Abstract
Environmental adaptation is considered as one of the most challenging subjects in biology to understand evolutionary or ecological diversification processes and in biotechnology to obtain useful microbial strains. Temperature is one of the important environmental stresses; however, microbial adaptation to higher temperatures has not been studied extensively. For industrial purposes, the use of thermally adapted strains is important, not only to reduce the cooling expenses of the fermentation system, but also to protect fermentation production from accidental failure of thermal management. Recent progress in next-generation sequencing provides a powerful tool to track the genomic changes of the adapted strains and allows us to compare genomic DNA sequences of conventional strains with those of their closely related thermotolerant strains. In this article, we have attempted to summarize our recent approaches to produce thermotolerant strains by thermal adaptation and comparative genomic analyses of Acetobacter pasteurianus for high-temperature acetic acid fermentations, and Zymomonas mobilis and Kluyveromyces marxianus for high-temperature ethanol fermentations. Genomic analysis of the adapted strains has found a large number of mutations and/or disruptions in highly diversified genes, which could be categorized into groups related to cell surface functions, ion or amino acid transporters, and some transcriptional factors. Furthermore, several phenotypic and genetic analyses revealed that the thermal adaptation could lead to decreased ROS generation in cells that produce higher ROS levels at higher temperatures. Thus, it is suggested that the thermally adapted cells could become robust and resistant to many stressors, and thus could be useful for high-temperature fermentations.
Graphical abstract
Experimental (in vitro) adaptation, as well as natural adaptation, could generate thermotolerant species, useful for high-temperature fermentation, from mesophiles.
The ability to maintain functional homeostasis despite changes in the external environments is essential for the survival of all microbes. Temperature is one of the most important environmental factors that affect growth and survival of microbes. The minimum, optimum, and maximum microbial growth temperatures vary greatly and usually reflect the temperature range and average temperature of their habitats.
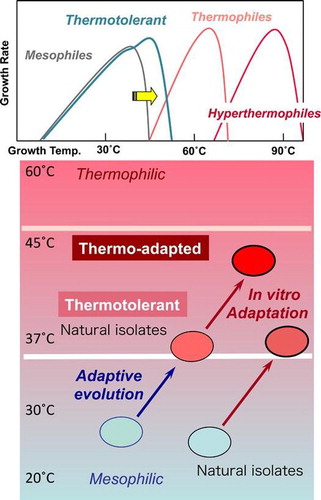
Many thermotolerant microbes have been isolated from tropical environments, including foods, plants, soils, and waters in Thailand.Citation1–3) Such thermotolerant microorganisms are mesophiles with optimum growth temperatures (typically 35–45 °C) that are 5–10 °C higher than those of the typical mesophilic strains belonging to the same genusCitation2–5) or even in the same species.Citation1,6) Thus, these strains are distinct from thermophiles, which are defined to have an optimum growth temperature above 50 °C (Fig. (A)). Therefore, it is conceivable that these thermotolerant strains acquired their distinctive growth phenotype by adapting to given habitats in relatively warm or hot environments, such as in the tropical regions,Citation1–3,5) specific local areas where heated water from a factory-cooling system is released,Citation4) areas that receive high levels of solar radiation,Citation6) and in natural or manmade fermentation systems.Citation7,8) Thus, such the “adaptive evolution” generates a lot of thermotolerant species which could be found as natural isolates in tropical area or closed fermentors (Fig. (B)).
Fig. 1. (A) Concept for how thermotolerant species differ from mesophiles, thermophiles, and hyperthermophiles, and (B) concept of adaptive evolution and experimental adaptation (in vitro adaptation) to produce naturally thermotolerant and thermo-adapted strains.
Genomic mutations are fundamental phenomena in the evolution of not only microorganisms but also all organisms. Bacteria may be more directly influenced by mutations than multicellular organisms because of their compact genomes and the density of information in bacterial genomes. Based on new information regarding genomic structures and mutational events, various genomic mutations in bacteria were classified into several systems, such as a horizontal gene transfer of mobile gene units (e.g. transposon, plasmid, and phage); hypermutable tandem repeats; and genomewide rearrangement and genome reduction,Citation9) in addition to the well-known mutation systems, including nucleotide substitution, insertion and deletion caused by inadequate replication or repair, and gene duplication.Citation10)
In order to examine whether genomic modification occur during adaptation to specific environments, an experimental adaptation becomes a very useful approach as next-generation sequencing (NGS) technology is now available for whole-genome analysis of laboratory-propagated bacterial strains.Citation11,12) Experimental adaptation to unviable high temperatures (see “in vitro adaptation” in Fig. (B)) has also been tried using several microorganisms including not only Escherichia coliCitation13,14) as a model strain but also practical fermentative microbes such as Saccharomyces cerevisiaeCitation15,16) and Acetobacter pasteurianusCitation11,17) for ethanol and acetic acid fermentation, respectively.
Fermentative microbes for food production, especially yeast, lactic acid bacteria, and acetic acid bacteria (AAB), are mostly found in plant habitats and utilized in human activities; they function naturally in mesophilic environments. However, they sometimes exposed to higher temperatures due to the fermentative heat generated during the fermentation process or due to the increase in ambient temperatures. Large-scale industrial fermentation is carried out in a closed fermentor, which generates a lot of heat, and thus requires strict temperature control. However, such systems sometimes experience fermentation failure due to temporary temperature increases that occur from power failure, mechanical defects, or operator errors during the fermentation process. Recent global warming affects such large-scale fermentation processes and necessitates the use of more thermotolerant microbes to achieve stable fermentation.
In this article, we have shown our recent attempts to experimentally adapt several fermentative microbes to higher temperatures to obtain thermo-adapted strains (Fig. (B)) useful for acetic acid and ethanol fermentations; we also performed comparative genomewide analyses between wild and adapted strains or between conventional strains and closely related thermotolerant strains to elucidate mechanisms of thermotolerance. We have shown the results we obtained from an acetic acid producer, A. pasteurianus, and ethanol producers, Zymomonas mobilis and Kluyveromyces marxianus, including both naturally thermotolerant species isolated in Thailand,Citation1,3,18) and the corresponding mesophilic species obtained from culture collections. In addition, we have shown two examples for the development of high-temperature ethanol and acetic acid fermentation systems with thermotolerant yeast and thermally adapted AAB.
I. Genetic instability of A. pasteurianus
I.i. Acetobacter strains and their physiological instabilities
AAB are a widely divergent group within the alpha-proteobacteria and are isolated from a variety of natural habitats, such as fruits, flowers and fermented foods; they are rarely found in soil and insect guts.Citation19,20) AAB incompletely oxidize a variety of sugars and alcohols and accumulate large amounts of the corresponding oxidation products in their environments or culture media. These traits of AAB, known as oxidative fermentation, are utilized in many industrial production processes, such as the manufacture of vinegar and L-sorbose.Citation21) In industrial production, AAB play a crucial role in the quantity, taste, nutrition and hygienic quality of the products, thus making the reliability of strains is indispensable with respect to functional properties and robustness of growth.
Acetobacter strains, which are the most popular AAB for the production of vinegar in many countries, have shown noticeable physiological instabilities, the characteristics of which were reported in at least two different contexts, specifically temporal acclimation and heritable mutation.Citation22–26) As an example of temporal acclimation (or physiological adaptation), Acetobacter strains may gradually acquire resistance against higher concentrations of acetic acid when properly adapted, but they rapidly lose the acquired phenotype when maintained without acetate.Citation23) This is why new tanks for fermentation are continuously inoculated with AAB from old tanks, but not with a preserved seed AAB strain in traditional vinegar production. Very little is known about the genetic properties of these instabilities, but phenotypic modifications by transposon insertion were reported in ethanol oxidation and acetic acid resistance,Citation22,24) and cellulose formation.Citation25,26)
I.ii. Transposons and plasmids in A. pasteurianus IFO 3283
Within the past decade, genomic DNA sequences of many AAB were published and relatively copious amounts of transposons were identified; these transposons influence AAB genomic features related to genome instability. We have clarified genomic features that may be attributed to genetic instability based on analyses of the A. pasteurianus IFO 3283 (now called NBRC 3283) genome.Citation11) A. pasteurianus IFO 3283 for genomic analysis was maintained by serial passage every three months from the deposit of the pure culture at Institute for Fermentation, Osaka (IFO) in 1954 until the establishment of the freeze-dry preservation method in 1974. Since no colony isolation was performed in maintenance to avoid loss of useful features of the strain, a multiphenotype cell complex was formed and characterized. It was estimated that the three-month slant passage produced 2.2 × 10Citation3 generations during 20 years of maintenance. Within this period, a multiphenotype cell population has been generated, resulting in having three SNPs and four transposon polymorphisms (Fig. ).
Fig. 2. Divergency of A. pasteurianus IFO 3283 (NBRC 3283) for 20 years.
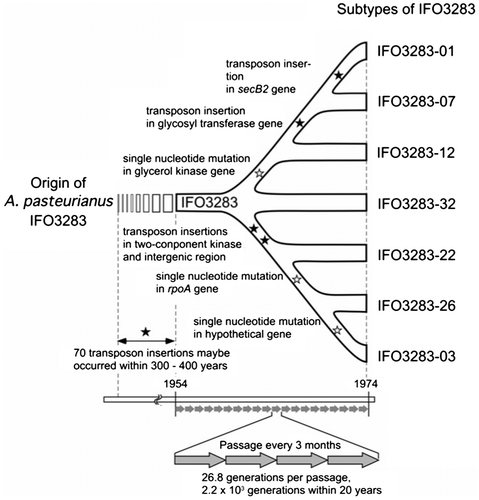
The A. pasteurianus genome contains more than 280 transposons comprising approximately 9% of total genes in the genome. Seventy-four transposons are classified into IS1380,Citation24) and 32 genes are truncated by the IS1380-type transposons. No gene truncations by other types of transposons were identified. About four transposon polymorphisms that occurred during 20 years of maintenance suggests the possibility of 32 gene truncations that may occur in the cell population within, approximately, two centuries (168 years). Establishment of modern vinegar fermentation in Japan developed about 200 years ago, suggesting that a unified environment for fermentation leads to an accumulation of mutations. The expansion ability of IS1380-type transposons is one of the most feasible reasons for genome instability.
In addition to the transposons, the A. pasteurianus genome contained six plasmids, which may act as factors corresponding to genome flexibility according to a fact that the three smaller plasmids seemed to be removable from cells during the prolonged thermal adaptation (Y. Azuma et al., unpublished). Plot analysis between the proportion of transposase genes and fragment numbers constituting chromosomes and plasmids indicated that the combination of relatively large amounts of plasmids and transposons is one of its genome characteristics, and it is also true for other AAB utilized in fermentation.Citation11) Two human pathogenic AAB, Granulibacter bethesdensisCitation27) and Asaia bogorensis,Citation28) both of which contain no plasmids and few transposases, were plotted in a far-flung corner from other fermentation AAB (data not shown). AAB species adapted or adaptable to fermentation and those capable of infecting humans could evolve in disparate evolutionary directions.
I.iii. Short tandem repeats in A. pasteurianus IFO 3283
Precise genomic analysis of the multiphenotype cell complex of A. pasteurianus indicated the existence of hyper-mutable tandem repeats (HTRs).Citation11) When DNA fragments were sequenced from many different single colonies, approximately 90% of the template DNA fragments including HTRs showed a highly repetitive size (e.g. (aggac)n), but 5% of the DNA templates were sequences either one repeat shorter (aggac)n-1 or longer (aggac)n+1. Therefore, it is possible that the repetitive numbers (n) quickly alter the coding frames or amino acid sequences following the HTR and thus influence its functions. HTRs were shown to play critical roles in phenotypic modification of programmed alterations in some bacteria. For instance, the Neisseria meningitidis gene nadA, which encodes a pathogenic adhesion protein, contains an HTR in its promoter sequence and the number of repeats present influences its gene expression.Citation29)
Interestingly, another AAB, Gluconacetobacter xylinus, contains a DNA helicase gene homologous to that of A. pasteurianus. Both genes include the same repetitive sequences in the same location of the genes. Moreover, we found that gamma-proteobacteria Azotobacter vinelandii DJ included a DNA helicase gene (Avin_22890) highly similar to the DNA helicase genes of the aforementioned species. Each gene contained a repetitive segment at a similar site, but the repetitive sequences were different. An ancestral gene of the DNA helicase genes may have been horizontally transferred between two independent genera or from another related organism with an alteration of repetitive sequence. This horizontal gene/plasmid transfer might be another mechanism in the genome instability of AAB. As in the case of A. pasteurianus, beside direct effects on functional modification by HTRs, expansion and deletion of tandem repeats in two genes encoding a DNA helicase and the DNA polymerase III exonuclease subunit epsilon could reduce the fidelity of replication and enhance mutation accumulation.Citation30) Thus, DNA helicase genes were significantly abundant in A. pasteurianus and one of them contained a HTR. These DNA helicases may influence the genome instability of A. pasteurianus. During a relatively short period (20 years), several single nucleotide mutations and transposons accumulated in the genomic DNA sequence (Fig. ). During culturing to establish the modern fermentation of acetic acid, A. pasteurianus IFO 3283 accumulated a variety of mutations, especially gene truncations by transposons, which altered the bacterium from strains found in natural habitats and enabled it to be an expert at fermentation. Thus, the genetic background of A. pasteurianus regarding its genome instability enabled us to attempt in vitro accelerated evolution to adapt the bacterium to industrial fermentation and to a previously unviable environment.
II. Thermal adaptation of A. pasteurianus
To clarify and utilize the genomic instability of A. pasteurianus, adaptation to unviable temperatures was carried out using A. pasteurianus IFO 3283-01, an isolate from the multi-phenotype cell complex of IFO3283, in addition to A. pasteurianus SKU 1108, which was isolated in Thailand under conditions accelerating evolution in vitro.Citation11,17)
II.i. Thermal adaptation of A. pasteurianus IFO 3283-01
The IFO 3283-01 strain can grow at 39 °C, but its growth is unstable at 40 °C, and no growth was observed at 40.5 °C or higher in nonfermentative conditions (without ethanol or acetic acid). Adaptation was initiated by long-term cultivation at 40 °C in nonfermentative conditions. After 27 days of cultivation at 40 °C, a strain able to grow at 42 °C was isolated and named IFO3283-01/42C. This strain was maintained the ability to grow at 42 °C after 13 serial passages at 30 °C. It demonstrated that the thermotolerant phenotype of this strain was heritable, and thus, genome DNA sequencing of IFO3283-01/42C was performed using the next-generation sequencer. Analyses of sequencing data and mutation mapping revealed that there were a genome truncation of 92 kb including the predicted replication terminal region and several point mutations in the genomic DNA of IFO 3283-01/42C (see Section 7).Citation11). By mutation analyses using genome DNA sequences of intermediate strains, which are not thermotolerant yet, during the process of thermotolerance acquirement, it was shown that the 92-kb truncation was uniquely detected in the IFO 3283-01/42C but no point mutations were specific to the strain.
Since it was reported that shorter plasmids and mitochondrial genomes showed faster replication,Citation31) the 92-kb deletion corresponding to approximately 3% of the intact genome may allow the bacterium to retain viability and growth ability in the thermally stressful environment by decreasing the stress from proliferation. Although the deletion-generating mechanism and genetic meaning of this large deletion is unknown, it seems that the smaller genome confers a survival advantage in highly stressful conditions, possibly because of faster replication or lower heat generation based on lower burden from DNA replication. Additional thermal adaptation with A. pasteurianus IFO 3283-32, one of the strains from the multi-phenotype cell complex of IFO3283, also showed a 64-kb deletion, part of which overlapped with the region of the 92-kb deletion in IFO 3283-01/42C (T. Yakushi et al., unpublished). Therefore, it is also possible that one or more genes present in the overlapping portion may affect the thermotolerant capacity of the strains.
II.ii. Omics analyses of the thermoadapted strain
Genomic analysis revealed that genome reduction was the most critical mutation for the thermotolerant phenotype in A. pasteurianus IFO 3283-01/42C. But it remains to be clarified whether or not the thermotolerant phenotype is only due to partial decrement of the replication burden. It is still possible that certain genes in the truncated region are involved in the phenotype. In order to examine this question, proteome, and transcriptome analyses were performed (Y. Azuma et al., unpublished). Comparative proteome analysis of soluble proteins indicated that proteins related to oxidoreduction and stress responses, including superoxide dismutase, thioredoxin, and osmotically inducible protein OsmC, accumulated more in the thermotolerant strain than in the parental ones. Comparative transcriptome analysis similarly showed higher expression of the genes for oxidoreduction and stress response in IFO 3283-01/42C grown at 42 °C than in the parental ones at 30 and 37 °C. In addition to these genes, several genes encoding transcriptional factors, including heat shock sigma factor RpoH and transcriptional regulator for starvation/low temperature, were also shown to be highly expressed in IFO 3283-01/42C grown at 42 °C than in the parental ones at 30 and 37 °C. Thus, the thermotolerant phenotype of the IFO 3283-01/42C is deemed not only due to partial decrement of the replication burden, also higher expression of the oxidoreduction and stress responsive genes might be involved in the thermotolerance.
II.iii. Thermal adaptation of A. pasteurianus SKU 1108
We isolated thermotolerant A. pasteurianus SKU1108 in Thailand.Citation1) This strain is able to perform acetic acid fermentation efficiently at high temperatures, even at 37 °C, but is unable to ferment effectively at 39 °C.Citation17,32) In order to obtain a thermotolerant strain useful for acetic acid fermentation at higher temperatures, we performed in vitro evolution experiments with A. pasteurianus SKU1108 by serial passage of the bacterial culture at growth-limiting temperatures under acetic acid fermentative conditions.Citation17) Single colonies from the culture grown for experimental evolution were screened with regard to thermotolerance in order to select the best clone. The two thermo-adapted acetic acid fermentation strains, TI and TH-3, were obtained from two independent experiments.
Following the adaptation, acetic acid fermentation properties of the adapted strains were examined in a medium containing 4% ethanol (i.e. acetic acid fermentation conditions).Citation17) The growth of the adapted strains was not significantly different from that of the wild-type strain up to 37 °C; the adapted strains exhibited better growth at temperatures over 39 °C under fermentation conditions. Even at 37 °C, the acetic acid production rate was significantly higher in TH-3 than in the wild and TI strains. At the later stages of fermentation, the wild-type strain consumed the produced acetic acid more rapidly than the adapted strains; thus, the adapted strains showed a delayed overoxidation of acetic acid. When the fermentation capacity of the wild and adapted strains was compared in a laboratory-scale fermentor at various growth temperatures, the adapted strains grew well and produced acetic acid even at 38.5 °C, while the SKU1108 strain showed delayed growth.Citation17) Although the wild-type strain cannot grow at 39.5 °C, the TI strain showed delayed growth at 39.5 °C but no growth at 40.5 °C. On the other hand, the TH-3 strain still performs effective fermentation at both temperatures and even at 41 °C, a temperature that is 10 °C higher than those usually used in industrial acetic acid fermentation. Thus, the adapted strains, particularly the TH-3 strain, have a 3 °C higher fermentation-limiting temperature than the wild-type strain.
II.iv. Mutations in the adapted strains, TI and TH-3
Table shows the list of mutations in the TI and TH-3 genomes with annotated functions for the mutated genes.Citation17) The six mutations in the TI strain consist of two base replacements, one frameshift, one 1-bp insertion, and two transposon insertions, while four base replacements, three deletions (two 6 bp and one 4 bp), two frameshifts, and two transposon insertions are present in the TH-3 strain. Of these mutations, an amino acid transporter (APT_1698), the transcriptional regulator MarR (APT_2081), and a C4-dicarboxylate transporter (APT_2237) are shared between the TI and TH-3 strains in spite of the different methods employed in the evolution of the two strains. In E. coli, the C4-dicarboxylate transporter is responsible for uptake of fumarate, malate, and succinate, and inactivation of the C4-dicarboxylate transporter gene resulted in growth defect on media with C4-dicarboxylates.Citation33) Proteins homologous to APT_1698 and APT_2081 are discussed later. Although APT_2081 in TH-3 has an amino acid substitution, the gene in TI is likely dysfunctional. These results suggest that disruption of these genes plays a crucial role in thermotolerance and/or fermentation at higher temperatures. On the other hand, we screened the genes responsible for the thermotolerance of Acetobacter tropicalis through random transposon mutagenesis.Citation34) However, there is no obvious overlap between the 24 genes for thermotolerance found by transposon mutagenesis and the 14 genes found in both TI and TH-3 strains from two rounds of in vitro evolution experiments.
Table 1. List of mutations or insertion regions in the adapted strains, TI and TH-3, of A. pasteurianus SKU1108.
The IFO 3283-01/42C strain that was thermally adapted under nonfermentative conditions has an amino acid mutation in the “two-component hybrid sensor histidine kinase and regulator”.Citation11) This corresponds to a mutated gene (APT_0997) in the TI strain, suggesting that mutations in this gene are involved in the thermotolerance of IFO 3283-01/42C and TI.
In order to evaluate whether the mutations of the adapted strains were responsible for the thermal adaptation, we eliminated the marR and amino acid transporter genes in the parental SKU1108 strain, the genes with mutations most likely to be related to thermotolerance. Growth of the ∆marR strain was similar to that of the wild-type strain under high temperatures. However, the ΔmarR strain showed higher acetic acid resistance and faster ethanol oxidation capacity than the wild type. It is known that some MarR family of regulatory proteins, which negatively regulate multidrug efflux systems, play important roles in antibiotic resistance and virulence regulation in bacteria.Citation35) In fact, our preliminary transcriptomic analysis revealed that expression of the same marR-regulated gene operon is increased in the ΔmarR strain as well as in the TH-3 strain (M. Matsutani et al., unpublished). We anticipate that the increased expression of these genes is related to the significant outcomes observed in the ΔmarR strain.
The ∆APT_1698 (amino acid transporter) strain grows better at high temperatures than the SKU1108 strain and produces acetic acid even at 39 °C, a temperature at which the wild-type strain cannot grow. Furthermore, the ∆APT_1698 strain and the TH-3 strain show a delayed acetic acid overoxidation at 37 °C. The ΔAPT_1698 strain has interesting features that are important for industrial vinegar production. A homologous gene to APT_1698 has been reported to be involved in Asn uptake into Salmonella cells.Citation36) It can be speculated that some specific compounds, presumably amino acids, are less readily incorporated into the ΔAPT_1698 cell. We are currently examining the effects of elimination of the APT_1698 gene using metabolomics to explore the role of the APT_1698 gene product. We found loss of marR or the amino acid transporter gene is independently related to the capacity of high temperature acetic acid fermentation. However, the effects of such single gene disruptions on thermotolerance and/or fermentation abilities are not as significant as those of the gene disruptions of the TI and TH3 strains. All the mutations present in both adapted strains may not necessarily be involved in thermotolerance, but multiple mutations likely have combined effects on relevant metabolism and physiology.
III. High-temperature acetic acid fermentation with thermally adapted A. pasteurianus
Industrial vinegar production is based on the incomplete oxidation of ethanol by AAB.Citation37) Acetobacter and Komagataeibacter (formerly a group of Gluconacetobacter) are the most prominent acetic acid producers and show high acetic acid resistance. Acetobacter species, particularly A. pasteurianus, are utilized for vinegar production in traditional processes where the concentration of acetic acid does not exceed 6% (v/v). Such industrial acetic acid fermentations are carried out at 25–30 °C and thus require a cooling system to maintain the temperature of the culture medium due to fermentation heat. The oxidation of ethanol to acetic acid is associated with a high Gibbs free energy of −493 kJ mol−1, which may produce a large amount of heat in acetic acid fermentation. High-temperature acetic acid fermentation has been described in a pioneering work by Beppu’s groupCitation8), and it is still of industrial and scientific interest.Citation38)
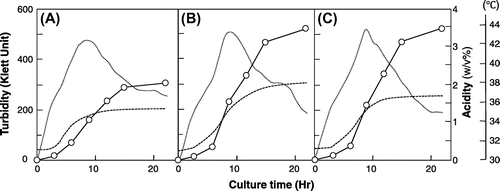
Industrial fermentation is carried out in a large-scale fermentor, which consequently generates fermentation heat. Moreover, large-scale fermentation in tropical areas and areas affected by climate change is more difficult to perform with proper temperature regulation. Thus, a strict temperature control system is required for fermentation using mesophilic microbes. More thermotolerant microbes would help to reduce the labor required to achieve stable fermentation. By using thermally adapted strains with increased fermentation abilities at higher temperatures, we developed a natural temperature fermentation system in a laboratory-scale fermentor without temperature control (Fig. ). Growth of the SKU1108 strain ceases due to heat at approximately 42 °C, and thus, acetic acid production ceases at around 2%. However, the thermally adapted strains TI and TH-3 keep growing even if the temperature is increased to 43.5 and 44 °C, respectively; at these temperatures, acetic acid can be produced a little faster in the TH-3 strain than in the TI strain (Fig. ). Thus, without temperature control of the fermentor, both the adapted strains produced approximately 3.5% acetic acid, which is comparable in productivity to the conventional temperature-controlled fermentation at 30 °C.
Fig. 3. Growth, temperature, and acetic acid fermentation of A. pasteurianus SKU1108 (A), TI (B), and TH-3 (C) strains in a natural temperature fermentation system.
We further adapted the TH-3 strain to higher concentrations of ethanol; the TH-3 strain was cultivated in a medium containing 5% ethanol and serial passages were subsequently performed to adapt TH-3 to high concentrations of ethanol. This process was repeated using 6% and 7% ethanol. Finally, strain 7E-13 was obtained, which can tolerate 7% ethanol (U. Masud-Tippayasak et al., unpublished). Vinegar production from Jasmine rice wine, with an ethanol concentration that had been adjusted to 6%, was tried using the 7E-13 strain by natural temperature fermentation in a 100 L fermentor without any temperature control. The fermentation was started under nonsterile conditions in the presence of 0.5% acetic acid to avoid contamination with other microorganisms. The temperature reached 40 °C transiently, but the fermentation was successfully finished in 21 h and yielded 6.6% acetic acid (U. Masud-Tippayasak et al., unpublished). Thus, the increased fermentation abilites acquired by the strain subjected to laboratory evolution will be useful for natural or high-temperature fermentation systems, as the adapted strains must sustain the heat generated by the mechanics of fermentors and microbes themselves. We have furthermore reported such natural high-temperature fermentation systems for sorbose production by the Gluconobacter strain.Citation39)
IV. Thermal adaptation of Z. mobilis strains and genomic analysis
Z. mobilis is a Gram-negative facultative anaerobe in the family of alpha-proteobacteria. Its ability to ferment sugars efficiently to ethanol makes it an attractive candidate for producing bioethanol. The strong ethanol-producing route from glucose, called the Entner–Doudoroff pathway including the pyruvate–ethanol (PE) pathway, generates most of the ATP required for cellular activities, in a ratio of one mole of ATP per mole of glucose, which is one half of that in S. cerevisiae. Due to the lack of key enzymes for the Embden–Meyerhof–Parnas pathway and the TCA cycle,Citation40) the PE pathway is indispensable for Z. mobilis and its production of NADH and ATP per glucose consumed is low.Citation41) It is thus assumed that NADH oxidation via the electron transport chain becomes unfavorable for ethanol production due to the competition for NADH from the PE pathway. The organism appears to maintain a high level of glucose flux through these pathways to compensate for its low ATP yield, as large amounts of enzymes related to these pathways are expressed. Consequently, it forms less biomass and produces ethanol more efficiently than S. cerevisiae.Citation42,43)
IV.i. Ethanol fermentation capability of thermotolerant Z. mobilis at relatively high temperatures
Z. mobilis, a mesophilic bacterium, generally grows at around 30 °C but some of its strains are relatively thermotolerant. Of these, TISTR 405 is able to grow and produce ethanol even at 39 °C to an extent similar to that of 30 °C under shaking conditions.Citation44) TISTR 405's growth and ethanol productivity at 39 °C are better than those of a well-known efficient strain, CP4, even at 30 °C. Genes directly related to ethanol formation or degradation, adhA, adhB, and pdc, encoding ethanol dehydrogenase A (AdhA), ethanol dehydrogenase B (AdhB) and pyruvate decarboxylase, respectively, are highly conserved between both strains. However, the total activity of AdhA and AdhB in TISTR 405 is much higher than that in CP4.Citation44) These results allow us to speculate that high activity of the PE pathway is a crucial trait of thermotolerant strains, in order to produce the ATP required for repairing macromolecules damaged by high temperatures.
Under static conditions, TISTR 548 exhibits the highest growth and ethanol production at 39 °C among TISTR Z. mobilis strains and is clearly thermotolerant when compared to CP4. We have developed a procedure to determine an upper temperature limit that is able to detect colony-forming activity, called a critical high temperature, in which the target strain is cultivated twice under the same conditions at the same temperature. This procedure ensures the presence of viable cells at least during the first cultivation at the tested temperature. This simple but reliable procedure has allowed us to determine the critical high temperatures of TISTR 548 and CP4 to be 38 and 37 °C, respectively (T. Kosaka et al., unpublished). The difference of 1° seems to be very crucial, as both strains exhibited large differences in cell growth. The followings thus show recent data on TISTR 548 as a thermotolerant strain and CP4 as a less-thermotolerant strain.
The draft genome sequence of TISTR 548 led us to notice that TISTR 548 is described as ATCC 29191 (ZM6) in public databases. In order to explore the 1° difference in the critical high temperatures of both strains, we compared the complete genome sequences of ATCC 29191 and CP4. ATCC 29191 contains a chromosome of 1,961,307 bp and three plasmids, p29191_1 to p29191_3 (18,350 bp, 14,947 bp, and 13,742 bp, respectively). The entire genome has 1765 protein-coding genes, 51 tRNA genes, and three rRNA gene clusters.Citation45) In comparison, CP4 consists of a chromosome of 1,998,637 bp and five plasmids, pCP4_1 to pCP4_5 (36,892 bp, 33,915 bp, 32,400 bp, 30,952 bp, and 30,440 bp, respectively). Its genome has 1860 protein-coding genes, 48 tRNA genes, and two rRNA gene clusters.Citation46) In total, the former has a roughly 37 kb smaller genome than the latter, and a lower number of plasmids. There are 71 and 68 genes unique for ATCC 29191 and CP4, respectively, but LAST alignment revealed that the two strains have similar gene organization to each other except for two short different regions. Therefore, the 1° critical temperature difference would be due to the differential expression of common genes, the presence of unique genes and/or the genome size including plasmids.
IV.ii. Development of mutants adapted to temperatures higher than the critical temperature of the parental strain
In order to understand the molecular mechanisms of thermotolerance of microbes, we chose two procedures: (1) screening of disrupted mutants sensitive specifically to critical high temperature followed by analysis of the mutants obtained, and (2) development of mutants adapted to temperatures higher than the critical temperature of the parental strain.Citation47) The former was applied to three microbes and provides information regarding thermotolerant genes that are essential for survival at their critical high temperatures.Citation34,48)
In the latter procedure, repeated cultivation with gradually increasing temperatures was carried out (T. Kosaka et al., unpublished). In the case of TISTR 548 (ATCC 29191), three adapted mutants isolated by 80 rounds of culturing (named 80 M), 130 rounds of culturing (130 M) and 200 rounds of culturing (200 M), exhibit much better growth and ethanol production at 40 °C than the parental strain. The last two mutants show nearly the same thermotolerance and possess the same mutations (see below), suggesting the saturation of mutations after at least 130 rounds of culturing. The critical high temperature of the three mutants is 40 °C though the maximum growth of 80 M at this temperature is significantly lower than that of 130 and 200 M.
In the case of CP4, four adapted mutants obtained by independent cultivation via 80 rounds of culturing in different test tubes, exhibited significantly higher growth than the parental strain. The critical high temperature of the four mutants is 39 °C, though they show slight differences in their maximum growth at this temperature. Taken together with the results of the TISTR 548 mutants, it is likely that the repeated cultivation method can improve thermotolerance in Z. mobilis up to 2 °C.
IV.iii. Characteristics of adapted Z. mobilis strains
Z. mobilis exhibits typical physiological features of cell elongation and accumulation of reactive oxygen species (ROS) at a critical high temperature. As common phenotypes, all adapted mutants suppress both the abnormal phenotypes at a critical high temperature for the corresponding parental strain. The fact that oxidative stress increases and accumulates as temperature is elevatedCitation49) suggests the possibility that DNA fragments generated because of DNA damage from oxidative stress causes an arrest in cell division leading to filamentous cells. We assume that thermal adaptation prevents accumulation of DNA damage by reducing oxidative stress, so that the cell division can continue even at elevated critical high temperature. On the other hand, each adapted strain exhibits specific phenotypes with respect to tolerance of high glucose concentration, H2O2 resistance, reduction of respiratory chain activity and resistance to antibiotics (T. Kosaka et al., unpublished). These facts lead us to hypothesize that thermal adaptation is an efficient producer to enhance cellular robustness. The enhancement of thermotolerance in the adapted mutants, though only at 2°, may thus be very beneficial for industrial applications and to reduce costs in such operations.
IV.iv. Genomic alteration of adapted Z. mobilis strains
The phenotypes acquired in strains adapted to high temperatures can be further understood by the determination of genomic alterations. Draft genome sequencing followed by confirmation of individual mutations via direct sequencing reveals that there are similar and unique mechanisms for exhibiting these phenotypes. The mutations in adapted strains can be classified into eight groups including unknown functions (Table ). Adapted mutants of 80M and 130 M (200 M) from TISTR 548 share the same mutations due to continuous repeated cultivation between 80 and 200 times. Z4-80a, Z4-80b, Z4-80c, and Z4-80d, derived independently from CP4, have mutations in common and fall into unique classification groups. The presence of genes related to signal transduction and transcriptional regulators might reflect alterations in local or global genomic gene expression. Membrane stabilization and transporters might prevent the accumulation of ROS. Detailed analysis of the individual genes in Table allows us to understand the molecular mechanisms of thermal adaptation in Z. mobilis strains, which might provide useful information to convert generally non-thermotolerant mesophiles to thermotolerant mesophiles.
Table 2. Classification of genes mutated during thermal adaptation in two Z. mobilis strains.
IV.v. Enhancement of thermotolerance by introduction of genes in Z. mobilis
ROS are mostly generated by electrons leaking from the respiratory chain in organisms.Citation50) Z. mobilis has a simple and strong NADH-oxidizing respiratory chain, consisting of type-II NADH dehydrogenase and only one terminal oxidase, cytochrome bd.Citation18) Respiration-deficient mutants increased in ethanol fermentation under aerobic conditions.Citation51) However, it is likely that some of these respiratory activities are crucial for high-temperature fermentation; for example, a disrupted mutant of cytC (cytochrome c peroxidase), which likely accepts electrons from the cytochrome bc1 complex, exhibits significant reductions in growth and filamentous shape under shaking conditions at a high temperature and shows hypersensitivity to exogenous H2O2.Citation52) In relation to the reduction of oxidative stress, cytC and ZMO1573 for iron-dependent peroxidase are upregulated at higher temperatures. In the cytC-disrupted mutant, sod for superoxide dismutase, ahpC for alkyl hydroperoxide reductase and ZMO1573 are complementarily expressed.Citation52)
On the basis of these findings and other evidence that disrupted E. coli mutants of about 60% of the thermotolerant genes essential for growth at critical temperature are sensitive to H2O2,Citation48) further enhancement of thermotolerance was examined by the overexpression of genes related to protection against oxidative stress. As expected, the overexpression of each gene of sod, cat for catalase, cytC, ahpC, and ZMO1573 gives rise to increase in cell growth at high temperature, indicating enhanced thermotolerance (M. Murata et al., unpublished). In addition, it may be possible that the exogenous addition of some compounds strengthens thermotolerance of Z. mobilis. Magnesium and sugar alcohols protected Z. mobilis from heat stress and promoted cell growth and ethanol production at high temperatures.Citation53) Experiments with a defective mutant of gfo-encoding glucose–fructose oxidoreductase suggest that sorbitol plays a crucial role not only in cell growth and ethanol production but also in the protection of cellular proteins from stresses including high temperature.Citation54)
V. High-temperature alcohol fermentation and genomic analysis of the thermotolerant yeast K. marxianus
High-temperature fermentation is also effective for cost saving in ethanol fermentation. In addition to the general advantages of high-temperature fermentation described above, ethanol fermentation receives the additional benefits at high temperatures of the simultaneous saccharification and fermentation (SSF) process.Citation55,56) The SSF process includes saccharification and fermentation in the same bioreactor. The optimum temperatures for fermentation are lower than those of saccharification enzymes, meaning that enzymes do not fully demonstrate their potential in SSF. Utilization of a thermotolerant yeast in SSF enables the temperature to be closer to the optimum temperature (50–60 °C) of the enzyme, resulting in decreased enzyme loading. Higher temperature of fermentation culture also decreases the heating energy for 5–10 °C in distillation (80 °C).Citation56) Recovery of actively evaporated ethanol under high temperature and reduced pressure has also been proposed as a cost-saving process.Citation56) The conventional yeast S. cerevisiae, the strain exclusively used for industrial fuel ethanol fermentation, is sensitive to high temperatures over 35 °C in the presence of ethanol, indicating that one or more ethanol-producing and thermotolerant yeast strains are strongly required to establish a cost-effective high-temperature ethanol fermentation process.
V.i. Yeast strains for high-temperature ethanol fermentation
One possible approach to obtain thermotolerant yeasts is improvement of S. cerevisiae by mutagenesis, breeding, in vitro adaptation, and genetic engineering. These techniques were applied and improved thermotolerance of S. cerevisiae. However, fermentation temperatures of the developed strains were still around 40 °C. Recently, Caspeta et al.Citation16) reported that laboratory thermal adaptation of S. cerevisiae enhanced ethanol production rate at 40 °C. The genome sequence of the adapted strains as analyzed by a next-generation sequencer identified the mutations in the genes involved in sterol biosynthesis, DNA repair, and respiration.
Another approach is the use of naturally thermotolerant yeast species for high-temperature fermentation. There are several reports describing high-temperature ethanol fermentation by using Hansenula polymorpha, Pichia kudriavzevii, and Candida spp. In these reports, however, the maximum fermentation temperature was around 40 °C and the ethanol productivity was not high. H. polymorpha showed the highest ethanol productivity (1.3%) at 37 °C and produced only 0.24% ethanol at 45 °C.Citation57) P. kudriavzevii produced about 8% ethanol from 18% glucose at 40 °C for 24 h, but at 45 °C ethanol production decreased to 3.8% in 30 h.Citation58) Ethanol concentrations produced by Candida species were greatly decreased over 38 °C.Citation59)
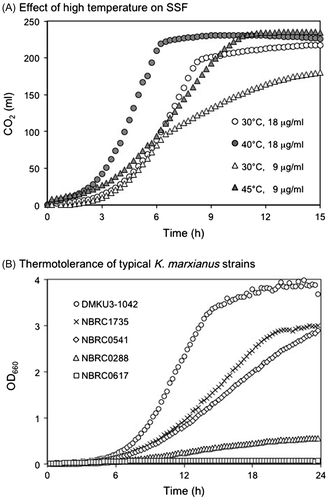
In contrast to these yeast species, K. marxianus seems to be the most promising species for use in industrial high-temperature ethanol fermentation. The maximum growth temperatures of K. marxianus strains are around 48 °C and growth at 52 °C is also reportedCitation60); these temperatures are significantly higher than those of other ethanol-producing yeasts. More importantly, K. marxianus produces ethanol even at 45 °C. The K. marxianus strains isolated from sugar mills in Australia produced 6% ethanol from 15% glucose within 24 h at 47 °C.Citation61) The strains isolated from an Indian distillery produced 7% ethanol from 14% glucose within 42 h at 45 °C.Citation60) The K. marxianus strain isolated in Thailand also showed high ethanol fermentation ability at 45 °C.Citation62) K. marxianus strains were often found in fermentative conditions in tropical areas, indicating that K. marxianus may have acquired thermotolerance and ethanol-producing abilities during long-term adaptation to high concentrations of sugars and ethanol at high temperatures. We demonstrated that a K. marxianus strain produced in excess of 8% ethanol from molasses in a 24-h period repeated batch fermentation at 40 °C in a 300 L scale reactor.Citation63) High-temperature ethanol fermentation from glucose or sucrose using K. marxianus seems to be ready for industrial application. We also tested the effects of high temperature on the SSF by using a K. marxianus strain, in which soluble starch was used as a substrate (Fig. (a)). Two types of positive effects were observed depending on the temperatures used. At 40 °C, fermentation speed was accelerated by 3 h and at 45 °C half the amount of glucoamylase was enough for complete fermentation compared with the amount used at 30 °C. In the case of S. cerevisiae, fermentation speed was delayed at 40 °C, and no ethanol production was observed at 45 °C (data not shown).
Fig. 4. SSF and thermotolerance of K. marxianus.
V.ii. Genome analysis of K.marxianus
Recent remarkable progress in sequencing technology enables us to reveal and examine microorganism genome sequences. In K. marxianus, four draft genome sequences were submitted to public genome databases during the last few years. The draft genome sequences were obtained by using different types of next-generation sequencers; some were assembled by combining the data gathered by two different sequencers or Sanger sequencing, and one was simply aligned to the complete genome described below. Compared with prokaryotes, yeasts have larger genome sizes and a single NGS read might be insufficient to obtain a highly assembled draft genome. Basically, the reported genome sizes, numbers of chromosomes, GC content and estimated numbers of open reading frames are the same or very similar. The reason why K. marxianus shows higher thermotolerance than the other yeast strains is not yet known from the draft genome sequences. However, the recently reported complete genome sequence and expression analysis data of a K. marxianus strain by our study may suggest some genetic backgrounds of its thermotolerance.Citation64) The genome size was 10.97 Mb consisting of eight chromosomes and a mitochondrial genome. The size was similar to the phylogenetically closest species Kluyveromyces lactis. The number of predicted genes in the genome is 4952, which is smaller than that of other hemiascomycete yeast species. The gene constituents in K. marxianus might have been simplified to adapt high temperature. Among the predicted genes, 193 genes have no homology, while the remaining 4759 genes have homologies with genes in some mesophilic yeast species. Some of these specific genes might be involved in thermotolerance. However, the possibility that a set of common genes has evolved in K. marxianus to confer thermotolerance cannot be excluded. Genomewide transcription analysis revealed that transcription of the genes for ribosome biogenesis, DNA repair and heat shock proteins were upregulated at 45 °C. These cellular functions may be sensitive to higher temperatures and could be reinforced by increased expression. In addition, some of the specific genes were upregulated at 45 °C compared with 30 °C.
Strains of K. marxianus show some strain-dependent abilities in inulinase production, lactose utilization, and xylose utilization. Thermotolerance is also different from strain to strain. We examined the growth of 15 strains obtained from culture collections and the complete genome strain DMKU3-1042. Fig. (b) shows growth of typical strains showing different growth speeds at 46 °C. The strain DMKU3-1042 showed fastest growth among the strains; NBRC1735 and NBRC0541 grew slower than DMKU3-1042, but NBRC0288 and NBRC0617 barely grew in these conditions. The NBRC0617 showed severe growth defect at 43 °C, and slow growth even at 40 °C. Comparative genomics between these K. marxianus strains could also be helpful to elucidate the genetic basis of thermotolerance. Since K. marxianus strains can be crossed and the resulting diploids can make viable spores,Citation65) repeated back crossing of these yeasts is a reliable way to obtain strains which show a specific phenotype with a limited number of causable genes. Our attempts to obtain thermotolerant progenies after repeated crossing between the strains demonstrated different thermotolerances among the progeny strains. NGS of the progeny will be helpful for quantitative trait locus analysis to uncover the genes involved in high-temperature growth.
VI. Aiming at development of stable & high-temperature fermentation system
We have succeeded in obtaining many strains adapted to higher temperatures from several fermentative bacteria, including both mesophilic and thermotolerant species. In addition to the strains, A. pasteurianus IFO 3283-01 and SKU1108, and Z. mobilis CP4 and TISTR548, as described above, we have also obtained thermoadapted strains from A. pasteurianus IFO 3283-32, Gluconacetobacter xylinus NBRC 3288, thermotolerant Gluconobacter frateurii CHM43,Citation39) Corynebacterium glutamicum KY9002, and thermotolerant C. glutamicum N24 (Table ). As shown, all thermally adapted strains acquired 2–3 °C higher growth temperatures. These thermally adapted strains could be useful in applications to high-temperature fermentation and for understanding the mechanisms of thermotolerance via genomic analysis. Genome modifications due to thermal adaptation varied from strain to strain in terms of the number of mutations, the modes of the mutations, and the expected functions of the mutated genes. As for the mode of mutation, there are several types, such as nucleotide insertions from a single base pair up to 12-bp, transposon insertions, and nucleotide deletions from a single bp up to 92-kbp, in addition to relatively large numbers of nucleotide substitutions (Table ). The genome truncation described previously in IFO 3283-01/42C is also observed in the adapted strains of A. pasteurianus IFO 3283-13 and C. glutamicum KY9002 (Table ). While the functions of the mutated genes are also highly diverse, the mutated genes could be categorized into several groups: genes related to cell surface functions (peptidoglycan synthesis, outer membrane protein, lipopolysaccharide synthesis), transporters for ion or amino acids, and two-component signal transduction systems. In addition, although the results are not shown here (only partly described in case of the IFO 3283-01/42C), gene expression and proteomic analysis showed, in all the strains we used, that several ROS-scavenging enzymes are increased when grown at higher temperatures. Furthermore, the overexpression of these corresponding genes enables the cell to grow at higher temperatures. This is in agreement with the findings that all these strains produce ROS at a higher level when grown at higher temperatures. Thus, thermal adaptation could lead to decreased ROS generation, suggesting that the thermally adapted cells could become robust and resist many stressors.
Table 3. Summary of thermal adaptation or cross breeding of several fermentative microbes and their genome modifications occurred in the breeding processes.Table Footnotea
These thermally adapted strains and naturally thermotolerant strains could be useful for high-temperature fermentations. In this review, we showed successful acetic acid fermentation with thermally adapted strains at 40 °C and higher without temperature control when the fermentation temperature increased up to 44 °C. High-temperature alcohol fermentation was achieved with the thermotolerant yeast K. marxianus at around 45 °C. Although the results are not shown in this review, large-scale alcohol fermentation with K. marxianus has been shown to be repeatable many times over without loss of the fermentation ability. These fermentations that could be carried out at higher temperatures reduce electricity consumption during the fermentation process, and it should be noted that natural nontemperature-control acetic acid fermentation was shown to reduce electricity usage by nearly 60% without any decreased fermentation ability. More importantly, such thermal adaptation may confer additional properties to the adapted strains, as shown in the case of thermally adapted A. pasteurianus TH-3, where acetic acid resistance was also acquired together with the thermotolerance.
Thus, these thermotolerant or thermally adapted microbes could be very useful for high temperature or robust fermentations, where an economical fermentation could be achieved through stably repeated fermentation processes and with reduced electricity costs, even without any cooling equipment during the fermentation process. Therefore, such fermentation systems could be useful, especially for bioenergy production or commodity chemical production while maintaining low-cost performance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Advanced Low Carbon Technology Research and Development Program (ALCA); Japan Society for the Promotion of Science (JSPS); National Research Council of Thailand (NRCT).
Acknowledgments
Part of the work described in this review paper were carried out by many collaborators, especially Minenosuke Matsutani (YU), Masayuki Murata (YU), Uraiwan Masud (Kasetsart U.), Kannikar Charoensuk (RMUTT), Kaewta Sootsuwan (RMUTI), Naoko Furuya (YU), Nobuyuki Fujita (NITE), Akira Hosoyama (NITE), Hirofumi Yoshikawa (TUA), Yutaka Suzuki (UT), Yuki Shite (YU). The authors deeply appreciate the efforts by all of these collaborators to accomplish this work continuously and successfully.
References
- Saeki A, Theeragool G, Matsushita K, et al. Development of thermotolerant acetic acid bacteria useful for vinegar fermentation at higher temperatures. Biosci. Biotech. Biochem. 1997;61:138–145.
- Moonmangmee D, Adachi O, Ano Y, et al. Isolation and characterization of thermotolerant Gluconobacter strains catalyzing oxidative fermentation at higher temperatures. Biosci. Biotechnol. Biochem. 2000;64:2306–2315.10.1271/bbb.64.2306
- Limtong S, Sringiew C, Yongmanitchai W. Production of fuel ethanol at high temperature from sugar cane juice by a newly isolated Kluyveromyces marxianus. Bioresour. Technol. 2007;98:3367–3374.10.1016/j.biortech.2006.10.044
- Manaia CM, Moore ER. Pseudomonas thermotolerans sp. nov., a thermotolerant species of the genus Pseudomonas sensu stricto. Int. J. Syst. Evol. Microbiol. 2002;52:2203–2209.
- Ndoye B, Lebecque S, Dubois-Dauphin R, et al. Thermoresistant properties of acetic acid bacteria isolated from tropical products of Sub-Saharan Africa and destined to industrial vinegar. Enzyme Microb. Technol. 2006;39:916–923.10.1016/j.enzmictec.2006.01.020
- Sikorski J, Brambilla E, Kroppenstedt RM, et al. The temperature-adaptive fatty acid content in Bacillus simplex strains from ‘Evolution Canyon’, Israel. Microbiology. 2008;154:2416–2426.10.1099/mic.0.2007/016105-0
- Illeghems K, De Vuyst L, Weckx S. Complete genome sequence and comparative analysis of Acetobacter pasteurianus 386B, a strain well-adapted to the cocoa bean fermentation ecosystem. BMC Genomics. 2013;14:526. doi: 10.1186/1471-2164-14-526.
- Ohmori S, Masai H, Arima K, et al. Isolation and identification of acetic acid bacteria for submerged acetic acid fermentation at high temperature. Agric. Biol. Chem. 1980;44:2901–2906.10.1271/bbb1961.44.2901
- Sallstrom B, Andersson SG. Genome reduction in the alpha-proteobacteria. Curr. Opin. Microbiol. 2005;8:579–585.
- Nobusato A, Uchiyama I, Ohashi S, et al. Insertion with long target duplication: a mechanism for gene mobility suggested from comparison of two related bacterial genomes. Gene. 2000;259:99–108.10.1016/S0378-1119(00)00456-X
- Azuma Y, Hosoyama A, Matsutani M, et al. Whole-genome analyses reveal genetic instability of Acetobacter pasteurianus. Nucleic Acids Res. 2009;37:5768–5783.10.1093/nar/gkp612
- Sjödin A, Svensson K, Lindgren M, et al. Whole-genome sequencing reveals distinct mutational patterns in closely related laboratory and naturally propagated Francisella tularensis strains. PLoS One. 2010;5:e11556.10.1371/journal.pone.0011556
- Rudolph B, Gebendorfer KM, Buchner J, et al. Evolution of Escherichia coli for growth at high temperatures. J. Biol. Chem. 2010;285:19029–19034.10.1074/jbc.M110.103374
- Rodriguez-Verdugo A, Carrillo-Cisneros D, Gonzalez-Gonzalez A, et al. Different tradeoffs result from alternate genetic adaptations to a common environment. Proc. Nat. Acad. Sci. USA. 2014;111:12121–12126.
- Wallace-Salinas V, Gorwa-Grauslund MF. Adaptive evolution of an industrial strain of Saccharomyces cerevisiae for combined tolerance to inhibitors and temperature. Biotechnol. Biofuels. 2013;6:151. doi: 10.1186/1754-6834-6-151.10.1186/1754-6834-6-151
- Caspeta L, Chen Y, Ghiaci P, et al. Biofuels. Altered sterol composition renders yeast thermotolerant. Science. 2014;346:75–78.10.1126/science.1258137
- Matsutani M, Nishikura M, Saichana N, et al. Adaptive mutation of Acetobacter pasteurianus SKU1108 enhances acetic acid fermentation ability at high temperature. J. Biotechnol. 2013;165:109–119.10.1016/j.jbiotec.2013.03.006
- Sootsuwan K, Lertwattanasakul N, Thanonkeo P, et al. Analysis of the respiratory chain in ethanologenic Zymomonas mobilis with a cyanide-resistant bd-type ubiquinol oxidase as the only terminal oxidase and its possible physiological roles. J. Mol. Microbiol. Biotechnol. 2008;14:163–175.10.1159/000112598
- Lambert B, Kersters K, Gosselé F, et al. Gluconobacters from honey bees. Antonie Van Leeuwenhoek. 1981;47:147–157.10.1007/BF02342197
- Yamada Y, Yukphan P. Genera and species in acetic acid bacteria. Int. J. Food Microbiol. 2008;125:15–24.10.1016/j.ijfoodmicro.2007.11.077
- Adachi O, Moonmangmee D, Toyama H, et al. New developments in oxidative fermentation. Appl. Microbiol. Biotechnol. 2003;60:643–653.10.1007/s00253-002-1155-9
- Beppu T. Genetic organization of Acetobacter for acetic acid fermentation. Antonie Van Leeuwenhoek. 1993;64:121–135.
- Steiner P, Sauer U. Proteins induced during adaptation of Acetobacter aceti to high acetate concentrations. Appl. Environ. Microbiol. 2001;67:5474–5481.10.1128/AEM.67.12.5474-5481.2001
- Takemura H, Horinouchi S, Beppu T. Novel insertion sequence IS1380 from Acetobacter pasteurianus is involved in loss of ethanol-oxidizing ability. J. Bacteriol. 1991;173:7070–7076.
- Coucheron DH. An Acetobacter xylinum insertion sequence element associated 21, lin inactivation of cellulose production. J. Bacteriol. 1991;173:5723–5731.
- Matsutani M, Ito K, Azuma Y, et al. Adaptive mutation related to cellulose producibility in Komagataeibacter medellinensis (Gluconacetobacter xylinus) NBRC 3288. Appl. Microbiol. Biotechnol. 2015;99:7229–7240.10.1007/s00253-015-6598-x
- Greenberg DE, Porcella SF, Zelazny AM, et al. Genome sequence analysis of the emerging human pathogenic acetic acid bacterium Granulibacter bethesdensis. J. Bacteriol. 2007;189:8727–8736.10.1128/JB.00793-07
- Kawai M, Higashiura N, Hayasaki K, et al. Complete genome and gene expression analyses of Asaia bogorensis reveal unique response to culture with mammalian cells as a potential opportunistic human pathogen. DNA Res. 2015;22:357–366.
- Martin P, Makepeace K, Hill SA, et al. Microsatellite instability regulates transcription factor binding and gene expression. Proc. Nat. Acad. Sci. USA. 2005;102:3800–3804.10.1073/pnas.0406805102
- Iyer RR, Pluciennik A, Rosche WA, et al. DNA polymerase III proofreading mutants enhance the expansion and deletion of triplet repeat sequences in Escherichia coli. J. Biol. Chem. 2000;275:2174–2184.10.1074/jbc.275.3.2174
- Casane D, Dennebouy N, de Rochambeau H, et al. Genetic analysis of systematic mitochondrial heteroplasmy in rabbits. Genetics. 1994;138:471–480.
- Kanchanarach W, Theeragool G, Yakushi T, et al. Characterization of thermotolerant Acetobacter pasteurianus strains and their quinoprotein alcohol dehydrogenases. Appl. Microbiol. Biotechnol. 2010;85:741–751.10.1007/s00253-009-2203-5
- Davies SJ, Golby P, Omrani D, et al. Inactivation and regulation of the aerobic C4-dicarboxylate transport (dctA) gene of Escherichia coli. J. Bacteriol. 1999;181:5624–5635.
- Soemphol W, Deeraksa A, Matsutani M, et al. Global analysis of the genes involved in the thermotolerance mechanism of thermotolerant Acetobacter tropicalis SKU1100. Biosci. Biotechnol. Biochem. 2011;75:1921–1928.10.1271/bbb.110310
- Alekshun MN, Levy SB. The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 1999;7:410–413.10.1016/S0966-842X(99)01589-9
- Jennings MP, Anderson JK, Beacham IR. Cloning and molecular analysis of the Salmonella enterica ansP gene, encoding an L-asparagine permease. Microbiology. 1995;141:141–146.10.1099/00221287-141-1-141
- Matsushita K, Toyama H, Adachi O. Respiratory chains and bioenergetics of acetic acid bacteria. Adv. Microb. Physiol. 1994;36:247–301.10.1016/S0065-2911(08)60181-2
- Perumpuli PA, Watanabe T, Toyama H. Identification and characterization of thermotolerant acetic acid bacteria strains isolated from coconut water vinegar in Sri Lanka. Biosci. Biotechnol. Biochem. 2014;78:533–541.10.1080/09168451.2014.882758
- Hattori H, Yakushi T, Matsutani M, et al. High-temperature sorbose fermentation with thermotolerant Gluconobacter frateurii CHM43 and its mutant strain adapted to higher temperature. Appl. Microbiol. Biotechnol. 2012;95:1531–1540.10.1007/s00253-012-4005-4
- Seo JS, Chong H, Park HS, et al. The genome sequence of the ethanologenic bacterium Zymomonas mobilis ZM4. Nat. Biotechnol. 2005;23:63–68.10.1038/nbt1045
- Sprenger GA. Carbohydrate metabolism in Zymomonas mobilis: a catabolic highway with some scenic routes. FEMS Microbiol. Lett. 1996;145:301–307.10.1111/fml.1996.145.issue-3
- Rogers PL, Lee KL, Tribe DE. High productivity ethanol fermentations with Zymomonas mobilis. Process Biochem. 1980;15:7–11.
- Thanonkeo P. Effect of ethanol and heat stress on physiological responses in Zymomonas mobilis. In: Yamada M, editor. Survival and death in bacteria. Kerala: Research Signpost; 2005. p. 183–196.
- Sootsuwan K, Irie A, Murata M, et al. Thermotolerant Zymomonas mobilis: comparison of ethanol fermentation capability with that of an efficient type strain. Open Biotechnol. J. 2007;1:59–65.10.2174/1874070700701010059
- Desiniotis A, Kouvelis VN, Davenport K, et al. Complete genome sequence of the ethanol-producing Zymomonas mobilis subsp. mobilis centrotype ATCC 29191. J. Bacteriol. 2012;194:5966–5967.10.1128/JB.01398-12
- Kouvelis VN, Teshima H, Bruce D, et al. Finished genome of Zymomonas mobilis subsp. mobilis strain CP4, an applied ethanol producer. Genome Announc. 2014;2:e00845-13.
- Yamada M, Akada R, Kosaka T, et al. Molecular mechanisms of thermotolerance of thermotolerant fermentation microorganisms. Kagakutoseibuts. Japanese. 2015;53:763–773.
- Murata M, Fujimoto H, Nishimura K, et al. Molecular strategy for survival at a critical high temperature in Eschierichia coli. PLoS One. 2011;6:e20063.10.1371/journal.pone.0020063
- Noor R, Murata M, Yamada M. Oxidative stress as a trigger for growth phase-specific sigmaE-dependent cell lysis in Escherichia coli. J. Mol. Microbiol. Biotechnol. 2009;17:177–187.10.1159/000236029
- Davies BW, Kohanski MA, Simmons LA, et al. Hydroxyurea induces hydroxyl radical-mediated cell death in Escherichia coli. Mol. Cell. 2009;36:845–860.10.1016/j.molcel.2009.11.024
- Hayashi T, Furuta Y, Furukawa K. Respiration-deficient mutants of Zymomonas mobilis show improved growth and ethanol fermentation under aerobic and high temperature conditions. J. Biosci. Bioeng. 2011;111:414–419.10.1016/j.jbiosc.2010.12.009
- Charoensuk K, Irie A, Lertwattanasakul N, et al. Physiological importance of cytochrome c peroxidase in ethanologenic thermotolerant Zymomonas mobilis. J. Mol. Microbiol. Biotechnol. 2011;20:70–82.10.1159/000324675
- Thanonkeo P, Laopiboon P, Sootsuwan K, et al. Magnesium ions improve growth and ethanol production of Zymomonas mobilis under heat or ethanol stress. Biotechnology. 2007;6:112–119.
- Sootsuwan K, Thanonkeo P, Keeratirakha N, et al. Sorbitol required for cell growth and ethanol production by Zymomonas mobilis under heat, ethanol, and osmotic stresses. Biotechnol. Biofuels. 2013;6:180. doi: 10.1186/1754-6834-6-180.10.1186/1754-6834-6-180
- Banat IM, Nigam P, Singh D, et al. Ethanol production at elevated temperature and alcohol concentrations: Part I-Yeasts in general. World J. Microbiol. Biotechnol. 1998;14:809–821.10.1023/A:1008802704374
- Abdel-Banat BM, Hoshida H, Ano A, et al. High-temperature fermentation: how can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl. Microb. Biotechnol. 2010;85:861–867.10.1007/s00253-009-2248-5
- Ryabova OB, Chmil OM, Sibirny AA. Xylose and cellobiose fermentation to ethanol by the thermotolerant methylotrophic yeast Hansenula polymorpha. FEMS Yeast Res. 2003;4:157–164.10.1016/S1567-1356(03)00146-6
- Yuangsaard N, Yongmanitchai W, Yamada M, et al. Selection and characterization of a newly isolated thermotolerant Pichia kudriavzevii strain for ethanol production at high temperature from cassava starch hydrolysate. Antonie Van Leeuwenhoek. 2013;103:577–588.10.1007/s10482-012-9842-8
- Tanimura A, Nakamura T, Watanabe I, et al. Isolation of a novel strain of Candida shehatae for ethanol production at elevated temperature. SpringerPlus. 2012;1:27. doi:10.1186/2193-1801-1-27.
- Banat IM, Nigam P, Marchant R. Isolation of thermotolerant, fermentative yeasts growing at 52°C and producing ethanol at 45°C and 50°C. World J. Microbiol. Biotechnol. 1992;8:259–263.10.1007/BF01201874
- Anderson PJ, McNeil K, Watson K. High-efficiency carbohydrate fermentation to ethanol at temperatures above 40 degrees C by Kluyveromyces marxianus var. marxianus isolated from sugar mills. Appl. Environ. Microbiol. 1986;51:1314–1320.
- Nonklang S, Abdel-Banat BM, Cha-aim K, et al. High-temperature ethanol fermentation and transformation with linear DNA in the thermotolerant yeast Kluyveromyces marxianus DMKU3-1042. Appl. Environ. Microbiol. 2008;74:7514–7521.10.1128/AEM.01854-08
- Research and Development of Cost-effective Fermentation Technology Using a Thermotolerant Yeast (FY2007–FY2010) Final Report: citied on 2011.06.29. http://www.nedo.go.jp/library/seika/shosai_201106/20110000000963.html. Japanese.
- Lertwattanasakul N, Kosaka T, Hosoyama A, et al. Genetic basis of the highly efficient yeast Kluyveromyces marxianus: complete genome sequence and transcriptome analyses. Biotechnol. Biofuels. 2015;8:47. doi: 10.1186/s13068-015-0227-x.10.1186/s13068-015-0227-x
- Yarimizu T, Nonklang S, Nakamura J, et al. Identification of auxotrophic mutants of the yeast Kluyveromyces marxianus by non-homologous end joining-mediated integrative transformation with genes from Saccharomyces cerevisiae. Yeast. 2013;30:485–500.10.1002/yea.v30.12
