Abstract
To prepare an aroma mixture of Japanese soy sauce by fewest components, the aroma concentrate of good sensory attributes was prepared by polyethylene membrane extraction, which could extract only the volatiles with diethyl ether. GC-MS-Olfactometry was done with the aroma concentrate, and 28 odor-active compounds were detected. Application of aroma extract dilution analysis to the separated fraction revealed high flavor dilution factors with respect to acetic acid, 4-hydroxy-2(or5)-ethyl-5(or2)-methyl-3(2H)-furanone (HEMF), 3-methyl-1-butanol (isoamyl alcohol), and 3-(methylsulfanyl)propanal (methional). A model aroma mixture containing above four odorants showed a good similarity with the aroma of the soy sauce itself. Consequently, the reminiscent aroma mixture of soy sauce was prepared in water. The ratio of acetic acid, HEMF, isoamyl alcohol, and methional was 2500:300:100:1.
Graphical abstract
Reminiscent aroma mixture of soy sauce was prepared by only four compounds such as acetic acid, HEMF, isoamyl alcohol, and methional.
Soy sauce is a fermented soy bean product that is traditionally used in eastern Asia as a seasoning or a condiment. Nowadays, its popularity is also growing in the Western part of the world due to its salty and intense umami taste accompanied by a very characteristic aroma.
Japanese soy sauce is traditionally produced by 2 steps fermentation, that is, koji fermentation (solid state fermentation) for 1st step and moromi fermentation (brine fermentation) for 2nd step. In the 1st step fermentation, a purely cultured starter of Aspergillus oryzae or Aspergillus sojae is used to ferment the mixture of cooked soy beans and roasted wheat. This step is marked by the production of extracellular enzymes consisting of protease, carbohydrase, etc. Prior to the 2nd step fermentation, an equal amount of koji is mixed with brine solution having salt concentration of 22–23% to obtain moromi with a salt concentration of 17–18%. The high salt concentration is believed to control the growth of undesirable microorganisms. In the 2nd step fermentation, Pediococcus halophilus grows and produces lactic acid in the earlier stage to drop the moromi pH to less than 5; Zygosaccharomyces rouxii or Saccharomyces rouxii (a salt-tolerant yeast) grows accompanying by the decrease in moromi pH, which develops a vigorous alcoholic fermentation at the middle stage; Torulopsis sp. or Candida sp. grows at the last stage to produce volatile compounds and adds characteristic aroma to soy sauce.
Many investigations on the volatile constituents of Japanese soy sauce have been performed, since soy sauce aroma is believed to be effective enhancer of umami and salty taste in foods.Citation1,2) Nunomura et al.Citation3) investigated the volatile compounds of Japanese soy sauce by GC-MS with the aroma concentrate prepared by the combination of vacuum distillation and solvent extraction. They identified over 90 compounds in its acidic fraction and over 140 compounds in its neutral fraction. In its basic fraction, various pyrazines were identified.Citation3) The tautomer of 4-hydroxy-5-ethyl-2-methyl-3(2H)-furanone and 4-hydroxy-2-ethyl-5-methyl-3(2H)-furanone (HEMF) was proposed to be major important compound for soy sauce aroma.Citation4) Steinhaus and Schieberle first applied aroma extract dilution analysis (AEDA) to the aroma concentrate of Japanese soy sauce, which was prepared by the combination of solvent extraction and solvent-assisted flavor evaporation (SAFE) distillation.Citation5) Recently, Kaneko et al. investigated the aromas of five different types of Japanese soy sauce. Their comparative AEDA experiment and sensory evaluation demonstrated that different types of soy sauce varied in their key aroma compounds.Citation6) We have reported that the odor concentrate from Japanese soy sauce showed a salty and umami enhancing effect.Citation2) Soy sauce contains many volatile flavor compounds including sweet odor, burnt odor, and vinegar-like odor.Citation7) We supposed that there was a compound which had suggestive salty and umami taste.Citation2) Steinhaus and Schieberle recombinated the soy sauce aroma with 13 odorants.Citation5) However, if the reminiscent aroma mixture of salty and umami can be prepared with fewer kinds of compounds, it is more useful as a food flavor. This study aimed to prepare the reminiscent aroma mixture of Japanese soy sauce with fewest odorants.
To accomplish this purpose, polyethylene (PE) membrane extractionCitation8) was done. PE membrane consists of two phases, an amorphous phase and a crystalline phase, or three phases, with a boundary region around the crystalline phase; hence, a PE membrane is called a semi-crystalline membrane.Citation9) Small molecules of low polarity such as aroma compounds can permeate through the amorphous phase. On the other hand, molecules such as triglycerides or sterols are too large to penetrate through the amorphous region. Moreover, ionic and extra polar compounds such as amino acids, peptides, and sugars cannot dissolve in PE membrane. Therefore, PE membrane of semi-permeable property acts as a molecular sieve, whose working factor is molecular size and polarity. Surprisingly, PE membrane, which mediated between butter oil and solvent, enabled to extract only odorants from the oil.Citation8) In this study, PE membrane extraction was applied to the extraction of aroma compounds from soy sauce.
Materials and methods
Soy sauce
Five commercial soy sauce (Koikuchi-shoyu) samples (A, B, C, D, and E) originating from different manufacturers in Japan were purchased from a local supermarket. The soy sauce A was used in all experiments, and the others were used for only quantification of key aroma compounds.
Chemicals
The following odorants were obtained from the commercial source (Nacalai Tesque Inc., Japan, Wako Chemical Industries, Ltd, Japan, Tokyo Chemical Industry Co., Ltd, Japan): 2-methylbutanal, ethanol, ethyl propionate, ethyl 2-methylpropanoate, 2-butanol, ethyl 3-methylbutanoate, 2-methylpropan-1-ol, 1-butanol, 3-methyl-1-butanol (isoamyl alcohol), 3-hydroxy-2-butanone, 1-hydroxy-2-propanone, 2,5-dimethylpyrazine, 2,6-dimethylpyrzine, ethyl 2-hydroxypropanoate, 1-hydroxy-2-butanone, 3-(methylthio)propanal (methional), acetic acid, propanoic acid, 2-methylpropanoic acid, butanoic acid, 2-furanmethanol, 3-(methylthio)propanol (methionol), 2-methoxyphenol, and HEMF.
PE membrane extraction of aroma compounds from soy sauce
The PE membrane was a low density polyethylene (LDPE) of specific gravity 0.92 g/cm3 and 40 μm thickness (Tamapoly Co. Ltd, Tokyo, Japan). LDPE membrane pouches (9 cm × 5 cm) were made using a heat-sealer. Then, the pouches were immersed in diethyl ether overnight to remove contaminants in the membrane. The decontaminated pouch was filled with soy sauce A (25 mL) and heat-sealed. As a blank test, water containing pouch was extracted with diethyl ether. And no degradation product was detected. The pouch containing soy sauce was put into a wide-mouthed bottle along with diethyl ether (40 mL) containing cyclohexanol 10 mg/L soy sauce as an internal standard (IS) as an extract solvent, and the bottle was capped tightly. For the extraction of volatile flavor compounds, the capped bottle was shaken at 70 rpm in a water bath at 30 °C for 60 min. After the extraction, the pouch was taken out and then small amount of anhydrous sodium sulfate was added to the ether extract for elimination of moisture. Dehydrated extract was concentrated under atmospheric pressure to about 50 μL for GC-MS analysis and applied to silica gel column chromatography for odor fractionation.
Fractionation of soy sauce aroma over silica gel
A glass column (6 mm i.d. × 100 mm) was packed with 3 g of silica gel (70–230 mesh) in pentane. After loading the aroma concentrate (50 μL), chromatography was performed with a stepwise gradient of pentane and diethyl ether, increasing the percentage of ether as follows: 0% (3 mL), 25% (3 mL), 50% (3 mL), 60% (1 mL × 3), 75% (3 mL), and finally 100% (3 mL). Obtained 8 fractions were concentrated and supplied to odor evaluation on a smelling strip. Sensory impact fraction, the third fraction of 60% ether eluents, was supplied to GC-MS analysis and AEDA. The concentrate of the fraction was stepwise diluted with diethyl ether to 1:2, 1:4, 1:8, 1:16, and 1:32. Aliquots (1 μL) of each dilution were analyzed by GC-O. An AEDA was performed three times with respect to each sample by two assessors. The detection of each compound was defined as not less than three detections in six analyses. FD factor was determined as the maximum dilution degree for detection.
Quantitative determination of key aroma compounds in soy sauce samples
Five soy sauce samples A, B, C, D, and E were used for the determination of four aroma compounds. The pouch filled with soy sauce 25 mL was extracted with diethyl ether (40 mL) containing cyclohexanol 10 mg/L soy sauce as an IS. For determination of four aroma compounds, spiked sample was prepared as follows: acetic acid, HEMF, isoamyl alcohol, and methional were added to soy sauce sample 25 mL by 100, 20, 4, and 0.004 μg, respectively. Their concentrations in soy sauce were calculated by a following equation.
where Aus is concentration of each component, Pus is a peak area ratio to IS of each component in sample, and Pss is a peak area ratio to IS in spiked sample. Ass is an added concentration of each component.
GC-MS analysis
A Shimadzu GC-MS QP2010 Plus was used. A fused silica column (30 m × 0.25 mm i.d. coated with a 0.25-μm film of DB-WAX, Agilent Technologies) was used. The column temperature was programmed from 40 to 230 °C at the rate of 4 °C/min and held for 15 min at final temperature. The injector temperature was 230 °C. Helium was used as the carrier gas at a linear flow velocity of 47 cm/s. Aliquots (1 μL) of the sample were injected, and a split ratio was 1:20. The mass spectrometer was operated under the following conditions: ionization voltage, 70 eV (EI); ion source temperature, 200 °C. The interface between GC and MS was heated at 230 °C. Linear retention indices (RI) of the compounds were calculated from the retention times of n-alkanes. RI were confirmed by comparing them to the database of Aroma Office (Nishikawa Keisoku Co. Ltd). Obtained mass spectra were compared to those in the NIST Library, 2008 edition (NIST 08) using a computer system, GC-MS solution.
GC-MS-O
GC-MS-Olfactometry (GC-MS-O) was performed by a Shimadzu GC-MS QP2010 Plus equipped with sniffer 9000. In the column oven, the end of capillary column was connected to a deactivated splitter, where makeup gas (helium) was added to the column effluent, and the flow was divided and transferred to a mass spectrometer and a sniffing port, respectively. The sniffing port connecting capillary was held at 230 °C and nitrogen gas was supplied to purge odorants emerged from the capillary. Samples were injected by splitless mode and the purge valve closed time was 60 s. The others were the same as GC-MS analysis. During an operation of GC-MS, the panelist placed assessors’ nose closely above the top of the sniffing port and evaluated the odor of the GC effluent.
Sensory evaluation
Seven assessors (four women and three men, aged 21–26 years) were recruited from the laboratory of food process engineering, graduate school of bioresource, and bioenvironmental sciences, Kyushu University. They were trained to recognize and describe the odor qualities using mixtures of odor components and commercial soy sauces. The assessors were subjected to odor profiling with six samples of two components mixtures, four samples of three components mixtures, and one sample of four components mixture. Total 11 mixtures were prepared by the combination of four key odorants, which were acetic acid (8000 mg/L), HEMF (960 mg/L), isoamyl alcohol (320 mg/L), and methional (3.2 mg/L) in aqueous solution.
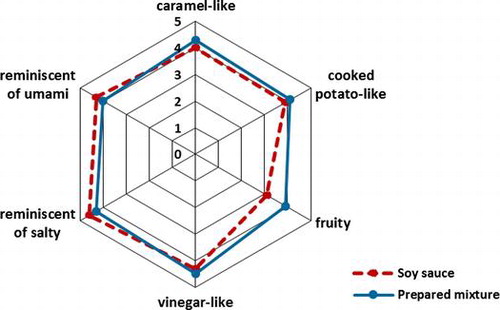
The assessors were required to sniff and score the six odor attributes of the prepared quaternary odorants mixture and the commercial soy sauce A on a five-point scale from 1 (not recognized) to 5 (clearly recognized). That is, attributes of caramel-like, cooked potato-like, fruity, vinegar-like, reminiscent of salty, and reminiscent of umami were evaluated. The attributes of “reminiscent of salty” and “reminiscent of umami” were defined as “odor intensity which made panelists feel salty or umami taste image.” The other attributes were represented by each compound, i.e., acetic acid (vinegar-like), HEMF (caramel-like), isoamyl alcohol (fruity), and methional (cooked potato-like). Ten milliliters of soy sauce A and odor mixture (with addition of brown color) were served in 100-mL amber glass bottles capped with Teflon-lined plastic lids. All samples were labeled with three-digit numbers and heated at 25 °C for 10 min before served. The sensory analyses were performed in triplicate with samples presented in random order. Statistical analysis of the sensory data was performed by Student’s t-test to evaluate the difference existed between each attributes of the aroma mixture and the soy sauce. Significance was established at p < 0.05.
Results and discussion
Isolation of volatile compounds from soy sauce by PE membrane extraction
In order to remove non-volatile compounds from the extract by solvent extraction or solid phase extraction of soy sauce aroma compounds, SAFE distillation has been carried out.Citation5,6) On the other hand, newly developed PE membrane extraction gave an aroma concentrate free from the taint of non-volatile compounds. To identify the characteristic aroma components of soy sauce A, GC-MS analysis was carried out. As shown in Table , total ion chromatogram consisted of 18 alcohols (12.5), 16 esters (11.1), 12 acids (8.3), 9 furans & furanones (6.2), 7 hydrocarbons (4.8), 5 aldehydes (3.5), 5 pyrazines (3.5), 6 ketones (4.2), 3 phenols (2.1), 2 sulfur-containing compounds (1.4), 2 pyrroles (1.4), 2 miscellaneous (1.4), and 57 unknown (39.6). The numbers in parentheses are the percentages of chemical groups for total peak area. It was a distinctive feature that the LDPE membrane extraction gave the aroma concentrate containing a large amount of alcohols and acids compared to a conventional solvent extraction and solid phase extraction. These compounds were identified by comparing the mass spectra, the retention indices, and the odor qualities with those of the respective reference compounds.
Table 1. Volatile flavor compounds separated by LDPE membrane extraction method from soy sauce.
Among these 144 peaks, 27 peaks were detected as odor-active compounds (Table ). Among these odor-active compounds such as isoamyl alcohol (fruity), 1-hydroxy-2-propanone (peanut-like), acetic acid (sour, vinegar-like), methional (cooked potato-like), 2-methylpropanoic acid (rancid, cheesy), 2-methoxyphenol (smoky), and HEMF (sweet, caramel-like), no odorant gave soy sauce-like odor.
Table 2. Odor-active compounds in soy sauce aroma concentrate by GC-MS-O.
Fractionation of soy sauce aroma concentrate and AEDA
It is important to separate the whole fraction of soy sauce odor, because such odor fraction is predicted to keep the olfactory interaction among the odorants. Therefore, the soy sauce A aroma concentrate was fractionated by silica gel column chromatography using a stepwise elution of pentane and diethyl ether. The fraction of 60% diethyl ether was separated into more specific three fractions in order to narrow down the target compounds because it had soy sauce-like aroma. The third fraction of 60% ether revealed soy sauce aroma. The second fraction of 60% ethyl ether possessed strong beany, sweet, and burnt odors together with slight savory aroma. In the fraction of 75% ethyl ether, weak savory aroma besides strong nutty and burnt odor was recognized. The fraction of 0 and 100% ethyl ether had stimulus, solvent odor. The fraction of 25 and 50% ethyl ether had smoky odor. The first fraction of 60% ethyl ether had burnt, smoky odor.
AEDA was done with the third fraction of 60% ethyl ether because it had strongest savory soy sauce aroma. As shown in Table , no compound revealed soy sauce aroma by single compound. Flavor Dilution (FD) factors higher than 32 were obtained by isoamyl alcohol (fruity), acetic acid (sour, vinegar-like), methional (cooked potato-like), and HEMF (sweet, caramel-like). As a result, we had no choice but to consider that the soy sauce aroma was a complex aroma of these odor impact compounds. Steinhaus and Schieberle also reported the FD factors of these compounds were over 32.Citation5) Kaneko et al. reported FD factors of key aroma compounds in five different types of Japanese soy sauce.Citation6) According to this, it is reported that the FD factors of methional and HEMF were 256 (>32).
Table 3. Odor-active compounds of the third fraction of 60% ether by AEDA.
To confirm the existence of four compounds whose FD factors were over 32 in soy sauce, we used soy sauce samples from other five different manufactures. As shown in Table , few differences were detected in the concentration of the four odor impact compounds from different manufactures. Each compound existed in the following ranges in soy sauce: acetic acid 1000–1400 mg/L, HEMF 24–36 mg/L, isoamyl alcohol 10–13 mg/L, and methional 0.17–0.38 mg/L.
Table 4. Determination of odor impact compounds in commercial soy sauce A, B, C, D, and E.
Preparation of reminiscent aroma mixture of soy sauce
We prepared aroma mixture after several attempts consisting of four compounds based on their concentration ratios in soy sauces. The ratio of acetic acid, HEMF, isoamyl alcohol, and methional was 2500:300:100:1. As shown in Table , six samples of two components mixture, four samples of three components mixture, and one sample of four components mixture were prepared in water. All of the two components mixtures and three components mixtures could not elicit soy sauce aroma, but the four components mixture gave the soy sauce aroma. It was fortunate for us that the savory aroma of soy sauce was prepared by only four odorants. The quaternary mixture reflects more an expected sensation rather than an actual aroma.
Table 5. Aroma profile of mixtures of odor impact compounds.
The aroma mixture consisting of acetic acid (8000 mg/L), HEMF (960 mg/L), isoamyl alcohol (320 mg/L), and methional (3.2 mg/L) was used as a sensory evaluation. We prepared this concentration to make panelists recognize the odor easily. Table lists sensory scores of soy sauce A and the aroma mixture. There was no significant difference between soy sauce A and the mixture with all aroma attributes. As a result, we could prepare reminiscent aroma mixture of soy sauce.
Table 6. Sensory scores of soy sauce A and prepared mixture.
Acetic acid, the most abundant component in the mixture, is produced as a by-product during lactic acid fermentation.Citation3) Since its odor threshold value, 22 mg/L,Citation10) is quite high, acetic acid has been rarely designated as an aroma impact compound of foods. The sour odor of acetic acid would be necessary to balance the sweet and caramel-like odor of HEMF.
HEMF is biosynthesized through a pentose-phosphate cycle and its highest productions occurred at the sodium chloride concentration of 17–18%.Citation11) Since a large amount of HEMF is produced in soy sauce and it smells a caramel-like odor, it has been considered as a significant contributor to soy sauce odor.Citation4,5)
Soy sauce yeasts produce fusel alcohols, such as isoamyl alcohol, n-butyl alcohol, and isobutyl alcohol, from branched-chain amino acids via an Ehrlich pathway.Citation12) The highest amount (40–50 mg/L) of isoamyl alcohol was produced at the sodium chloride concentration of 17–18% during the soy sauce brewing.
Saccharomyces cerevisiae catabolizes the transamination of methionine to α-keto-γ-(methylthio)-butyrate, and the decarboxylation of α-keto-γ-(methylthio)-butyrate to methional.Citation13) Although the effect of sodium chloride concentration on above methionine catabolism is not cleared, it is certain that methional is formed during brine fermentation.
Four compounds above mentioned were all bio-synthesized compounds during brine fermentation. These compounds could be signal products formed by microorganisms under such a high concentration of sodium chloride.
Recently, Lawrence et al.,Citation14) Nasri et al.,Citation15) and Seo et al.Citation16) investigated the validity of odor-induced saltiness enhancement strategy to compensate for sensory loss in low-salt foods with a salt-congruent odor such as soy sauce aroma. In this study, the reminiscent aroma mixture of soy sauce was successfully prepared with the fewest odorants. Hereafter, we investigate the feasibility of using the present aroma preparation to compensate for flavor decrease with salt reducing in model food systems.
Author contributions
Shimoda and Igura developed the study conception and design. Fuji, Nakao, and Bonkohara acquired the data. Bonkohara wrote the manuscript and critically revised.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Djordjevic J, Zatorre RJ, Jones-Gotman M. Odor-induced changes in taste perception. Exp. Brain Res. 2004;159:405–408.10.1007/s00221-004-2103-y
- Shimoda M. Does shoyu odor strengthen salty flavor? Nippon Aji to Nioi Gakkaishi. 2007;14:3–8. Japanese.
- Nunomura N, Sasaki M. Japanese soy sauce flavor with emphasis on off-flavors. Dev. Food Sci. 1992;28:287–312.10.1016/B978-0-444-88558-6.50016-7
- Nunomura N, Sasaki M, Yokotsuka T. Studies on flavor components in shoyu. VI. Agric. Biol. Chem. 1980;44:339–351.10.1271/bbb1961.44.339
- Steinhaus P, Schieberle P. Characterization of the key aroma compounds in soy sauce using approaches of molecular sensory science. J. Agric. Food Chem. 2007;55:6262–6269.10.1021/jf0709092
- Kaneko S, Kumazawa K, Nishimura O. Comparison of key aroma compounds in five different types of Japanese soy sauces by aroma extract dilution analysis (AEDA). J. Agric. Food Chem. 2012;60:3831–3836.10.1021/jf300150d
- Nunomura N, Sasaki M, Asao Y, et al. Studies on flavor components in shoyu. I. Agr. Biol. Chem. 1976;40:485–490.10.1271/bbb1961.40.485
- Chongcharoenyanon B, Yamashita N, Igura N, et al. Extraction of volatile flavour compounds from butter oil in a low-density polyethylene membrane pouch. Flavour Fragr. J. 2012;27:367–371.10.1002/ffj.v27.5
- Kim MH, Glinka CJ. Correlation between structure and vapor sorption in semicrystalline linear polyethylene: one dimensional nano-swelling measured using in situ vapor sorption small angle neutron scattering (iVSANS). Macromolecules. 2009;42:2618–2625.10.1021/ma802363d
- Rychlik M, Schieberle P, Grosch W. Compilation of odor thresholds, odor qualities and retention indices of key food odorants. Garching: German Research Center for Food Chemistry; 1998.
- Sasaki M, Nunomura N, Matsudo T. Biosynthesis of 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone by yeasts. J. Agric. Food Chem. 1991;39:934–938.10.1021/jf00005a027
- Jansen M, Veurink JH, Euverink GW, et al. Growth of the salt-tolerant yeast Zygosaccharomyces rouxii in microtiter plates: effects of NaCl, pH and temperature on growth and fusel alcohol production from branched-chain amino acids. FEMS Yeast Res. 2003;3:313–318.
- Perpete P, Duthoit O, De Maeyer S, et al. Methionine catabolism in Saccharomyces cerevisiae. FEMS Yeast Res. 2006;6:48–56.10.1111/fyr.2006.6.issue-1
- Lawrence G, Salles C, Septier C, et al., Odour-taste interactions: a way to enhance saltiness in low-salt content solutions. Food Qual. Pref. 20, 241–248 (2009).10.1016/j.foodqual.2008.10.004
- Nasri N, Beno N, Septier C, et al. Cross-modal interactions between taste and smell: odour-induced saltiness enhancement depends on salt level. Food Qual. Pref. 2011;22:678–682.10.1016/j.foodqual.2011.05.001
- Seo H, Iannilli E, Hummel C, et al. A salty-congruent odor enhances saltiness: functional magnetic resonance imaging study. Hum. Brain Mapp. 2013;34:62–76.10.1002/hbm.v34.1
