Abstract
The improved salt tolerance effects of He–Ne laser were further studied through the estimation of ROS levels, cell viability, DNA damage phenomena, physicochemical properties, and monosaccharide compositions of cell wall polysaccharides in tall fescue seedlings. Salt stress produced deleterious effects on seedlings growth and development. ROS levels and genomic DNA damage were markedly increased compared with controls. Physicochemical activities and monosaccharide proportions of cell wall polysaccharide were also pronouncedly altered. He–Ne laser irradiation improved plant growth retardation via increasing cell viability and reverting physicochemical parameters. According to the results of Fourier transform infrared (FTIR) scanning spectra and DNA apopladder analysis, He–Ne laser was showed to efficiently ameliorate cell wall polysaccharide damage and DNA fragmentation phenomena. The treatment with DNA synthesis inhibitor further demonstrated that DNA damage repair was correlated with the improvement effects of the laser. Therefore, our data illustrated that He–Ne laser irradiation resulted in cell wall reconstruction and genomic DNA injury repair in vivo in salt-stressed seedlings, then enhanced salt tolerance probably via interactions between plant cell wall and related resistance gene expression pattern.
Graphical abstract
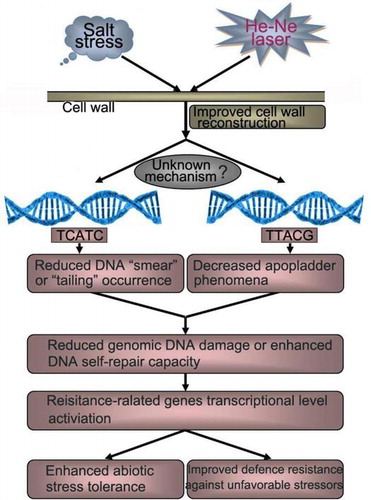
A proposed model regarding the improved salt resistance of He–Ne laser irradiation through modulating plant cell wall reconstruction and genomic DNA damage repair capacity.
The cell wall of plants, as a dynamic and crucial component of the plant cell, generally regulates the morphogenesis, physiological processes, and biochemical metabolisms in plants,Citation1−3) and also mediates interactions between the cell and its environment, including a variety of environmental stresses.Citation4–6) The cell wall is the major location of plant responses to a variety of environmental signals due to such is the site where the plant cell first meets these signals.Citation7) It is well known that plant cells consist of two types of cell walls: primary and secondary cell walls. Primary cell walls are found at the surface of plant cells and composed of celluloses, pectins, hemicelluloses, proteins, and phenolic compounds.Citation8) The primary walls can generate turgor pressure, thus resisting tensile forces, accommodating cell expansion and mediating cell adhesion. While secondary cell walls are formed in specific types of differentiated cells and consist of celluloses and hemicelluloses, often being encrusted with lignin compositions. The secondary walls are usually thicker than the primary walls and are more resistant to various compressive forces from external environment.Citation9,10) Cellulose forms the main structural component of the cell wall and is a relatively homogenous polymer of β-1,4-linked glucose. The pectins and hemicelluloses polysaccharides are heterogeneous in nature and comprise polymers of different sugar compositions. Therefore, cell wall polysaccharides, the predominant compositional polymers of plant cell wall, exert spatial and temporal control on cellular morphogenesis and plant developmental processes.Citation11) Although cell walls are generally referred to sugar-rich structures, their composition and chemical structure vary highly among different species and even among different organs or various tissues or among the same tissue under different growth conditions.Citation12) To our knowledge, there is still a lack of information regarding monosaccharide compositions and physicochemical characteristics of cell wall polysaccharides of tall fescue seedlings. In addition, recently, carbon-rich materials of plant cell wall components derived from the straw and husk have been converted into bio-ethanol and other invaluable, renewable materials, such as fiber, fuel, and paper.Citation13,14) To facilitate plant cell wall-derived resources usages and rational manipulation, it is imperative to further gain better insight into the details of the monosaccharide compositions and physicochemical characteristics of cell wall polysaccharides in many plants including tall fescue seedlings, in particular under unfavorable environment stress.
Salt stress is one of the major environmental stressors throughout the world that can cause multifarious adverse effects on plant physiological processes and biochemical metabolisms.Citation13,14) In recent years, soil salinity drastically restricts agricultural crops or commercial crops productivity in many countries due to the increased salinization of arable land that would result in about thirty percent land loss within the next fifteen years all over the world.Citation10,15–17) The effects of salt stress on plant cell wall polysaccharide compositions, cell wall degrading enzymes, and organelle ultra-structure have been extensively studied in many plants species, such as blueberries fruit,Citation18) coffee leaf,Citation10) oryza sativa plants,Citation19) cucumber seedlings,Citation20) and soybean roots.Citation21) Furthermore, previous studies have illustrated that the suitable doses of He–Ne and CO2 laser, commonly applied in crops and many plants, play a positive role in accelerating plant growth and metabolismCitation22–25) and also protect plants seedlings against cellular damages induced by unfavorable environmental stressors like salt stress,Citation26) enhanced UV-B radiation,Citation27–29) chilling stress,Citation30) osmotic stress,Citation23) drought stress,Citation22) or cadmium stress.Citation25) These reports demonstrated that the laser treatment enhanced plants tolerance mainly through improving seed germination rates, plant height, root length and numbers, seedlings biomass, enzymatic activities, antioxidant compounds biosynthesis, and chlorophyll contents in seedlings subjected to environmental stressors.Citation25,26,28,31) However, some researchers have assumed that there is a link between the use of He–Ne laser irradiation as a protective method in enhancing resistance to a variety of environmental stressors and cell wall reconstruction mediating by cell wall polysaccharides.Citation10) Thus, it is also urgent to obtain information on plant cell wall polymers, in particular cell wall polysaccharides, responses to a combination treatment of low power of He–Ne laser irradiation and salt stress.
Salt stress also caused the formation of cell apoptosis that could be visualized by DNA fragmentation or DNA laddering analysis. As oxidation of DNA bases is the main source of DNA damage, a significant increase in intracellular ROS levels induced by a variety of unfavorable stressors would further induce DNA strand breakage and DNA fragmentation, leading to enhanced DNA damage, and finally resulting in the formation of programmed cell death (PCD). These results suggested that the structure and arrangement of plant cell genomic DNA can be significantly altered or damaged under different environmental stresses conditions, such as salt stress.Citation32,33) In preliminary studies, we found that He–Ne laser pre-illumination enhanced salt tolerance in tall fescue seedlings via up-regulation expression level of some antioxidant enzyme genes and phytochrome B gene.Citation26) Then, under the combined treatments of salt stress and He–Ne laser irradiation, whether DNA fragmentation induced by salt stress can be repaired by He–Ne laser or if there is a link between the occurrence of PCD and the related resistance gene expression levels, the resistance of plants to salt stress induced by the laser. These problems were also the primary objective of our investigations.
Taken together, this article describes the monosaccharide compositions and physicochemical characteristics of cell wall polysaccharides in tall fescue leaf and root tissues under salt stress alone or a combination treatment of He–Ne laser irradiation and salt stress and assesses the compositional proportions and chemical properties of cellulose, hemicelluloses, and pectic fractions polysaccharides. The primary aim was to study the effect of He–Ne laser irradiation on cell wall reconstruction mediating by cell wall polysaccharides and DNA fragmentation in tall fescue seedlings, and evaluate the role of cell wall reconstruction and DNA damage repair in the induction of enhanced adaption capacity to saline conditions by the laser irradiation, and further explore the physicochemical mechanism of the protective effects of He–Ne laser illumination on plants under unfavorable growth conditions.
Materials and methods
Plant materials and culture condition
Mature seeds of tall fescue (Festuca arundinacea Schreb. cvs. Houndog5) obtained from Beijing Clover Seed&Turf Co., Ltd., China, were surface sterilized for 10 min in 6.0% (v/v) sodium hypochlorite, rinsed three times in distilled water, and then germinated in petri dishes with moistened filter paper for 7 d under darkness at room temperature (24 ± 2 °C), a 70% of relative humidity. After germination of 3 d, tall fescue seedlings were cultured hydroponically in 5L black plastic containers containing suitable volume of Hoagland’s nutrient solution for 10 d in a plant incubator (PGX-250B, AC 220 V/50HZ, 600 W, Shanghai Fuma Laboratory Instrument Co., Ltd.) at 24 ± 2 °C, 70% relative humidity, a photoperiod 14/10 h day/night cycle, and a photosynthetic photo flux density (PPFD) 100 μmol m−2 s−1.Citation34,35) Light was provided by 20 W conventional white fluorescent lamps. Each container contained thirty seedlings, and Hoagland’s nutrient solution was renewed once a week according to the method of Gao et al.Citation26)
Salt stress and He–Ne laser irradiation
Salt stress and He–Ne laser irradiation were carried out with 13 d-old seedlings after pre-culture of 10 d according to the presupposed treatment procedure as shown in Table . There were nine different treatments with five replications. Seedlings were stressed with 150 mM sodium chloride (NaCl) alone for 10 d,Citation26) or pretreated with a continuous wave of He–Ne laser or a combination of He–Ne laser and hydroxyurea (U, 10 mM) for 7 d prior to salt stress. He–Ne laser was obtained with the He–Ne laser generator (wavelength λ = 632.8 nm, flux rate 5.0 mW mm−2,Citation26) initial beam diameter 2.0 mm, and final diameter 100.0 mm via amplifier, made by Nanjing Laser Instrument Factory, Nanjing City, China), and the irradiation duration was 2, 4, 6 min per day, respectively. Laser irradiation treatments were performed at 06:00–06:30 am in darkness to prevent the influence of stray light. To gain better insight into the role and physicochemical mechanism of He–Ne laser radiation in improvement salt resistance of tall fescue seedlings, He–Ne laser treatment alone was also applied in tall fescue seedlings for 2 min (H2), 4 min (H4), and 6 min (H6) per day, respectively. The combined treatment with He–Ne laser and 10 mM hydroxyurea prior to salt stress was used for demonstrating the role of genomic DNA in the effect of the laser on salt-stressed seedlings. At the same time, control seedlings were cultured in Hoagland’s nutrient solution and not treated with any stresses. Finally, leaves and roots from the 30 d-old seedlings were harvested and frozen in liquid nitrogen (N2), and then stored at −80 °C for further analysis of physiological parameters and biochemical parameters.
Table 1. Treatment procedure of different groups of tall fescue seedlings.
Growth parameters of tall fescue seedling measurement
Leaf and root development in response to the combined treatments of salt stress and He–Ne laser irradiation were measured with an Portable Scanning Measuring Instrument (Canon, CanoScan LiDE 90), and the values of fresh weight, leaf length, leaf width, leaf area index, plant height, and root length were recorded, respectively. Leaf length and root length were measured with the 30 d-old seedlings using a meter stick.
ROS levels assay
Hydrogen peroxide (H2O2) contents were determined by the peroxidase-coupled assay according to the method of Zhao et al.Citation36) Superoxide radical (O2•−) production was measured according to Gao et al.Citation26) Membrane lipid peroxidation was estimated by the level of malondialdehyde (MDA) production by the method of Xu et al.Citation35)
Cell viability and apopladder assay
Since cell viability was usually used as an index of cellular necrosis rates or cellular damage degree, it was also determined in leaves and roots material with the trypan blue staining assay kit (Beyotime Institute of Biotechnology, Shanghai, China) according to the specification protocol and instructions. After being immersed for 5–8 min with trypan blue dye in 10-ml eppendorf centrifuge tubes, fresh leaves and roots material were observed under optical microscopy (Olympus BX53, Japan, 10×, bar = 500 μm).
Prevailing research results have shown that diverse environmental stressors, including salt stress, can induce cell apoptosis formation and DNA laddering phenomena. Thus, it is necessary to further perform programmed cell death assay and DNA fragmentation determination. The total genomic DNA from 0.5 g fresh plant materials was extracted using the Plant MiniBEST Genomic DNA Extraction Kit (TaKaRa, Japan) according to the manufacturer’s instructions. The completed DNA relative concentration was measured by ultraviolet–visible (UV) spectrophotometer (Model U-2900, Hitachi, Japan). The DNA sample was diluted with TE buffer at the same time, and further analyzed with 5 μL diluted sample using 0.8% agarose gel electrophoresis in the presence of 2 μg/mL ethidium bromide (EB). DNA fragmentation pattern or apopladdering was separated and detected by ApopLadder Ex Reagent (TaKaRa, Japan). Dilute DNA sample with elution buffer at 1.0 μg/μL ratio, then the 5 μL sample from different treatment groups mixed with 6× Loading buffer at a proportion of 5:1, was loaded on 1.5% agarose gel containing 2 μg/mL EB dye for DNA laddering analysis. The signal strength of DNA bands from different treatments in agarose gel was evaluated by gel analysis software (Quantity One, Bio-Rad, USA).
Cell wall sequential extraction
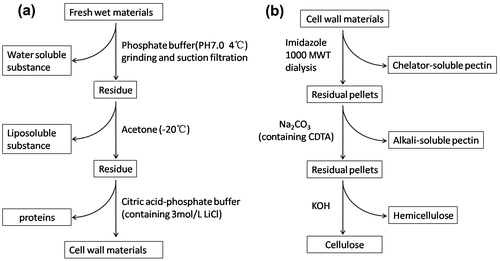
The cell wall compositions of leaf and root materials, including celluloses, hemicelluloses, and pectins, were evaluated by sequentially extracting cell wall materials as described by Albini et al.Citation37) The process of the preparation and sequential extraction of cell wall materials is illustrated in Fig. . Cell wall materials (3.0 g) were extracted with 0.5 M imidazole solution (pH 7.0) in a thermostat oscillator (HY-5, Changzhou, Jiangsu) at room temperature for 24 h, and salt in the supernatant and solid pellets were removed by dialysis with Visking dialysis tubing (1000MWT, USA) in 3-L flasks of distilled water, followed by reduced pressure condense, to recover the chelator-soluble pectins. The residual pellet was then extracted with 50 mM Na2CO3 (containing 20 mM Cyclohexane-diamine-tetraacetic Acid, CDTA) to obtain the alkali-soluble pectins. Finally, the pellet was extracted using 4.0 M concentration of KOH to solubilize hemicelluloses, and recovered celluloses. The dialysis water in all extractants was renewed twice daily, and the process was carried out for 10 d. The resulting samples were freeze-dried for subsequent analysis.
Monosaccharide compositions assessment
The monosaccharide compositions and yields of cell wall samples from different treatments were evaluated by gas chromatography (GS) analysis technology. Samples were prepared with trifluoroacetic acid (5 h, 100 °C), evaporated to dryness, then dissolved in water, and finally acetylated with absolute acetic anhydride P. A. as described methods of Lima et al.Citation10) and Zhao et al.Citation38) for 30 min at 100 °C. The resulting products were determined by GS analysis system (Model Agilent 7890, USA). 2-Deoxyglucose was added as the internal standard, and nitrogen was employed as the carrier gas.
Fourier transform infrared spectroscopy (FTIR) analysis
For FTIR analysis, cell wall material samples were dried at 55 °C in an Abderhalden dryer and stored in desiccators. Agarose gel electrophoresis was performed for the detection of nucleic acid in cell wall polysaccharide samples (2 mg/ml, 5 μL).Citation39) Pellets or granules of different treatments were prepared and ground with a agate mortar and pestle from mixtures of the samples with potassium bromide (KBr, SP, spectrum pure) at a 1:100 (w/w) ratio. FTIR spectra bands were recorded over a region of 2000–500 cm−1 at a resolution of 4 cm−1 in absorbance mode with a Varian 660-IR Fourier transform infrared spectrometer (USA) equipped with a MCT detector. The spectra were averaged using 64 scans.
Statistical design
All data from five replicates were expressed as means ± SD (standard deviations). Statistical analysis was performed by the student’s t-test. Differences among treatment samples were considered significant at P < 0.05.
Results
Effects of He–Ne laser irradiation on plant growth
Salt stress remarkably inhibited leaf and root development of 30-d-old tall fescue seedlings (Table ). Compared to controls, the whole plant fresh weight under salt stress conditions reduced by 60.71%, and leaf area index also decreased from 2.3 to 0.8. Significant decreases regarding leaf length, leaf width, root length, and plant height were observed in seedlings treated with 150 mM NaCl. However, He–Ne laser irradiation significantly improved deleterious effects of salt stress on plant growth and development. The laser irradiation alone also accelerated the expansion and development of leaf and root. The 2 min per day or 4 min per day of He–Ne laser irradiation was the most effective treatment for plant growth when under non-saline condition or saline condition, respectively. So we concluded that the 4 min per day of He–Ne laser improved salt tolerance of seedlings, whereas the 2 min per day of the laser alone was advantageous to plant growth under normal conditions.
Table 2. Effects of He–Ne laser irradiation on plant growth and development.
Effects of He–Ne laser irradiation on ROS levels
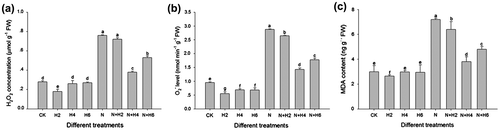
Fig. showed the effects of salt stress and He–Ne laser irradiation on ROS production and physiological parameters. Salt stress resulted in significant increases in H2O2 level, O2•− generation rates, and MDA contents, whereas He–Ne laser irradiation significantly decreased H2O2 level, O2•− generation rates, and MDA relative amounts compared to salt-stressed plants, suggesting that plants with the laser irradiation might face less stress because He–Ne laser improved ROS level and membrane lipid peroxidation degree. The 4 min per day of He–Ne laser was the most efficient treatment among all groups. Moreover, the 2 min per day of the laser treatment alone also significantly decreased ROS accumulation and lipid peroxidation in plants when under normal conditions. In addition, the combined treatments with DNA synthesis inhibitor (U) and He–Ne laser (2, 4, 6, 8, 10 min per day, respectively) had not altered higher ROS levels and lipid peroxidation injury in plant cells under salt stress (Supplementary materials Fig. 1), suggesting that the combined treatments with DNA synthesis inhibitor and He–Ne laser had not attenuated the detrimental effects on seedlings growth caused by salt stress. Thus, we assumed that the genomic DNA might be involved in the progress of improved salt tolerance by He–Ne laser irradiation.
Fig. 2. ROS level (a, H2O2 relative content; b, the generation rate of O2•−) and MDA contents (c) in tall fescue seedlings under He–Ne laser irradiation and salt stress.
Notes: Data are presented as the means ± SD from five independent experiments (n = 5). Different letters followed with bars indicate significant differences at p < 0.05 according to Duncan’s multiple range test. CK: controls without any stress treatment; H2, H4, H6: He–Ne laser illumination alone for 2, 4, and 6 min d−1 under normal conditions, respectively; N: salt stress (150 mM) for 10 d; N+H2, N+H4, N+H6: He–Ne laser illumination for 2, 4, and 6 min d−1 prior to salt stress.
Cell viability assay
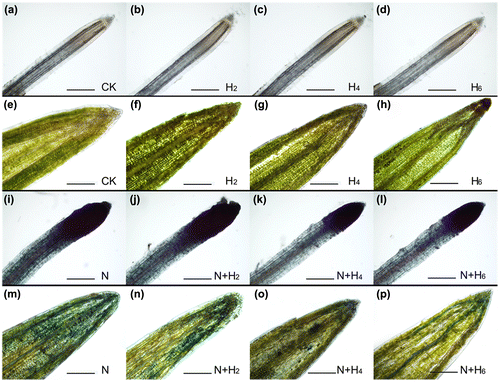
The effects of He–Ne laser irradiation on plant cell toxicity in vivo induced by salt stress were evaluated through cell viability assay with trypan blue staining method (Fig. ). Salt stress resulted in an obvious reduction of cell viability in leaves and roots of tall fescue seedlings (Fig. (N)). Furthermore, cell viability in roots was much lower than that in leaves under the same NaCl treatment. While He–Ne laser treatment significantly enhanced cell viability in both leaves and roots from salt-stressed seedlings, and this mitigated effect was much pronounced in leaves than in roots (Fig. N+H2, N+H4, N+H6). In addition, He–Ne laser had no effect on cell viability under normal conditions, except for 2 min per day of the laser that remarkably enhanced cell viability compared with controls (Fig. H2, H4, H6).
Fig. 3. Effects of salt stress and He–Ne laser irradiation on cell viability with trypan blue staining assay kit in tall fescue seedlings (a–d, i–l: root tissues; e–h, m–p: leaf tissues).
Notes: CK: controls without any stress treatment; H2, H4, H6: He–Ne laser illumination alone for 2, 4, and 6 min d−1 under normal conditions, respectively; N: salt stress (150 mM) for 10 d; N+H2, N+H4, N+H6: He–Ne laser illumination for 2, 4, and 6 min d−1 prior to salt stress. 10×, bar = 500 μm.
Apopladder assay and DNA damage analysis
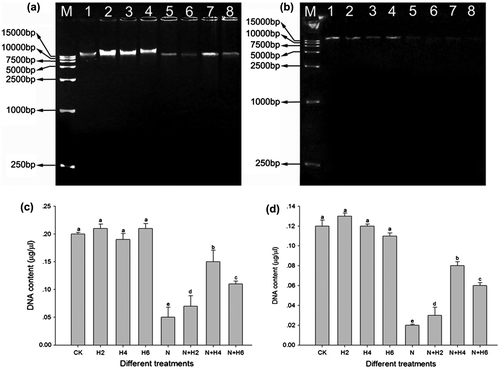
The completed genomic DNA relative contents analysis further established significant protection effects of the laser against stress damage. Salt stress significantly decreased completed genomic DNA concentrations in leaves (Fig. (a) and (c)) and roots (Fig. (b) and (d)) material. Under normal conditions, He–Ne laser treatment alone had no effect on completed genomic DNA relative amounts that were similar with controls. However, He–Ne laser irradiation prior to salt stress remarkably increased DNA contents compared with salt-stressed plants. And the 4 min per day of the laser irradiation was still more efficient when under salt stress.
Fig. 4. The agarose gel electrophoresis analysis and the completed genomic DNA relative contents determination in leaves and roots of tall fescue seedlings (a,c, leaf tissues; b,d, root tissues).
Notes: The results of agarose gel electrophoresis analysis (a, leaf tissues; b, root tissues). M: DNA marker; 1: controls without any stress treatment; 2, 3, 4: He–Ne laser illumination alone for 2, 4, and 6 min d−1 under normal conditions, respectively; 5: salt stress (150 mM) for 10 d; 6, 7, 8: He–Ne laser illumination for 2, 4, and 6 min d−1 prior to salt stress, respectively. The completed genomic DNA relative contents (c, leaf tissues; d, root tissues) according to signal strength of DNA on the agarose gel. Data are presented as the means ± SD from five independent experiments (n = 5). Different letters followed with bars indicate significant differences at p < 0.05 according to Duncan’s multiple range test. CK: controls without any stress treatment; H2, H4, H6: He–Ne laser illumination alone for 2, 4, and 6 min d−1 under normal conditions, respectively; N: salt stress (150 mM) for 10 d; N+H2, N+H4, N+H6: He–Ne laser illumination for 2, 4, and 6 min d−1 prior to salt stress.
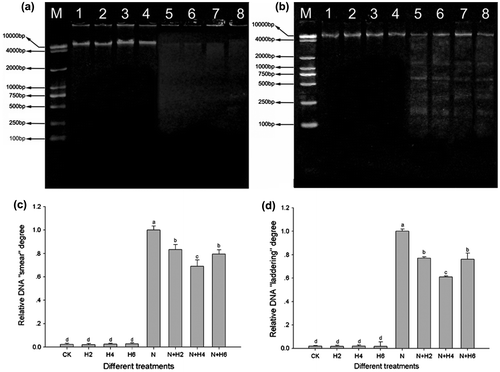
In preliminary studies, we found that the enhanced salt tolerance of plants was correlated with resistance-related gene up-regulation expression induced by He–Ne laser.Citation26) It is very clear that prolonged salt stress would induce DNA damage and DNA fragmentation phenomena. Therefore, it is worth exploring whether the laser can protect genomic DNA against salt stress, and if resistance-related gene up-regulation expression has correlation with the protection of He–Ne laser on genomic DNA. The results of agarose gel electrophoresis showed that salt stress resulted in a significant “smear” phenomena in leaf and root samples, while DNA laddering or DNA fragmentation (<2000 bp) (Fig. ) only in root samples, suggesting that PCD only happened in roots, but not in leaves. However, both DNA smear phenomena (in leaves and roots) and DNA laddering brand (in roots) significantly reduced with He–Ne laser irradiation prior to salt stress, and the 4 min per day of the laser was the most effective treatment (Fig. a-7, b-7, c-N+H4, d-N+H4). These observations illustrated that He–Ne laser irradiation ameliorated genomic DNA injury via reducing DNA “smear” phenomena (in leaves and roots) and DNA laddering brand (in roots), finally modulated some stress resistance-related genes transcriptional levels. In addition, DNA “smear” phenomena and DNA apopladdering band (in leaves and roots) had not been significantly detected in plants under normal conditions or He–Ne laser treatment alone (Fig. a,b-1, 2, 3, 4; c,d-CK,H2,H4,H6), further demonstrating that the laser had no obvious effects on genomic DNA under normal conditions.
Fig. 5. Apopladder assay and DNA damage analysis under salt stress and He–Ne laser irradiation in leaves and roots of tall fescue seedlings (a, c, leaf tissues; b, d, root tissues).
Notes: The results of agarose gel electrophoresis analysis (a, leaf tissues; b, root tissues). M: 1 kb DNA ladder marker; 1: controls without any stress treatment; 2, 3, 4: He–Ne laser illumination alone for 2, 4, and 6 min d−1 under normal conditions, respectively; 5: salt stress (150 mM) for 10 d; 6, 7, 8: He–Ne laser illumination for 2, 4, and 6 min d−1 prior to salt stress, respectively. Relative DNA “smear” degree (c, leaf tissues) and DNA “laddering” degree (d, root tissues). Data are presented as the means ± SD from five independent experiments (n = 5). Different letters followed with bars indicate significant differences at p < 0.05 according to Duncan’s multiple range test. CK: controls without any stress treatment; H2, H4, H6: He–Ne laser illumination alone for 2, 4, and 6 min d−1 under normal conditions, respectively; N: salt stress (150 mM) for 10 d; N+H2, N+H4, N+H6: He–Ne laser illumination for 2, 4, and 6 min d−1 prior to salt stress.
Effects of He–Ne laser on cell wall polysaccharide properties and monosaccharide composition yield
To investigate the effects of salt stress on the yield of monosaccharide composition, cell wall monosaccharide composition was determined and analyzed after cell wall polysaccharides had been completely hydrolyzed, and monosaccharide composition and relative contents were presented in Table . The results showed that prolonged salt stress significantly enhanced glucose and mannose proportions and decreased fucose, arabinose, and galatose yields. There was no obvious effect on the xylose and rhamnose contents of cell wall polysaccharides. He–Ne laser irradiation further remarkably increased glucose and mannose amounts and also enhanced arabinose proportions in cell wall polysaccharides. These results implied that glucose, mannose, and arabinose might have a link or play an important role in enhancement of salt tolerance with He–Ne laser in tall fescue seedlings. However, the laser alone had no effect on all monosaccharide amounts. Therefore, seedlings (only H4 group and N+H4 group) irradiated with He–Ne laser were used for FTIR spectrum of cell wall polysaccharide determination at the end of experiment.
Table 3. Effects of He–Ne laser irradiation on the monosaccharide composition proportions of cell wall polysaccharide of tall fescue seedlings.
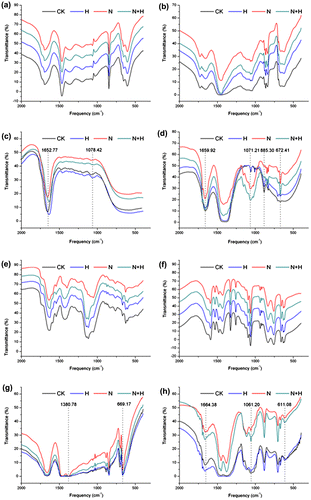
The FTIR spectrum of cell wall materials showed cell wall polysaccharides bands (at 1652.77, 1078.42, 1659.92, 1071.21, 885.30, and 672.41 cm−1) in leaf cell hemicellulosic and cellulosic fractions, and at 1380.78, 669.17, 1664.38, 1061.20, and 611.08 cm−1 in root cell hemicellulosic and cellulosic fractions extracted from plants subjected to salt stress alone were remarkably increased or displaced compared to controls (Fig. ). There was no obvious alteration in the pectin fractions (including the chelating pectin and the alkali-soluble pectin) from both leaves and roots of seedlings under salt stress. These results are consistent with the data of monosaccharide composition analysis (Table ), suggesting that salt stress primarily caused changes in physicochemical property in the hemicelluloses and celluloses fractions in the cell wall materials of tall fescue seedlings. Bands near 1645, 1420, and 1043 cm−1 are typical absorbance of polysaccharides, indicating C–H bending or C–O or C–C stretching modes. The results of agarose gel electrophoresis illustrated that there were no nucleic acids in all samples from different treatments.
Fig. 6. The FTIR spectra analysis of cell wall polysaccharides (a, e, the chelating pectin fraction; b, f, the alkali-soluble pectin fraction; c, g, hemicelluloses; d, h, celluloses) from leaves (a–d) and roots (e–h) materials of tall fescue seedlings.
Notes: CK: controls without any stress treatment; H: He–Ne laser illumination alone for 4 min d−1 under normal conditions; N: salt stress (150 mM) for 10 d; N+H: He–Ne laser illumination for 4 min d−1 prior to salt stress.
He–Ne laser irradiation prior to salt stress alleviated abnormal changes of cell wall polysaccharides FTIR spectrum bands in the “fingerprint” regions in both leaf and root materials. Thus, we supposed that the He–Ne laser irradiation might protect hemicelluloses and celluloses fractions from cell wall polysaccharides against salt stress-induced physicochemical injuries, and it was contributed to cell wall reconstruction in plants.
Discussion
This study has demonstrated that a low power of continuous wavelength of He–Ne laser (632.8 nm) irradiation would be contributed to enhance salt tolerance in tall fescue seedlings and also might accelerate plant growth and development, and biochemical metabolism under normal conditions. This phenomenon was supported by the changes of agronomic traits (Table ) and physiological parameters (including ROS levels, MDA content, Fig. ) induced by He–Ne laser in our experiments, and the results were in agreement with our observations in preliminary studies.Citation26) Similar observations on the effect of He–Ne laser irradiation were reported by Chen and HanCitation40,29) in triticum aestivum L. seedlings under enhanced ultraviolet-B radiation,Citation29,40) and also by Qiu et al. in wheat seedlings subjected to osmotic stressCitation23) or cadmium (Cd2+) stress environments.Citation25)
Enhanced tolerance of plants to salt stress is usually viewed as being closely associated with the increased cell viability.Citation41–43) In this study, both leaves and roots of tall fescue seedlings with He–Ne laser irradiation exhibited pronounced increase in cell viability via trypan blue staining assay kit (Fig. ), suggesting that the laser treatment protected plant seedlings against physiological damages and cytotoxicity effects induced by salt stress.Citation44,45) Moreover, under salt stress conditions, a greater increase in cell viability was found in leaves than roots, indicating that the protective effects of He–Ne laser against salt stress might seem more effective in leaves than in roots. These results further demonstrated that He–Ne laser irradiation could enhance salt tolerance of seedlings to salt stress. Our data also demonstrated that the 2 min per day or 4 min per day of the laser was the most efficient in improved cell viability of plants when under normal conditions or under salt stress, respectively.
In preliminary experiments, we found that plant antioxidant systems played an important role in the progress of improved salt resistance induced by He–Ne laser irradiation. He–Ne laser reduced oxidative damage of seedlings due to the interactions between ROS productions accumulation, and antioxidant compounds biosynthesis induction and antioxidant enzyme genes expression up-regulation by the laser.Citation26) Then, whether the expression up-regulation of related resistance gene had a correlation with genomic DNA damage repair, or if He–Ne laser can alleviated DNA sequence and structure injuries induced by salt stress? These problems need to be further investigated and analyzed. The data of Fig. (c) and (d) showed that He–Ne laser irradiation significantly enhanced completed genomic DNA relative amounts in leaves and roots of seedlings under salt stress, whereas had no obvious effects on DNA contents in plants under normal conditions. The agarose gel electrophoresis results of Fig. (a) and (b) further supported these findings, and the 4 min per day of the laser irradiation was the most efficient treatment for salt-stressed plants among all groups. This is consistent with the cell viability assay results (Fig. ).
In subsequent experiment, we also evaluated programmed cell death occurrence by apopladder assay. The results showed that salt stress caused significant DNA “smear” or “tailing” phenomena in both leaves and roots cells, the occurrence of which could be ameliorated by He–Ne laser irradiation. DNA fragmentation or DNA laddering formation was only observed in roots, but not in leaves, indicating that salt stress resulted in programmed cell death only in root tissues, while cell death in both roots and leaves. We assumed that the main reason might be related to the higher resistance to stress environment in roots than in leaves due to roots absorbed more water through stretching to the remote area. However, He–Ne laser treatment improved DNA “smear” and DNA “laddering” phenomena in leaves and roots, and the 4 min per day of the laser was the most efficient treatment. Our data implied that He–Ne laser irradiation could protect seedlings genomic DNA sequence and structure against different degree of damages caused by salt stress. Chen and Han also found that He–Ne laser had obviously reduced DNA laddering area in wheat seedlings under enhanced UV-B radiation.Citation24) Under normal conditions, the laser had no effect on genomic DNA structure and contents. Hence, we concluded that the laser treatment probably maintained the structural integrity of plant genomic DNA under unfavorable growth conditions, and consequently activated relevant gene transcriptional levels, finally improved tall fescue seedlings stress-resistance capability (Fig. ). The results of the combination with DNA synthesis inhibitor and He–Ne laser irradiation on lipid peroxidation (MDA contents and relative electrolyte leakage) and ROS metabolism (Supplementary materials Fig. 1) further showed genomic DNA expression pattern was essential for salt tolerance of seedlings. At the same time, we also assumed that the 2 min per day of He–Ne laser irradiation alone was contributed to plant growth under normal condition, which was most likely attributed to accelerating physicochemical metabolisms in vivo in plants by the laser, but not relevant gene transcriptional level activation and the integrity of genomic DNA. However, the detailed mechanism of the improvement of plant growth by He–Ne laser irradiation under non-saline conditions needs to be further investigated in further experiments.
Consequently, in order to further clarify the role of cell wall in enhanced salt tolerance effects of He–Ne laser irradiation, cell wall primary compositions, that is cell wall polysaccharides, including pectins, hemicelluloses, and celluloses physicochemical properties and yields extracted from plants under salt stress or combined treatments of salt stress and He–Ne laser irradiation, have been evaluated to obtain information regarding the changes in the cell wall materials. The results illustrated that glucose, fucose, arabinose, mannose, galatose, xylose, and rhamnose were the dominant monosaccharide compositions among the cell wall polysaccharides for tall fescue seedlings. Compared to control plants, prolonged salt stress caused a remarkable increase in glucose and mannose proportions. On the contrary, fucose, galatose, and arabinose proportions were reduced under long-range salt stress environment, whereas there were no significant effects in xylose and rhmnose contents. This result demonstrated that glucose and mannose biosynthesis in tall fescue were induced, while fucose, galatose, and arabinose were inhibited by salt stress. Based on monosaccharide yield analysis, the higher relative concentrations of glucose and mannose were supposed to be likely linked with salt resistance of tall fescue seedlings. He–Ne laser irradiation alleviated the inhibitory effects of arabinose biosynthesis, and also further enhanced glucose and mannose contents. And the 4 min per day of the laser was the most effective for the biosynthesis of three sugars. This is consistent with the postulations that glucose and mannose are probably the predominant monosaccharides being correlated with salt tolerance.
According to the results of Fig. , we found that salt stress primarily induced FTIR spectral increases or displaces in hemicelluloses and celluloses fractions, which implied that cell wall polysaccharides physicochemical properties were affected. However, the laser treatment mitigated these increments or displaces, indicating that He–Ne laser also protected cell wall materials against physicochemical injury, consequently improved cell wall reconstruction under salt stress, maintained higher physiological activity of intracellular compartmentation and genomic DNA integrity, and finally enhanced salt tolerance of plants (Fig. ).
Fig. 7. Schematic of a putative mechanism through which cell wall polysaccharides are protected against physicochemical damages induced by salt stress that result in plant cell wall structure reconstruction.
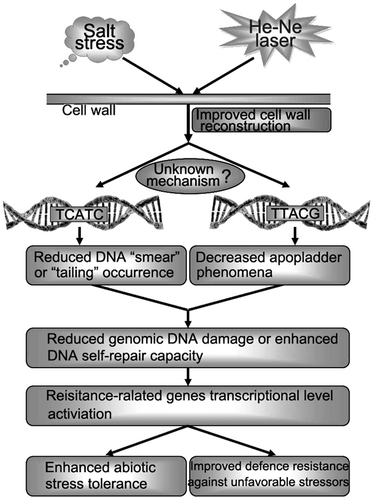
In conclusion, the two important findings of this study were the protection effects of He–Ne laser on genomic DNA damage, and cell wall polysaccharides physicochemical properties and monosaccharide composition proportion alterations induced by salt stress. He–Ne laser irradiation attenuated genomic DNA damage due to decreasing DNA “smear” phenomena and DNA laddering area. Based on polysaccharide properties and compositional analysis, glucose and mannose were observed to have significantly accumulated when under salt stress, even further enhanced when under combined treatments of salt stress and He–Ne laser irradiation. In addition, arabinose was also induced by the laser irradiation. These data establish a basis for further evaluations of which monosaccharide in cell wall polysaccharides contributes to variations in early growth stage of plants under salt stress, and its role in He–Ne laser improved plant’s adaptive capability to salt stress environment.
Authors contributions
Limei Gao, Yongfeng Li, and Rong Han conceived and designed the experiments. Limei Gao and Yongfeng Li performed the experiments. Limei Gao and Yongfeng Li analyzed the data. Yongfeng Li contributed reagents/materials/analysis tools. Limei Gao and Yongfeng Li wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the National Nature Science Foundation of People’s Republic of China [grant number 31301245]; Shanxi Province Nature Science Foundation [grant number 2014011028-5]; Nature Science Foundation of Shanxi Normal University [grant number ZR1414].
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2015.1101335.
A_final_version_of_supplementary_materials.docx
Download MS Word (133.2 KB)Acknowledgements
We thank Analysis and Testing Center, Shanxi Normal University, for helpful comments on data determination and analysis.
Notes
Abbreviations: ROS, reactive oxygen species; FTIR, Fourier transform infrared; MDA, malondialdehyde; GS, gas chromatography; PPFD, photosynthetic photo flux density; H2O2, hydrogen peroxide; O2•−, superoxide radical; PCD, programmed cell death.
References
- Albersheim P, An J, Freshour G, et al. Structure and functions studies plant cell wall polysaccharides. Biochem. Soc. Trans. 1994;22:374–378.10.1042/bst0220374
- Mierczyńska J, Cybulska J, Sołowiej B, et al. Effect of Ca2+, Fe2+ and Mg2+ on rheological properties of new food matrix made of modified cell wall polysaccharides from apple. Carbohyd. Polym. 2015;133:547–555.10.1016/j.carbpol.2015.07.046
- Martínez-Sanz M, Gidley MJ, Gilbert EP. Application of X-ray and neutron small angle scattering techniques to study the hierarchical structure of plant cell walls: a review. Carbohyd. Polym. 2015;125:120–134.10.1016/j.carbpol.2015.02.010
- Domon JM, Baldwin L, Acket S. Cell wall compositional modifications of Miscanthus ecotypes in response to cold acclimation. Phytochemistry. 2013;85:51–61.10.1016/j.phytochem.2012.09.001
- Fernandes JC, García-Angulo P, Goulao LF. Mineral stress affects the cell wall composition of grapevine(Vitis vinifera L.) callus. Plant Sci. 2013;205:111–120.10.1016/j.plantsci.2013.01.013
- Zagorchev L, Kamenova P, Odjakova M. The role of plant cell wall proteins in response to salt stress. Sci. World J. 2014. Available from: http://dx.doi.org/10.1155/2014/764089
- Hoson T, Wakabayashi K. Role of the plant cell wall in gravity resistance. Phytochemistry. 2015;112:84–90.10.1016/j.phytochem.2014.08.022
- Francoz E, Ranocha P, Nguyen-Kim H, et al. Roles of cell wall peroxidases in plant development. Phytochemistry. 2015;112:15–21.10.1016/j.phytochem.2014.07.020
- Doblin MS, Pettolino F, Bacic A. Evans Review: Plant cell walls: the skeleton of the plant world. Funct. Plant Biol. 2010;37:357–381.10.1071/FP09279
- de Lima RB, dos Santos TB, Vieira LGE, et al. Salt stress alters the cell wall polysaccharides and anatomy of coffee (Coffea arabica L.) leaf cells. Carbohyd. Polym. 2014;112:686–694.10.1016/j.carbpol.2014.06.042
- Altartouri B, Geitmann A. Understanding plant cell morphogenesis requires real-time monitoring of cell wall polymers. Curr. Opinin. Plant Biol. 2015;23:76–82.10.1016/j.pbi.2014.11.007
- Vogel J. Unique aspects of the grass cell wall. Curr. Opinin. Plant Biol. 2008;11:301–307.10.1016/j.pbi.2008.03.002
- Ye ZH, York WS, Darvill AG. Important new players in secondary wall synthesis. Trends Plant Sci. 2006;11:162–164.10.1016/j.tplants.2006.02.001
- Oh C-S, Kim H, Lee C. Rice cell wall polysaccharides: structure and biosynthesis. J. Plant Biol. 2013;56:274–282.10.1007/s12374-013-0236-x
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681.10.1146/annurev.arplant.59.032607.092911
- Rady MM. Mohamed Gamal F. Modulation of salt stress effects on the growth, physio-chemicalattributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci. Hortic. 2015;193:105–113.10.1016/j.scienta.2015.07.003
- Alvarez-Gerding X, Espinoza C, Inostroza-Blancheteau C, et al. Molecular and physiological changes in response to salt stress in Citrus macrophylla W plants overexpressing Arabidopsis CBF3/DREB1A. Plant Physiol. Biochem. 2015;92:71–80.10.1016/j.plaphy.2015.04.005
- Chen H, Cao S, Fang X, et al. Changes in fruit firmness, cell wall composition and cell wall degrading enzymes in postharvest blueberries during storage. Sci. Hortic. 2015;188:44–48.10.1016/j.scienta.2015.03.018
- Xavier R. Role of exogenous proline in ameliorating salt stress at early stage in two rice cultivars. J. Stress Physiol. Biochem. 2011;7:157–174.
- Zhen A, Bie Z, Huang Y. Effects of salt-tolerant rootstock grafting on ultrastructure, photosynthetic capacity, and H2O2-scavenging system in chloroplasts of cucumber seedlings under NaCl stress. Acta Physiol. Plant. 2011;33:2311–2319.10.1007/s11738-011-0771-3
- Neves GYS, Marchiosi R, Ferrarese MLL, et al. Root growth inhibition and lignification induced by salt stress in soybean. J. Agron. Crop Sci. 2010;196:467–473.10.1111/jac.2010.196.issue-6
- Qiu ZB, Liu X, Tian XJ, et al. Effects of CO2 laser pretreatment on drought stress resistance in wheat. J. Photochem. Photobiol. B: Biol. 2008;90:17–25.10.1016/j.jphotobiol.2007.09.014
- Qiu ZB, Li JT, Yue M. The damage repair role of He–Ne laser on wheat exposed to osmotic stress. Can. J. Plant Sci. 2010;90:691–698.10.4141/CJPS09118
- Perveen R, Jamil Y, Ashraf M, et al. He–Ne laser-induced improvement in biochemical, physiological, growth and yield characteristics in sunflower (Helianthus annuus L.). Photochem. Photobiol. 2011;87:1453–1463.10.1111/php.2011.87.issue-6
- Qiu ZB, Li JT, Zhang MM, et al. He–Ne laser pretreatment protects wheat seedlings against cadmium-induced oxidative stress. Ecotox. Environ. Safe. 2013;88:135–141.10.1016/j.ecoenv.2012.11.001
- Gao LM, Li YF, Han R. He–Ne laser preillumination improves the resistance of tall fescue (Festuca arundinacea Schreb.) seedlings to high saline conditions. Protoplasma. 2015;252:1135–1148.10.1007/s00709-014-0748-3
- Chen YP. Isatis indigotica seedlings derived from laser stimulated seeds showed improved resistance to elevated UV-B. Plant Growth Regul. 2008;55:73–79.10.1007/s10725-008-9258-7
- Yang LY, Han R, Sun Y. Damage repair effect of He–Ne laser on wheat exposed to enhanced ultraviolet-B radiation. Plant Physiol. Biochem. 2012;57:218–221.10.1016/j.plaphy.2012.06.003
- Chen HZ, Han R. He–Ne laser influenced actin filaments alleviate the damage of UV-B in wheat. Laser Phys. 2015;25:5601.
- Chen YP, Jia JF, Yue M. Effect of CO2 laser radiation on physiological tolerance of wheat seedlings exposed to chilling stress. Photochem. Photobiol. 2010;86:600–605.10.1111/php.2010.86.issue-3
- Laspina NV, Groppa MD, Tomaro MT, et al. Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci. 2005;169:323–330.10.1016/j.plantsci.2005.02.007
- Miyake H, Mitsuya S, Rahman MDS. Ultrastructural effects of salinity stress in higher plants. In: Rai AK, Takabe T, editors. Abiotic stress tolerance in plants. Netherlands: Springer; 2006. p. 215–226.
- Andrea B, Tani C. Ultrastructural effects of salinity in Nicotiana bigelovii var. bigelovii callus cells and Allium cepa roots. Caryologia. 2009;62:124–133.10.1080/00087114.2004.10589677
- Xu YF, Sun XL, Jin JW, et al. Protective effect of nitric oxide on high light-induced oxidative damage in leaves of tall fescue. J. Plant Physiol. 2010;167:512–518.10.1016/j.jplph.2009.10.010
- Xu Y, Fu J, Chu X, et al. Nitric oxide mediates abscisic acid induced light-tolerance in leaves of tall fescue under high-light stress. Sci. Hortic. 2013;162:1–10.10.1016/j.scienta.2013.07.042
- Zhao L, He JX, Wang XM, et al. Nitric oxide protects against polyethylene glycol-induced oxidative damage in two ecotypes of reed suspension cultures. J. Plant Physiol. 2008;165:182–191.10.1016/j.jplph.2007.03.002
- Albini FM, Murelli C, Patritti G, et al. Low-molecular weight substances from the resurrection plant Sporobolus stapfianus. Phytochemistry. 1994;37:137–142.10.1016/0031-9422(94)85013-5
- Zhao X, Moates GK, Wellner N, et al. Chemical characterisation and analysis of the cell wall polysaccharides of duckweed (Lemna minor). Carbohyd. Polym. 2014;111:410–418.10.1016/j.carbpol.2014.04.079
- Chen XQ, Fang YP, Nishinari K, et al. Physicochemical characteristics of polysaccharide conjugates prepared from fresh tea leaves and their improving impaired glucose tolerance. Carbohyd. Polym. 2014;112:77–84.10.1016/j.carbpol.2014.05.030
- Chen HZ, Han R. He–Ne laser treatment improves the photosynthetic efficiency of wheat exposed to enhanced UV-B radiation. Laser Phys. 2014;24. Available from: http://doi:10.1088/1054-660X/24/10/105602
- Chanaj-Kaczmarek J, Wysocki M, Karachitos A, et al. Effects of plant extract antioxidative phenolic compounds on energetic status and viability of Saccharomyces cerevisiae cells undergoing oxidative stress. J. Funct. Foods. 2015;16:367–377.
- Zhang XG, Wang YH, Han C, et al. Effects of trehalose supplementation on cell viability and oxidative stress variables in frozen-thawed bovine calf testicular tissue. Cryobiology. 2015;70:246–252.10.1016/j.cryobiol.2015.03.004
- Gandhi A, Shah NP. Effect of salt on cell viability and membrane integrity of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium longum as observed by flow cytometry. Food Microbiol. 2015;49:197–202.10.1016/j.fm.2015.02.003
- Yi S, Chen Y, Wen L, et al. Downregulation of nucleoporin 88 and 214 induced by oridonin may protect OCIM2 acute erthroleukemia cells from apoptosis through regulation of nucleocytoplasmic transport of NF-kB. Int. J. Mol. Med. 2012;30:877–883.
- Peng Y, Zheng Y, Zhang Y, et al. Different effects of omega-3 fatty acids on the cell cycle in C2C12 myoblast proliferation. Mol. Cell Biochem. 2012;367:165–173.10.1007/s11010-012-1329-4