation7), etc. including probiotics for animals and humans,Citation8) promoters of plant growth,Citation9), and biocontrollers of plant diseaseCitation10) in the broad areas of food, cosmetics, medicine, agriculture, and chemistry. The first Bacillus strain was discovered in 1872Citation1), and new Bacillus strains have been continuously isolated from different sources.Citation11) The number of Bacillus strains is presently around 300 species (http://www.bacterio.net/bacillus.html). This strongly encourages researchers around the world to screen for novel Bacillus isolates and to determine new functions or applications. Among the ca. 2277 total genera in the domain bacteria (http://www.bacterio.net), those of the genus Bacillus can be considered to not only be among the best studied and most commonly used, but also the most still unexplored and most wide-applicable potent bacteria for human life. Recently, production of optically pure lactic acid (LA) has attracted attention as a feasible application of Bacillus strains.Citation7)
LA has applications in the agricultural, medicinal, food, pharmaceutical, and chemical industries,Citation12–14) in particular, there is much interest in optically pure (l or d-isomer) LA for the synthesis of high-quality polylactic acid (PLA). PLA is used for the production of biodegradable and environmentally friendly bio-plastics as a substitute for petrochemical-based plastics.Citation15) Due to the numerous applications for this material, it has been estimated that global LA production demand is 130,000–150,000 tons per year.Citation16) Currently, LA is mainly produced by microbial fermentation processes rather than by traditional chemical synthesis. The traditional chemical synthesis of LA is based on the hydrolysis of lactonitrile derived from acetaldehyde and hydrogen cyanide, which leads to the formation of racemic mixtures of dl-LA.Citation12) Optically pure d-LA or l-LA can only be produced by microbial fermentation using specific microbes.Citation17–19) Another significant advantage of microbial fermentation over traditional LA chemical synthesis is that various plant-derived renewable biomass sources can be used as substrates for LA production by microbial fermentation.
In general, fermentative LA production is mostly accomplished by members of the domain bacteria. A few micro-organisms in the domains archaea (Scenedesmus sp. and Nannochlorum sp.) and eukaryota (fungi such as Rhizopus oryzae) have been shown to produce LA.Citation7) Within the domain bacteria, all the wild-type LA producers belong to the phylum Firmicutes and the class Bacilli that includes two orders: the Lactobacillales and Bacillales. The order Lactobacillales contains lactic acid bacteria (LAB), which are known as LA producers, including the genera Lactobacillus (l-, d- or dl-LA), Enterococcus (l-LA), Lactococcus (l-LA), Carnobacterium (d-LA), Pediococcus (l- or dl-LA), Leuconostoc (d-LA), Streptococcus (l-LA), Weissella (d- or dl-LA) and so on. On the other hand, within the order Bacillales and the family Bacillaceae (48 genera to date), most bacteria in the genus Bacillus have been reported as potent l-LA producers. Only two species of genus Geobacillus (G. stearothermophilus)Citation20) and Halolactibacillus (H. halophilus)Citation21) have been reported as l-LA producers. In the domain Eukaryota, several wild-type fungi, especially R. oryzaeCitation22) and Rhizopus microsporus,Citation23) are known as potent l-LA producers. In addition, some genetically modified bacteria and yeast have been developed for either d-LA or l-LA production depending upon the genetic manipulation.Citation24) In the future, approaches to screen for new wild-type LA-producers and to genetically modify LA producers for targeted processes will be performed actively and continuously.
Fermentative LA production by LABCitation25,26) and fungiCitation22) have been already reviewed and their recent issues have been addressed. In contrast, despite their interesting features such as high growth rates, tolerance to high temperatures, growth in simple nutritional conditions, and the ability to ferment a wide range of sugars including pentoses and hexoses to produce optically pure l-lactic acid (up to 100%), the recent developments in LA fermentation by Bacillus strains have not yet been reported.Citation27) Therefore, in this review article, we will provide an overview of the special features of LA-producing Bacillus strains in comparison to other LA producers, report the efficient utilization of various biomass resources by Bacillus strains, elucidate the major metabolic pathways involved and the genetic approaches for improving LA production, as well as make concluding remarks and summarize the future prospects.
I. Special features of LA-producing Bacillus strains and comparison to other LA producers
Here, we compared special features (nutritional requirement, tolerance to high temperature, tolerance to pH, ability to produce optical pure LA, oxygen demand, and sugar metabolism) among Bacillus, LAB, and Rhizopus sp. (Table ), and described them in each section.
Table 1. Special features of LA-producing Bacillus strains and comparison to other LA producers.
I.i. Nutritional requirements
Bacillus strains are commonly isolated from nutritionally poor environments such as soil under various climatic conditions.Citation28) In contrast, LAB are only isolated from nutritionally rich environments that contain high amounts of amino acids, vitamins, and nucleotides in addition to a carbon source,Citation29,30) while Rhizopus sp. requires fewer nutrients for the growth compared to LAB and have the ability to grow in simple inorganic salt containing media.Citation22) Yeast extract and peptone are rich in nitrogen, vitamins, and other nitrogenous growth-stimulating factors. Culture media for LAB require supplementation with organic nitrogen sources such as yeast extract, peptone, and other proteinous substances, which ultimately increases the cost of the medium formulation.Citation15,28) Altaf et al.Citation31) reported that yeast extract accounted for ca. 38% of the total medium cost for LA production by LAB. Therefore, it is necessary to use cheap substitutes for these expensive substances or to employ LA producers that can grow well at low concentrations of organic nitrogen sources.
Interestingly, Bacillus strains profusely grow and produce l-LA in a simple mineral salt medium containing a very small amount of yeast extract.Citation32,33) Many researchers have already described the minimal nutritional requirements for growth and LA production by Bacillus strains.Citation33,34) For Bacillus strains, ammonium sulfate,Citation35) NH4Cl,Citation36) low grade peanut meal,Citation37) corn steep liquor,Citation38) excess sludge hydrolysate,Citation39) and yeast autolysateCitation32) have been reported as cheap alternative nitrogen supplements for LA fermentation. In addition, Meng et al.Citation37) reported that Bacillus sp. strain WL-S20 efficiently produced l-LA using inexpensive peanut meal as a nitrogen source and yields 20% higher LA concentration than that using yeast extract. Furthermore, among the several sugars (glucose, fructose, mannose, arabinose, xylose, lactose, sucrose, maltose, and starch), it is reported that glucose and sucrose exhibited the highest LA concentration of ca. 18 g/L by Bacillus sp. strain WL-S20.Citation37) Meantime, LA production by Bacillus sp. WL-S20 LA using various glucose concentrations (40, 60, 80, 100, 110, and 120 g/L) results in different LA concentrations (16, 17, 19, 17, 17, and 16 g/L, respectively) and LA yield (0.73, 0.85, 0.95, 0.74, 0.78, and 0.67 g/g, respectively) using the same strains.Citation37) These findings reveal that types of carbon sources affect LA production as well as sugar concentrations. On the other hand, Ma et al.Citation39) reported that LA production (98.1 g/L of l-LA, 0.98 g/g yield, 99.6% optical purity, 3.63 g/L h productivity) using 10 g/L excess sludge hydrolysate was comparable to LA production (98.9 g/L l-LA, 0.98 g/g yield, 99.8% optical purity, 3.92 g/L h productivity) using 2 g/L yeast extract by B. coagulans NBRC12583T. Therefore, it is better to develop microbial strains that can produce LA in a simple and nutritionally low medium.
I.ii. Tolerance to high temperature
LA-producing Bacillus strains show optimum growth temperatures between 45 and 60 °C and limited growth up to 70 °C, whereas the optimum growth temperatures for most LAB range from 30 to 43 °C.Citation27) As exceptions, the thermotolerant d-LA producer Lactobacillus delbrueckii subsp. lactis QU 41Citation40) and the l-LA producer Enterococcus faecium QU 50Citation41) are reported to be tolerant to temperatures up to 55 °C. In case of Rhizopus sp., optimum temperatures for LA production range from 27 to 35 °C, and the maximum temperature at 45 °C for cell growth is reported for Rhizopus oligosporus TISTR 3518.Citation22,23) Many thermotolerant LA-producing Bacillus strains have been isolated from various environments. For instance, Poudel et al.Citation33) isolated thermotolerant Bacillus sp. MC-07 from compost and produced l-LA directly from starch at 50 °C. High temperature LA fermentation has several advantages compared to mesophilic fermentation.
First, high-temperature fermentation would significantly reduce the cooling cost compared to that incurred using mesophiles, which require temperature control, facilitated by the inclusion of additional cooling systems to maintain growth during fermentation.Citation7) Second, high-temperature fermentation minimizes contamination; B. coagulans JI12 produced 137.5 g/L l-LA with a high yield of 0.98 g/g, productivity of 4.4 g/L h, and optical purity of 99.6% from d-xylose at 50 °C even under non-sterile conditions.Citation42) Third, the fermentation temperature affects microbial activity and substrate conversion rate.Citation43) Heriban et al.Citation32) reported that a high temperature is beneficial for the synthesis of LA, as it improves the rate of the biochemical reactions and results in the higher activity of micro-organisms. Fourth, a high temperature is beneficial for simultaneous saccharification and fermentation (SSF) because several hydrolytic enzymes (cellulase, amylase) exhibit optimum temperatures higher than ca. 50 °C.Citation39,44) According to Ou et al.Citation44), thermophilic B. coagulans 36D1 (ca. 26.99 g/L LA and 0.903 g/g LA yield) showed better LA production performance than did mesophilic LAB (Lactococcus lactis subsp. lactis NRRL B-4449; ca. 20.34 g/L LA and 0.709 g/g LA yield) by SSF of 40 g/L crystalline cellulose carried out using 20 FPU-cellulase/g-cellulose at 50 and 40 °C, respectively. Therefore, high-temperature fermentation minimizes cooling cost, has no need for sterilization, and improves the efficiency of enzymatic hydrolysis in SSF.
I.iii. Tolerance to pH
One of the key parameters in LA fermentation is pH. Similar to LAB,Citation7) Bacillus strains also show an optimum pH for growth or LA production at neutral, but are sensitive to acidic pH (under pH 5.0). The pH for LA production by Bacillus strains is 5.0–9.0, whereas the pHs for LA production by most of LAB and Rhizopus species are 4.0–7.0Citation16,26,45) and 5.0–6.0,Citation22) respectively. The ability of some Bacillus (tolerance up to pH 10.0 with Bacillus sp. WL-S20) and LAB (tolerance up to pH 10.8 with Alkalibacterium olivoapovliticus)Citation46) strains to tolerate alkaline pH can minimize contamination.Citation21) At pH 9.0, Bacillus sp. WL-S20Citation37) and H. halophilusCitation21) efficiently produced l-LA (225 g/L, optical purity >99%, yield 0.99 g/g, productivity 1.04 g/L h; 65.8 g/L, optical purity of 98.3%, yield 0.76 g/g, productivity 0.83 g/L h, respectively) from d-glucose. Fermentation at acidic pH values close to the pKa of LA (3.86) can reduce the amount of agents added to neutralize the LA produced; however, most LA-producing Bacillus strains are more sensitive to acidic conditions than LAB (tolerance up to pH ~4.0 with Lactobacillus plantarum ATCC 21028)Citation45) and fungi (tolerance up to pH ~3.5 with R. oryzae ATCC 52311).Citation47) Comparison of one species in the genus Bacillus, Bacillus acidicola, was reported to tolerate pH values up to 3.5 lower than the pKa value of LA; however, the efficiency of LA fermentation by this strain under acidic conditions has not been well studied.Citation48) Therefore, additional studies are required to improve l-LA fermentation by Bacillus strains under acidic conditions, using techniques such as genome shuffling, which has enhanced the pH tolerance of Lactobacillus rhamnosus ATCC 11443 by up to 3.6.Citation49)
Under non-controlled pH conditions, Bacillus strains are not able to accumulate high concentrations of LA because of their intolerance to acidic pH values. Under controlled pH, the LA concentration and productivity in batch fermentation by Bacillus strains were found to be improved. The pH of the fermentation broth is controlled by various neutralizing agents such as CaCO3,Citation50) Ca(OH)2,Citation50) NaOH,Citation42) KOH,Citation51) and NH3.Citation18) Among these neutralizing agents, CaCO3 and Ca(OH)2Citation42,50) have been widely used. However, one disadvantage of using them is the formation of calcium salts such as calcium lactate in the fermentation broth, which requires an additional acidification procedure using H2SO4 during the recovery process to obtain free LA.Citation52) This disadvantage can be minimized using NH3 as neutralizing agent because calcium lactate is not formed and NH3 can be recovered in the supernatant of culture broth. Sakai and EzakiCitation18) reported l-LA fermentation (concentration, 86 g/L; productivity, 0.72 g/L h, optical purity, 97%, yield, 0.97 g/g) by B. coagulans NBRC 12583T using NH3 as a neutralizing agent to maintain the pH at 7.0 from food waste. Furthermore, a total recycled PLLA production process has been proposed, in which NH3 is recycled and utilized as the neutralizing agent for the subsequent LA fermentation after recovery of the free l-LA.Citation53)
I.iv. Ability to produce optical pure LA
Optical purity of LA produced in fermentation is mainly dependent on gene-expression levels and the enzyme activities of l-lactate dehydrogenase (l-LDH), d-lactate dehydrogenase (d-LDH), and lactate racemase. l-LDH and d-LDH catalyze the conversions of pyruvic acid to l-LA and d-LA, respectively, and almost all LAB possess both d-LDH and l-LDH.Citation54) In contrast, a few LAB have been reported to possess lactate racemase, which transforms d-LA to l-LA.Citation55) Therefore, the optical purity of LA varies considerably among LAB including l-LA producers (>95%; genera of Lactococcus, some Lactobacillus, and Enterococcus, etc.), dl-LA producers, and d-LA producers (>99%; Leuconostoc genus, and Lactobacillus delbrueckii subsp. lactis, etc. Majority of Rhizopus sp. produce l-LA with an optical purity 98.5–100%.Citation22) In contrast, most LA-producing Bacillus strains possess both l-LDH (encoded by the ldhL gene) and d-LDH (encoded by the ldhD gene), the enzymes responsible for the production of l-LA and d-LA, respectively, but lack the lactate racemase enzyme,Citation54) even though they produce l-LA with an optical purity of greater than 97%. Furthermore, it has been reported that the optical purities of l-LA produced by Bacillus strains are variable even within same species, such as the potent l-LA-producing B. coagulans strains, which ranges from 97–100%.Citation50,56) However, the mechanism underlying the synthesis of high optical purity LA by Bacillus strains has not been studied well.
I.v. Oxygen demand
LA-producing Bacillus strains and LAB are facultative anaerobes, whereas Rhizopus species are obligatory aerobes (Table ) and the demands of oxygen for cell growth and LA production are different among three LA-producers. LA-producing Bacillus strains aerobically grow very well using oxygen as the electron acceptor with less LA productionCitation56), while anaerobic culture exhibit less cell growth, but mainly produce LA. Although aerobic culture by LAB show cell growth due to their tolerance to oxygen, LAB cannot utilize oxygen as the electron acceptor, which results in lower LA production than anaerobic culture.Citation12,57) On the other hand, Rhizopus species can grow under only aerobic condition.Citation22)
One of the most interesting findings is that B. coagulans can produce l-LA as a major fermentative product either under strict anaerobic or aerobic (high oxygen) conditions. In particular, B. coagulans strain M21, accumulated 100% optically pure l-LA (12.5 g/L, yield 0.62 g/g, productivity 0.31 g/L h) from 20 g/L d-glucose under high oxygen conditions.Citation56) In contrast, it has been reported that LAB (L. plantarum, L. rhamnosus) degrade LA, especially in presence of oxygen as an electron accepter, and convert LA produced from sugars (glucose and cellobiose) to acetic acid, H2O2, and CO2.Citation26,58)
I.vi. Sugar metabolism
LA-producing Bacillus strains metabolize pentose and hexose sugars by the pentose phosphate pathway (PPP) and the Embden-Meyerhof-Parnas pathway (EMP), respectively, to produce LA as a major end product homofermentatively (Fig. ).Citation59) In EMP, hexose sugars such as glucose (six carbons) in the presence of ATP are phosphorylated by hexokinase to form glucose 6-phosphate, which is then isomerized by phosphoglucose isomerase to fructose 6-phosphate (F6P). F6P is further phosphorylated to fructose 1,6-bisphosphate (FBP) by phosphofructokinase with ATP consumption. FBP is then split into glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), which is catalyzed by fructose bisphosphate aldolase. GAP is converted to pyruvate via two substrate-level phosphorylations. Ultimately, pyruvate is reduced together with NADH oxidation to produce l-LA or d-LA by l-LDH or d-LDH, respectively. In some LAB, lactate racemase, which catalyzes the interconversion of l-LA/d-LA, has been reported to result in the formation of a racemic mixture of LA in EMP.Citation55) During EMP, the theoretical yield of LA from glucose is 1 g/g (2 mol/mol) in homolactic fermentation.
Fig. 1. Metabolic pathway for LA production from several sugars (xylose, glucose, arabinose), cellulose, and starch derived from various biomasses via homofermentative (EMP/PPP) (A) and heterofermentative (PK) (B) pathways.
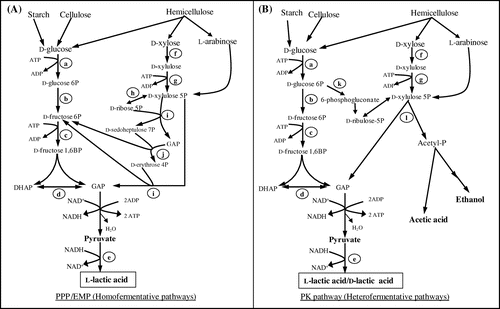
In PPP, pentose sugars, such as d-xylose, are converted to d-xylulose by xylose isomerase, which is further phosphorylated to xylulose 5-phosphate (X5P) by xylulose kinase. X5P is subsequently metabolized by transaldolase and transketolase to form GAP. GAP is then converted to pyruvate and then to LA via EMP. In the PPP, 3 mol of d-xylose is converted to 5 mol of LA, with a theoretical yield of 1 g/g (1.67 mol/mol),Citation60) and the highest yield (0.98 g/g) of LA was reported for B. coagulans JI12.Citation41) In contrast, a few LAB can metabolize pentose sugars (d-xylose).Citation7) Most pentose-utilizing LAB mainly utilize the phosphoketolase (PK) pathway to convert pentose sugar to LA and byproducts (acetate or ethanol) with a theoretical yield of only 0.6 g/g (1 mol/mol), which leads to heterofermentation.Citation61) As exceptions, the LAB Enterococcus mundtii QU 25,Citation26) E. faecium QU 50,Citation41) and some metabolically engineered LAB such as L. plantarumCitation62) have been reported to produce l-LA from d-xylose in a homofermentative manner via PPP/EMP with yields of 0.904, 0.89, and 0.89 g/g, respectively. In Rhizopus sp., pentose (xylose) sugar is metabolized by PPP to produce LA with a yield of 0.54–0.65 g/g and while hexose (d-glucose) is metabolized via EMP and tricarboxylic acid pathway to produce LA and other byproducts (ethanol, CO2, fumaric acid, citric acid, malic acid) with LA of yield 0.71–0.79 g/g.Citation22,63,64) Therefore, EMP and PPP are much better suited for increasing LA yield, which are common pathways in LA-producing Bacillus strains.
Tongpim et al.Citation56) recently proposed that l-LA-producing thermotolerant B. coagulans strains formed a distinct cluster in a phylogenetic tree based on their 16S rRNA gene sequence and showed heterogeneity in sugar metabolism. Although it has been reported that a few LAB utilize xylose as a carbon source, 8 of the 11 tested B. coagulans strains were able to metabolize xylose (one of the major sugar components in lignocelluloses).Citation56) In addition, De Clerck et al.Citation65) also reported that half of the B. coagulans strains (15 strains) metabolize d-xylose. These phenomena suggest the feasibility of efficient l-LA production from various lignocelluloses using B. coagulans strains.
Additionally, much progress has been made in our understanding of LA metabolism in Bacillus strains. The draft genome sequences of potent l-LA-producing B. coagulans strains (2–6, 36D1, DSM1, H-1, XZL4, and XZL9) showed the presence of xylose-utilizing genes encoding the enzymes xylose isomerase, ribulokinase, and ribulose 5-phosphate 4-epimerase involved in PPP.Citation66) In contrast, the genes involved in the PK pathway were absent from all tested B. coagulans strains, which may result in high LA yield from xylose. Actually, Ye et al.Citation42) reported that B. coagulans C106 produced 83.5 g/L of l-LA from 85 g/L of d-xylose, with an l-LA yield of 0.98 g/g, productivity of 7.5 g/L h, optical purity of 99.6% via PPP.
II. Available biomass for LA production by Bacillus species
LA production by Bacillus strains has mainly been investigated using expensive refined sugars (e.g. d-glucose) as a sole carbon source. However, substitutes for these expensive refined sugars, including cheap and widely abundant biomass resources, such as lignocellulose (e.g. wood and crop residues), sucrose (e.g. sugarcane and beet molasses), and starches (e.g. tapioca starch and corn starch) are rapidly gaining importance for the production of LA.Citation15)
II.i. Lignocellulosic biomass
Lignocelluloses consists of cellulose (insoluble fibers of β-1,4-glucan), hemicellulose (non-cellulosic polysaccharides of xylan, arabinose, glucan, and mannan), and lignin (a complex aromatic polymer).Citation26) The compositions of cellulose (35–50%), hemicellulose (20–40%), and lignin (10–30%) vary depending on plant type.Citation67–70) In enzyme hydrolysis of the lignocelluloses, cellulose mainly liberates d-glucose and cellobiose, whereas several sugars are obtained from hydrolysis of hemicellulose, including hexoses (d-mannose, d-galactose, and d-glucose) and pentoses (d-xylose and l-arabinose). d-xylose is the dominant sugar in hemicellulose. Thus far, wheat straw,Citation71) paper sludge,Citation72) corn fiber hydrolysate,Citation73) corncob molasses,Citation74) empty fruit bunches (EFB) of palm oil,Citation75,76) Jerusalem artichoke powder,Citation77) and Siberian larchCitation52) have already been efficiently used as raw materials for LA production by Bacillus strains. For example, B. coagulans JI12 utilized EFB hydrolysate (containing 4.7 g/L d-glucose, 48.8 g/L of d-xylose, and 9.6 g/L of l-arabinose) and produced 59.2 g/L of l-LA, with an l-LA yield of 0.97 g/g and a productivity of 6.2 g/L h and optical purity of 99.6%.Citation75)
However, one of the major challenges in fermentative production using lignocelluloses as substrates exist in the pretreatment process required for generating fermentable sugars and efficient LA production. The pretreatment of lignocelluloses also liberates phenolic compounds (furfural and 5-hydroxy methyl furfural [HMF]), furan derivatives (ferulic acid and vanillin), and weak organic acid (acetic acid).Citation78) These compounds are known to inhibit microbial growth and LA production at concentrations ranging from 0.08 g/L to 5.3 g/L.Citation78) Three main approaches have been investigated to improve LA production from pretreated lignocelluloses containing inhibitors: usage of LA-producing Bacillus strains that are tolerant to these inhibitors, introduction of removal process before fermentation, and conversion of these inhibitors into less-toxic substances by LA-producing Bacillus strains. Some B. coagulans strains have been reported to be resistant to certain levels of these inhibitors,Citation50,73,76,79) for instance, Bacillus sp. P38, which tolerates 6 g/L 2-furfural in corn stover hydrolysate, produced 180 g/L of l-LA with a productivity of 2.4 g/L h and a yield of 0.96 g/g under a fed-batch fermentation process (first approach).Citation50) By using LAB, there are a few reports on tolerance to fermentation inhibitors,Citation80,81) for instance, Lactobacillus brevis S3F4 with the tolerance of 10 mM (ca. 1.94 g/L) ferulic acid and 15 mM (ca. 1.44 g/L) furfural could produce 18.7 g/L LA from 25 g/L xylose.Citation80) Meantime, removal processes using chemicals, such as overliming,Citation73,76) prior to fermentation have also been reported to remove inhibitors from pretreated lignocelluloses for LA fermentation using Bacillus strains (second approach). Overliming decreased to ca. 27% furfural in EFB hydrolysate (63.1 g/L total sugar), which resulted in 59.2 g/L LA with 0.97 g/g yield by B. coagulans JI12.Citation76) To our knowledge, there is no report on LA fermentation using LAB by similar approach. As the third approach, successful simultaneous detoxification, saccharification, and fermentation has only been reported using B. coagulans JI12 by converting furfural (an inhibitor) to 2-furoin acid (a lessor inhibitor), producing 73.9 g/L of l-LA, with an l-LA yield of 1.09 g/g from EFB hydrolysate,Citation75) while we found no reports of LAB converting inhibitors to non-inhibitors. This process has become attractive in terms of not only overcoming the crucial issue of toxins produced by pretreatment of lignocelluloses, but also simplifying the overall processes in an environment-friendly method that requires less energy.Citation67)
II.ii. Sucrose-based biomass
Molasses from sugarcane and beet are sucrose-rich biomasses obtained from sucrose-based industries as waste byproducts. Sugarcane molasses contains 40–60% sucrose that can be utilized by micro-organisms to produce LA.Citation82) A few researchers have investigated the feasibility of using sucrose as a raw material for LA production using Bacillus strains.Citation83,84) Payot et al.Citation83) reported that B. coagulans TB/04 produced 55 g/L of LA from 60 g/L of sucrose derived from sugarcane molasses. In contrast, beet molasses containing 47% sucrose, 0.5% nitrogen, and 3% betaine has been shown to be used not only as a fermentation substrate, but also as an enhancer of LA fermentation by B. coagulans H-1.Citation84) The addition of beet molasses to a glucose solution increased l-LA productivity by 22% compared to that observed with fermentation using only d-glucose as a sole carbon source. Unlike other carbohydrates (such as cellulose and starch), these sucrose-containing molasses have a significant advantage: direct utilization for LA production without any requirements for saccharification because many B. coagulans strains can convert sucrose to LA.Citation56)
II.iii. Starchy biomass
Starch is a polysaccharide consisting of d-glucose with α-1,4 or α-1,6 bonds. It is usually found in cereal-based products (rice, corn, and wheat) and tuber plants (potato and cassava). Starchy biomasses are cheap alternatives to refined sugars. Inexpensive starches, starch-containing wastes, and starch derivatives obtained from many starch industries are attractive carbon sources for LA production even though starch requires saccharification by enzymes such as α-amylase and glucoamylase before fermentation.Citation85,86) Several enzymatic hydrolysates of corn starchCitation87) and tapioca starchCitation39) have been used as substrates for LA production by Bacillus strains. Similarly, a considerable number of starch-containing wastes such as food wastes have also been utilized for LA production by B. coagulans and Bacillus licheniformis strains.Citation88)
Conversely, direct fermentation from starch using amylolytic LA producers has the advantage of skipping the saccharification process. Although some amylolytic LAB and fungi (R. oryzae) have been reported to produce LA from starch without any enzymatic hydrolysis,Citation15,22) there are still the several problems regarding low optical purity of LA (for LAB; <98%), low yield (for Rhizopus; 0.79 g/g), mesophilic conditions (≤45 °C), and requirement for abundant nutrients (for LAB).Citation26) Recently, Poudel et al.Citation33) have established a direct starch fermentation process using Bacillus sp. MC-07 to obtain optically pure l-LA (purity 100%, yield 0.977 g/g). Furthermore, fermentation using strain MC-07 could be performed in a simple mineral salt medium containing only 0.001% yeast extract as an organic nitrogen source, at a high temperature (50 °C). This finding shows that some low-cost starchy biomasses can be directly fermented to l-LA with high yield and optical purity by omitting the addition of enzymes and nitrogen sources, a relatively simple methodology for LA fermentation.
III. Fermentative production of LA by Bacillus strains
III.i. Bacillus strains
As shown in Table , B. coagulans, B. licheniformis, B. subtilis, B. thermoamylovorans and some yet to be identified Bacillus species have been reported to be potent l-LA producers. All these Bacillus strains accumulated optically pure l-LA (97–100%). Among these reported Bacillus species, B. coagulans accumulated a higher amount of l-LA and showed thermotolerant behavior (Table ).
Table 2. Lactic acid production efficiencies among the Bacillus strains under batch and repeated batch fermentation modes.
III.ii. Fermentation modes
During LA fermentation, high LA yield, productivity, and the concentration of the final accumulated product are important factors for evaluating fermentation efficiency. To achieve such economically important parameters, numerous investigations on LA fermentation have been conducted under batch, fed-batch, and continuous fermentation processes (Tables and ).
Table 3. Lactic acid production in fed-batch and continuous fermentation processes.
Batch fermentation is the most frequently used for LA production.Citation25) The advantage of using batch fermentation is the low risk of contamination compared with other fermentation approaches because it is a closed system in which no nutrients are added during the fermentation period.Citation93,94) High LA production (210 g/L from d-glucoseCitation87) and 140.9 g/L from d-xylose)Citation42) has been obtained using Bacillus strains in batch fermentation under non-sterile conditions (Table ). These LA concentrations are the highest in the published literature using Bacillus strains under batch fermentation. In repeated batch mode, microbial cells can be repeatedly used for subsequent batch fermentations.Citation79) This method led to improved LA productivity and reduced fermentation time and does not require inoculum preparation. B. coagulans strain 2–6 accumulated the highest l-LA concentration (107 g/L, with an optical purity of 99.8%) under open repeated batch fermentation from d-glucose, in which the yield and productivity were improved to 0.95 g/g and 2.9 g/L h, respectively, compared to batch fermentation (63 g/L, yield up to 0.94 g/g, productivity of 1.4 g/L h).Citation51) However, various problems, such as substrate inhibition due to a high initial concentration of sugar, carbon catabolite repression (CCR) in mixed sugar fermentation caused by the consumption of more preferable sugars and lagging of the less preferable sugars, and product inhibition caused by LA accumulation still exist in batch fermentation.
In fed-batch fermentation, fresh medium or nutrients is continuously or sequentially fed into the fermentation broth without removal of culture broth mainly to overcome the problems of substrate inhibition and nutrient deficiencies that occur in batch fermentation.Citation26) Fermentative LA production by Bacillus strains under fed-batch fermentation is illustrated in Table . Several feeding strategies, including pulse feeding, constant feeding, and exponential feeding, have been developed to increase the LA concentration in fed-batch fermentation. Among these, pulse feeding is considered easier and more suitable for industrial scale.Citation93) When a pulse feeding strategy was used, the highest reported l-LA concentration (225 g/L) was obtained using Bacillus sp. WL-S20 under fed-batch fermentation.Citation37)
Compared to batch and fed-batch fermentation, continuous fermentation in Bacillus strains has been less studied. Continuous fermentation is adopted to overcome the issue of product inhibition that can occur in batch and fed-batch fermentation. In continuous fermentation, products, such as inhibitors, are constantly diluted by adding fresh medium at the same rate as that of the outflow of the broth, to maintain a steady volume in the fermentor. In addition, cells can be maintained in a stable physiological state and at a constant growth rate; thereby, attaining steady maximum productivity. Continuous fermentations using B. laevolacticus NCIB 10269,Citation17) B. coagulans TB/04,Citation83) and B. subtilis MUR13Citation5) have been investigated. Gao and HoCitation35) reported an average LA productivity of 16.8 g/L h at a dilution rate of 0.4 h−1, using glucose under continuous fermentation, which resulted in 4.8-fold increase in productivity compared to that obtained in fed-batch fermentation.Citation35,36) De Boer et al.Citation17) also showed that Bacillus laevolaticus NCIB 10269 have the ability to produce d-LA with a productivity of 13.1 g/L h at dilution rate of 0.07 h−1. In continuous mode, selection of the optimum dilution rate for attaining maximum cell growth and LA productivity is important in Bacillus strains. LA productivity was highly improved for a long period in continuous fermentation compared to that in batch and fed-batch fermentation.
Bacillus strains have been also employed to produce LA from mixed sugars derived from lignocelluloses.Citation52,59,60,75–77,79) However, a major obstacle for using mixed sugars is the difficulty in simultaneous utilization of each sugar during fermentation. Several researchers have reported on CCR in B. coagulans strains, i.e. initial consumption of more preferable sugars like glucose and lagging utilization of less preferable sugars like d-xylose and l-arabinose.Citation44,72,75) CCR with mixed sugars has mainly been reported for batch fermentation using LA-producing Bacillus strains.Citation75) This indicated that repeated batch fermentation by B. coagulans strains showed simultaneous utilization of mixed sugars (d-glucose, d-xylose, and l-arabinose) to some extent.Citation59,77) Zhang et al.Citation79) investigated repeated batch fermentation using various substrates (d-glucose, d-xylose, and l-arabinose) by B. coagulans IPE22 for up to six consecutive batches. In the first batch, fermentation exhibited a lag phase of 5 h, and then pentose sugars (d-xylose and l-arabinose) were utilized to produce 54.14 g/L of LA in 24 h, whereas in the sixth batch, the lag phase was eliminated and all sugars were simultaneously utilized to produce 56.27 g/L of LA in 17 h. However, complete avoidance of CCR has not been solved using LA-producing Bacillus strains. Therefore, more potent CCR-negative LA-producers must be procured, either by isolation of wild strains or through genetic manipulations, or by optimizing the fermentation conditions.
IV. Metabolic engineering of Bacillus species for LA production
Metabolic engineering is being applied to improve the cellular properties of Bacillus strains through alteration of specific biochemical reactions or introduction of new genes by DNA recombination.Citation95–99) However, attempts to engineer the genus Bacillus metabolically for LA production are far fewer than those with LAB,Citation62) Escherichia coli,Citation97) and fungi.Citation98) This section is focused on recent studies on the establishment of genetically modified Bacillus strains to improve LA fermentation.
Among the LA-producing Bacillus strains, B. subtilis is being widely studied for its good performance and established genetic engineering methods.Citation100) Zhang et al.Citation96) reported the direct fermentation of cellulose to l-LA by recombinant cellulolytic B. subtilis XZ7. In this study, overexpression of β-endoglucanase-coding genes by two-round directed evolution was performed, and the gene encoding α-acetolactate synthase (alsS) in the 2, 3-butanediol pathway was knocked out to increase the l-LA yield. As a result, B. subtilis XZ7 could digest up to 92% of the insoluble regenerated amorphous cellulose and produced l-LA with a yield of up to 0.63 g/g. This study demonstrated that B. subtilis is useful for genetic manipulation to generate excellent LA-producing Bacillus strains for the desired processes.
Genetic tools for l-LA-producing B. coagulans have been gradually developing; however, compared to B. subtilis, highly efficient genetic tools are still lacking. Basically, wild-type B. coagulans strains produce l-isomer efficiently from several sugars, while d-LA-producers are also required for the synthesis of stereocomplex PLA, consisting of PLLA and PDLA.Citation101,102) For the fermentative production of d-LA, some B. coagulans strains have been genetically modified using specific genetic tools.Citation95,99) Kovács et al.Citation95) successfully applied the widely used Cre-lox system for genomic modifications and removal of specific genes. The native ldhL gene of B. coagulans DSM1 (a potent l-LA producer) was disrupted, and the d-lactate dehydrogenase (ldhD) gene was overexpressed to generate d-LA. The engineered strain could produce 16.9% d-LA and 83.1% l-LA. However, optically pure d-LA could not be obtained from the engineered strain; therefore, further improvements are required. Conversely, Wang et al.Citation99) engineered a thermotolerant B. coagulans strain P4-102B (a potent l-LA producer, optical purity >99%) by targeting the genes encoding the enzyme d-LDH for d-LA production with high yield by deleting the native ldhL (l-lactate dehydrogenase) and alsS (acetolactate synthase) genes. The engineered strain produced optically pure d-LA (90 g/L of d-LA, optical purity >99%) from d-glucose at 50 °C, and use of this strain can be expected to reduce the cost of LA production for biopolymers.
Aside from genetic engineering, another approach that has been investigated is to breed thermotolerant l-LA-producing B. licheniformis, as the wild-type BL1 strain could not efficiently ferment xylose to LA. Wang et al.Citation34) transferred BL1 strain into medium containing xylose as the sole carbon source, and the evolved BL2 strain, obtained after 13 transfers, showed improved l-LA production (concentration, 24.5 g/L; yield, 79.5%; maximum LA productivity, 7.0 g/L h) from 30 g/L xylose compared with the parent strain (0.6 g/L, 60.0%, and 0, respectively). Furthermore, transcriptional analysis revealed 5.1- and 6.2-fold higher mRNA expression levels of xylA (xylose isomerase) and xylB (xylulose kinase), respectively, in the BL2 strain compared to the levels in the BL1 strain using xylose. Although further work is needed to understand the mechanism, including other genes and enzymes in this strain, this method is also a new and easy technique for modifying the metabolism of LA-producing Bacillus strains.
In future, progress toward more efficient and easy methods for genetic manipulation and evolution will lead to more studies in the field of metabolic engineering on LA-producing Bacillus strains.
V. Future prospects for LA-producing Bacillus species
Today’s society is more concerned with climate change because the majority of the industrial economies are largely dependent on fossil resources, which provide the basis for almost all of our energy and chemical feedstocks. In order to develop a sustainable society, we need to substantially reduce our dependence on fossil resources by establishing bio-based systems.Citation103) Thus, microbial technologies to produce value-added chemicals, such as optically pure LA, would be the best alternative strategy to overcome our dependency on fossil resources.Citation104)
Recently, many Bacillus strains have been shown to possess the ability to utilize a wide variety of biomass resources to produce optically pure l-LA. In particular, B. coagulans, B. subtilis, B. licheniformis, and B. thermoamylovorans strains are promising LA producers. These strains also have the ability to tolerate high temperatures, form spores, grow at high growth rates, and to produce highly optically pure l-LA. The basic physiological features of these strains indicate that Bacillus species can be an important l-LA producer. In addition, many Bacillus strains have advantages for the homofermentative conversion of the pentose sugars predominantly available in lignocelluloses. Most recently, B. coagulans strains have also been determined to be for use as safe probiotics.Citation105) In addition, many Bacillus strains have been adapted for the production of commercially valuable products such as thermostable enzymes and antimicrobial peptides.Citation66) However, due to limited studies on the metabolic pathways and metabolic engineering in LA-producing Bacillus strains, more investigations should be done to enhance the efficiency of LA production. Besides, there are extremely few investigations in terms of selection of nutrients (i.e. carbon and nitrogen sources) and available biomasses followed by several pretreatment and saccharification methods and improvement of production processes dealing with LA-producing Bacillus strains, compared with studies on LA production using LAB. Further studies should focus on the development of highly efficient strains and production processes that are able to produce optically pure l-LA as well as d-LA either by selective isolation and screening or through genetic engineering approaches.
Authors contribution
Poudel P, Tashiro Y, Sakai K, designed this review and Poudel P, drafted the review manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported partly by the JSPS KAKENHI [grant number 26740050].
References
- Logan NA, De Vos P, Genus I. Bacillus. In: De Vos P, Garrity GM, Jones D, et al., ed. Vol. 3. Bergey’s manual of systematic bacteriology 2nd ed. Vol. 3. New York (NY): Springer; 2009. p. 21–128.
- Parkouda C, Nielsen DS, Azokpota P, et al. The microbiology of alkaline-fermentation of indigenous seeds used as food condiments in Africa and Asia. Crit. Rev. Microbiol. 2009;35:139–156.10.1080/10408410902793056
- Schallmey M, Singh A, Ward OP. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004;50:1–17.10.1139/w03-076
- Sauer U, Cameron DC, Bailey JE. Metabolic capacity of Bacillus subtilis for the production of purine nucleosides, riboflavin, and folic acid. Biotechnol. Bioeng. 1998;59:227–238.10.1002/(ISSN)1097-0290
- Roh JY, Choi JY, Li MS, et al. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J. Microbiol. Biotechnol. 2007;17:547–559.
- Ogawa Y, Yamaguchi F, Yuasa K, et al. Efficient production of γ-polyglutamic acid by Bacillus subtilis (natto) in jar fermenters. Biosci. Biotechnol. Biochem. 1997;61:1684–1687.10.1271/bbb.61.1684
- Abdel-Rahman MA, Tashiro Y, Sonomoto K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013;31:877–902.10.1016/j.biotechadv.2013.04.002
- Cutting SM. Bacillus probiotics. Food Microbiol. 2011;28:214–220.10.1016/j.fm.2010.03.007
- Lucy M, Reed E, Glick BR. Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek. 2004;86:1–25.10.1023/B:ANTO.0000024903.10757.6e
- Sharma RR, Singh D, Singh R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol. Control. 2009;50:205–221.10.1016/j.biocontrol.2009.05.001
- Poudel P, Miyamoto H, Miyamoto H, et al. Thermotolerant Bacillus kokeshiiformis sp. nov. isolated from marine animal resources compost. Int. J. Syst. Evol. Microbiol. 2014;64:2668–2674.10.1099/ijs.0.059329-0
- Hofvendahl K, Hahn-Hägerdal B. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb. Technol. 2000;26:87–107.10.1016/S0141-0229(99)00155-6
- Wee Y, Kim J, Ryu H. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 2006;44:163–172.
- Castillo Martinez FA, Balciunas EM, Salgado JM. Lactic acid properties, applications and production: a review. Trends Food Sci. Technol. 2013;30:70–83.
- John RP, Nampoothiri KM, Pandey A. Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007;74:524–534.10.1007/s00253-006-0779-6
- Ghaffar T, Irshad M, Anwar Z, et al. Recent trends in lactic acid biotechnology: a brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014;7:222–229.10.1016/j.jrras.2014.03.002
- De Boer JP, de Mattos MJT, Neijssel OM. d(−)Lactic acid production by suspended and aggregated continuous cultures of Bacillus laevolacticus. Appl. Microbiol. Biotechnol. 1990;34:149–153.10.1007/BF00166771
- Sakai K, Ezaki Y. Open l-lactic acid fermentation of food refuse using thermophilic Bacillus coagulans and fluorescence in situ hybridization analysis of microflora. J. Biosci. Bioeng. 2006;101:457–463.10.1263/jbb.101.457
- Chen L, Zhou C, Liu G, et al. Application of lactic acid bacteria, yeast and Bacillus as feed additive in dairy cattles. J. Food Agri. Environ. 2013;11:22–25.
- Danner H, Neureiter M, Madzingaidzo L, et al. Bacillus stearothermophilus for thermophilic production of l-lactic acid. Appl. Biochem. Biotechnol. 1998;70–72:895–903.10.1007/BF02920200
- Calabia BP, Tokiwa Y, Aiba S. Fermentative production of l-(+)-lactic acid by an alkaliphilic marine microorganism. Biotechnol. Lett. 2011;33:1429–1433.10.1007/s10529-011-0573-0
- Zhang ZY, Jin B, Kelly JM. Production of lactic acid from renewable materials by Rhizopus fungi. Biochem. Eng. J. 2007;35:251–263.10.1016/j.bej.2007.01.028
- Kitpreechavanich V, Maneeboon T, Kayano Y, et al. Comparative characterization of l-lactic acid-producing thermotolerant Rhizopus fungi. J. Biosci. Bioeng. 2008;106:541–546.10.1263/jbb.106.541
- Litchfield JH. Lactic acid, microbially produced. In: Schaechter Mosel O, editor. Encyclopedia of microbiology. Oxford: Academic Press; 2009. p. 362–372.
- Mazzoli R, Bosco F, Mizrahi I, et al. Towards lactic acid bacteria-based biorefineries. Biotechnol. Adv. 2014;32:1216–1236.10.1016/j.biotechadv.2014.07.005
- Abdel-Rahman MA, Tashiro Y, Sonomoto K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J. Biotechnol. 2010;156:286–301.
- Sakai K, Poudel P, Shirai Y. Advances in applied biotechnology. In: Petre M, editor. Total recycle system of food waste for poly-l-lactic acid output. Croatia: Intech; 2012. p. 23–40.
- Vilain S, Luo Y, Hildreth MB, et al. Analysis of the life cycle of the soil Saprophyte Bacillus cereus in liquid soil extract and in soil. Appl. Environ. Microbiol. 2006;72:4970–4977.10.1128/AEM.03076-05
- Hébert EM, Raya RR, De Giori GS. Nutritional requirements of Lactobacillus delbrueckii subsp. lactis in a chemically defined medium. Curr. Microbiol. 2004;49:341–345.10.1007/s00284-004-4357-9
- Florou-Paneri P, Christaki E, Bonos E. Lactic acid bacteria as source of funtional ingredients. In: Marcelino K, editor. Lactic acid bacteria- R & D for food, health and livestock purposes. Croatia: Intech; 2013. p. 589–614, doi: 10.5772/2825
- Altaf M, Naveena BJ, Reddy G. Use of inexpensive nitrogen sources and starch for l(+) lactic acid production in anaerobic submerged fermentation. Bioresour. Technol. 2007;98:498–503.10.1016/j.biortech.2006.02.013
- Heriban V. Process and metabolic characteristics of Bacillus coagulans as a lactic acid producer. Lett. Appl. Microbiol. 1993;16:243–246.10.1111/j.1472-765X.1993.tb01409.x
- Poudel P, Tashiro Y, Miyamoto H, et al. Direct starch fermentation to l-lactic acid by a newly isolated thermophilic strain, Bacillus sp. MC-07. J. Ind. Microbiol. Biotechnol. 2015;42:143–149.10.1007/s10295-014-1534-0
- Wang Q, Zhao X, Chamu J, et al. Isolation, characterization and evolution of a new thermophilic Bacillus licheniformis for lactic acid production in mineral salts medium. Bioresour. Technol. 2011;102:8152–8158.10.1016/j.biortech.2011.06.003
- Gao T, Ho KP. l-Lactic acid production by Bacillus subtilis MUR1 in continuous culture. J. Biotechnol. 2013;168:646–651.10.1016/j.jbiotec.2013.09.023
- Gao T, Wong Y, Ng C, et al. l-Lactic acid production by Bacillus subtilis MUR1. Bioresour. Technol. 2012;121:105–110.10.1016/j.biortech.2012.06.108
- Meng Y, Xue Y, Yu B, et al. Efficient production of l-lactic acid with high optical purity by alkaliphilic Bacillus sp. WL-S20. Bioresour. Technol. 2012;116:334–339.10.1016/j.biortech.2012.03.103
- Ou MS, Ingram LO, Shanmugam KT. l(+)-Lactic acid production from non-food carbohydrates by thermotolerant Bacillus coagulans. J. Ind. Microbiol. Biotechnol. 2011;38:599–605.10.1007/s10295-010-0796-4
- Ma K, Maeda T, You H, et al. Open fermentative production of l-lactic acid with high optical purity by thermophilic Bacillus coagulans using excess sludge as nutrient. Bioresour. Technol. 2014;151:28–35.10.1016/j.biortech.2013.10.022
- Tashiro Y, Kaneko W, Sun Y, et al. Continuous d-lactic acid production by a novel thermotolerant Lactobacillus delbrueckii subsp. lactis QU 41. Appl. Microbiol. Biotechnol. 2011;89:1741–1750.10.1007/s00253-010-3011-7
- Abdel-Rahman MA, Tashiro Y, Zendo T, et al. Enterococcus faecium QU 50: a novel thermophilic lactic acid bacterium for high-yield l-lactic acid production from xylose. FEMS Microbiol. Lett. 2015;362:1–7.10.1093/femsle/fnu030
- Ye L, Zhou X, Bin Hudari MS, et al. Highly efficient production of l-lactic acid from xylose by newly isolated Bacillus coagulans C106. Bioresour. Technol. 2013;132:38–44.10.1016/j.biortech.2013.01.011
- Ramos HC, Hoffmann T, Marino M, et al. Fermentative metabolism of Bacillus subtilis: physiology and regulation of gene expression. J. Bacteriol. 2000;182:3072–3080.10.1128/JB.182.11.3072-3080.2000
- Ou MS, Mohammed N, Ingram LO, et al. Thermophilic Bacillus coagulans requires less cellulases for simultaneous saccharification and fermentation of cellulose to products than mesophilic microbial biocatalysts. Appl. Biochem. Biotechnol. 2009;155:379–385.
- Fu W, Mathews AP. Lactic acid production from lactose by Lactobacillus plantarum: kinetic model and effects of pH, substrate, and oxygen. Biochem. Eng. J. 1999;3:163–170.10.1016/S1369-703X(99)00014-5
- Ntougias S, Russell NJ. Alkalibacterium olivoapovliticus gen. nov., sp. nov., a new obligately alkaliphilic bacterium isolated from edible-olive wash-waters. Int. J. Syst. Evol. Microbiol. 2001;51:1161–1170.10.1099/00207713-51-3-1161
- Domínguez JM, Vázquez M. Effect of the operational conditions on the l-lactic acid production by Rhizopus oryzae. Cienc. Tecnol. Aliment. 1999;2:113–118.10.1080/11358129909487590
- Albert RA, Archambault J, Rosselló-Mora R, et al. Bacillus acidicola sp. nov., a novel mesophilic, acidophilic species isolated from acidic Sphagnum peat bogs in Wisconsin. Int. J. Syst. Evol. Microbiol. 2005;55:2125–2130.10.1099/ijs.0.02337-0
- Wang Y, Li Y, Pei X, et al. Genome-shuffling improved acid tolerance and l-lactic acid volumetric productivity in Lactobacillus rhamnosus. J. Biotechnol. 2007;129:510–515.10.1016/j.jbiotec.2007.01.011
- Peng L, Wang L, Che C, et al. Bacillus sp. strain P38: an efficient producer of l-lactate from cellulosic hydrolysate, with high tolerance for 2-furfural. Bioresour. Technol. 2013;149:169–176.10.1016/j.biortech.2013.09.047
- Zhao B, Wang L, Ma C. Repeated open fermentative production of optically pure l-lactic acid using a thermophilic Bacillus sp. strain. Bioresour. Technol. 2010;101:6494–6498.10.1016/j.biortech.2010.03.051
- Walton SL, Bischoff KM, van Heiningen ARP, et al. Production of lactic acid from hemicellulose extracts by Bacillus coagulans MXL-9. J. Ind. Microbiol. Biotechnol. 2010;37:823–830.10.1007/s10295-010-0727-4
- Sakai K, Taniguchi M, Miura S, et al. Making plastics from garbage: a novel process for poly-l-lactate production from municipal food waste. J. Ind. Ecol. 2003;7:63–74.10.1162/108819803323059406
- Wang L, Cai Y, Zhu L, et al. Major role of NAD-dependent lactate dehydrogenases in the production of l-lactic acid with high optical purity by the thermophile Bacillus coagulans. Appl. Environ. Microbiol. 2014;80:7134–7141.10.1128/AEM.01864-14
- Sakai K, Fujii N, Chukeatirote E. Racemization of l-lactic acid in pH-swing open fermentation of kitchen refuse by selective proliferation of Lactobacillus plantarum. J. Biosci. Bioeng. 2006;102:227–232.10.1263/jbb.102.227
- Tongpim S, Meidong R, Poudel P, et al. Isolation of thermophilic l-lactic acid producing bacteria showing homo-fermentative manner under high aeration condition. J. Biosci. Bioeng. 2014;117:318–324.10.1016/j.jbiosc.2013.08.017
- Bobillo M, Marshall VM. Effect of salt and culture aeration on lactate and acetate production by Lactobacillus plantarum. Food Microbiol. 1991;8:153–160.10.1016/0740-0020(91)90008-P
- Quatravaux S, Remize F, Bryckaert E, et al. Examination of Lactobacillus plantarum lactate metabolism side effects in relation to the modulation of aeration parameters. J. Appl. Microbiol. 2006;101:903–912.10.1111/jam.2006.101.issue-4
- Patel MA, Ou MS, Harbrucker R, et al. Isolation and characterization of acid-tolerant, thermophilic bacteria for effective fermentation of biomass-derived sugars to lactic acid. Appl. Environ. Microbiol. 2006;72:3228–3235.10.1128/AEM.72.5.3228-3235.2006
- Patel M, Ou M, Ingram LO, et al. Fermentation of sugar cane bagasse hemicellulose hydrolysate to l(+)-lactic acid by a thermotolerant acidophilic Bacillus sp. Biotechnol. Lett. 2004;26:865–868.10.1023/B:bile.0000025893.27700.5c
- Tanaka K, Komiyama A, Sonomoto K, et al. Two different pathways for d-xylose metabolism and the effect of xylose concentration on the yield coefficient of l-lactate in mixed-acid fermentation by the lactic acid bacterium Lactococcus lactis IO-1. Appl. Microbiol. Biotechnol. 2002;60:160–167.
- Okano K, Tanaka T, Ogino C, et al. Biotechnological production of enantiomeric pure lactic acid from renewable resources: recent achievements, perspectives, and limits. Appl. Microbiol. Biotechnol. 2010;85:413–423.10.1007/s00253-009-2280-5
- Xiao Z, Wu M, Beauchemin M, et al. Direct fermentation oftriticale starch to lactic acid by Rhizopus oryzae. Ind. Biotechnol. 2011;7:129–134.10.1089/ind.2011.7.129
- Maas RHW, Springer J, Eggink G, et al. Xylose metabolism in the fungus Rhizopus oryzae: effect of growth and respiration on l(+)-lactic acid production. J. Ind. Microbiol. Biotechnol. 2008;35:569–578.10.1007/s10295-008-0318-9
- De Clerck E, Vanhoutte T, Hebb T, et al. Isolation, characterization, and identification of bacterial contaminants in semifinal gelatin extracts. Syst. Appl. Microbiol. 2004;27:50–60.
- Su F, Xu P. Genomic analysis of thermophilic Bacillus coagulans strains: efficient producers for platform bio-chemicals. Sci. Rep. 2014;4:1–10.
- Anuj KC, Silvio SS, Om VS. Detoxification of lignocellulosic hydrolysates for improved bioethanol production. In: Marco Aurelio DBS, editor. Biofuel production-recent developments and prospects. Croatia: Intech; 2011, p. 225–246.
- Saha B. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003;30:279–291.10.1007/s10295-003-0049-x
- Neureiter M, Danner H, Madzingaidzo L, et al. Lignocellulose feedstocks for the production of lactic acid. Chem. Biochem. Eng. Q. 2004;18:55–63.
- Pandey A, Soccol CR, Nigam P, et al. Biotechnological potential of agro-industrial residues. I: sugarcane bagasse. Bioresour. Technol. 2000;74:69–80.10.1016/S0960-8524(99)00142-X
- Maas RHW, Bakker RR, Jansen MLA, et al. Lactic acid production from lime-treated wheat straw by Bacillus coagulans: neutralization of acid by fed-batch addition of alkaline substrate. Appl. Microbiol. Biotechnol. 2008;78:751–758.10.1007/s00253-008-1361-1
- Budhavaram NK, Fan Z. Production of lactic acid from paper sludge using acid-tolerant, thermophilic Bacillus coagulan strains. Bioresour. Technol. 2009;100:5966–5972.10.1016/j.biortech.2009.01.080
- Bischoff KM, Liu S, Hughes SR, et al. Fermentation of corn fiber hydrolysate to lactic acid by the moderate thermophile Bacillus coagulans. Biotechnol. Lett. 2010;32:823–828.10.1007/s10529-010-0222-z
- Wang L, Zhao B, Liu B, et al. Efficient production of l-lactic acid from corncob molasses, a waste by-product in xylitol production, by a newly isolated xylose utilizing Bacillus sp. strain. Bioresour. Technol. 2010;101:7908–7915.10.1016/j.biortech.2010.05.031
- Ye L, Bin Hudari MS, Zhi L, et al. Simultaneous detoxification, saccharification and co-fermentation of oil palm empty fruit bunch hydrolysate for l-lactic acid production by Bacillus coagulans JI12. Biochem. Eng. J. 2013;83:16–21.
- Ye L, Bin Hudari MS, Zhou X, et al. Conversion of acid hydrolysate of oil palm empty fruit bunch to l-lactic acid by newly isolated Bacillus coagulans JI12. Appl. Microbiol. Biotechnol. 2013;97:4831–4838.10.1007/s00253-013-4788-y
- Wang L, Xue Z, Zhao B, et al. Jerusalem artichoke powder: a useful material in producing high-optical-purity l-lactate using an efficient sugar-utilizing thermophilic Bacillus coagulans strain. Bioresour. Technol. 2013;130:174–180.10.1016/j.biortech.2012.11.144
- Palmqvist E, Hahn-Hägerdal B. Fermentation of lignocellulosic hydrolysates II: inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000;74:25–33.10.1016/S0960-8524(99)00161-3
- Zhang Y, Chen X, Qi B, et al. Improving lactic acid productivity from wheat straw hydrolysates by membrane integrated repeated batch fermentation under non-sterilized conditions. Bioresour. Technol. 2014;163:160–166.
- Guo W, Jia W, Li Y, et al. Performances of Lactobacillus brevis for producing lactic acid from hydrolysate of lignocellulosics. Appl. Biochem. Biotechnol. 2010;161:124–136.10.1007/s12010-009-8857-8
- Boguta A, Bringel F, Martinussen J, et al. Screening of lactic acid bacteria for their potential as microbial cell factories for bioconversion of lignocellulosic feedstocks. Microb. Cell Fact. 2014;13:1–16.10.1186/s12934-014-0097-0
- Dumbrepatil A, Adsul M, Chaudhari S, et al. Utilization of molasses sugar for lactic acid production by Lactobacillus delbrueckii subsp. delbrueckii mutant Uc-3 in batch fermentation. Appl. Environ. Microbiol. 2008;74:333–335.10.1128/AEM.01595-07
- Payot T, Chemaly Z, Fick M. Lactic acid production by Bacillus coagulans—kinetic studies and optimization of culture medium for batch and continuous fermentations. Enzyme Microb. Technol. 1999;24:191–199.10.1016/S0141-0229(98)00098-2
- Xu K, Xu P. Betaine and beet molasses enhance l-lactic acid production by Bacillus coagulans. PLoS One. 2014;9:e100731.10.1371/journal.pone.0100731
- Shimizu-Kadota M, Kato H, Shiwa Y, et al. Genomic features of Lactococcus lactis IO-1, a lactic acid bacterium that utilizes xylose and produces high levels of l-lactic acid. Biosci. Biotechnol. Biochem. 2014;77:1804–1808.
- Im DHK, Orimoto NM, Aburi WS, et al. Purification and characterization of a liquefying α-amylase from alkalophilic thermophilic Bacillus sp. AAH-31. Biosci. Biotechnol. Biochem. 2012;76:1378–1383.
- Zhou X, Ye L, Wu JC. Efficient production of l-lactic acid by newly isolated thermophilic Bacillus coagulans WCP10-4 with high glucose tolerance. Appl. Microbiol. Biotechnol. 2013;97:4309–4314.10.1007/s00253-013-4710-7
- Sakai K. Production of optically active lactic acid by using non-LAB microorganisms. In: Ohara H, Kimura Y, editors. White biotechnology; the front of energy and material development. Tokyo: CMC Publisher; 2008. p. 98–108. ( Japanese).
- Combet-Blanc Y, Ollivier B, Streicher C, et al. Bacillus thermoamylovorans sp. nov., a moderately thermophilic and amylolytic bacterium. Int. J. Syst. Bacteriol. 1995;45:9–16.10.1099/00207713-45-1-9
- Sakai K, Yamanami T. Thermotolerant Bacillus licheniformis TY7 produces optically active l-lactic acid from kitchen refuse under open condition. J. Biosci. Bioeng. 2006;102:132–134.10.1263/jbb.102.132
- Xue Z, Wang L, Ju J, et al. Efficient production of polymer-grade l-lactic acid from corn stover hydrolyzate by thermophilic Bacillus sp. strain XZL4. SpringerPlus. 2012;1:1–7.
- Ouyang J, Ma R, Zheng Z, et al. Open fermentative production of l-lactic acid by Bacillus sp. strain NL01 using lignocellulosic hydrolyzates as low-cost raw material. Bioresour. Technol. 2013;135:475–480.10.1016/j.biortech.2012.09.096
- Qin J, Zhao B, Wang X, et al. Non-sterilized fermentative production of polymer-grade l-lactic acid by a newly isolated thermophilic strain Bacillus sp. 2–6. PLoS One. 2009;4:e4359.
- Xu K, Xu P. Efficient production of l-lactic acid using co-feeding strategy based on cane molasses/glucose carbon sources. Bioresour. Technol. 2013;153:23–29.
- Kovács AT, van Hartskamp M, Kuipers OP, et al. Genetic tool development for a new host for biotechnology, the thermotolerant bacterium Bacillus coagulans. Appl. Environ. Microbiol. 2010;76:4085–4088.10.1128/AEM.03060-09
- Zhang XZ, Sathitsuksanoh N, Zhu Z, et al. One-step production of lactate from cellulose as the sole carbon source without any other organic nutrient by recombinant cellulolytic Bacillus subtilis. Metab. Eng. 2011;13:364–372.10.1016/j.ymben.2011.04.003
- Mazumdar S, Clomburg JM, Gonzalez R. Escherichia coli strains engineered for homofermentative production of d-lactic acid from glycerol. Appl. Environ. Microbiol. 2010;76:4327–4336.10.1128/AEM.00664-10
- Ishida N, Saitoh S, Tokuhiro K, et al. Efficient production of l-lactic acid by metabolically engineered Saccharomyces cerevisiae with a genome-integrated l-lactate dehydrogenase gene. Appl. Environ. Microbiol. 2005;71:1964–1970.10.1128/AEM.71.4.1964-1970.2005
- Wang Q, Ingram LO, Shanmugam KT. Evolution of l-lactate dehydrogenase activity from glycerol dehydrogenase and its utility for d-lactate production from lignocellulose. Proc. Natl. Acad. Sci. USA. 2011;108:18920–18925.10.1073/pnas.1111085108
- Romero S, Merino E, Bolivar F, et al. Metabolic engineering of Bacillus subtilis for ethanol production: lactate dehydrogenase plays a key role in fermentative metabolism. Appl. Environ. Microbiol. 2007;73:5190–5198.10.1128/AEM.00625-07
- Hirata M, Kimura Y. Thermomechanical properties of stereoblock poly(lactic acid)s with different PLLA/PDLA block compositions. Polymer. 2008;49:2656–2661.10.1016/j.polymer.2008.04.014
- Nishida H, Fan Y, Mori T, et al. Feedstock recycling of flame-resisting poly (lactic acid)/ aluminum hydroxide composite to l, l–lactide. Polym. Degrad. Stab. 2004;86:197–208.
- Cherubini F. The biorefinery concept: using biomass instead of oil for producing energy and chemicals. Energy Convers. Manage. 2010;51:1412–1421.10.1016/j.enconman.2010.01.015
- Tashiro Y, Matsumoto H, Miyamoto H, et al. A novel production process for optically pure l-lactic acid from kitchen refuse using a bacterial consortium at high temperatures. Bioresour. Technol. 2013;146:672–681.10.1016/j.biortech.2013.07.102
- Endres JR, Clewell A, Jade KA, et al. Safety assessment of a proprietary preparation of a novel Probiotic, Bacillus coagulans, as a food ingredient. Food Chem. Toxicol. 2009;47:1231–1238.10.1016/j.fct.2009.02.018
