Abstract
The identification of disease-specific alterations in miRNA expression and the ability to detect miRNAs in serum furnish the basis for identified potential research value. This study was aimed to characterize the expression of miRNAs in the serum samples from people with type 2 diabetes mellitus (T2DM) and healthy individuals in order to detect the differential expression of miRNAs in T2DM. In total, 582 participants were recruited. Microarray-based miRNA expression profiles were screened in pooled serum samples from two groups (T2DM and healthy control). The candidates’ miRNAs were validated by reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR). Five significantly different serum miRNAs were identified in T2DM patients (hsa-miR-320d, hsa-miR-4534, hsa-miR-3960, hsa-miR-451a, and hsa-miR-572) compared to those in the serum of healthy controls. This study provided evidence that serum miRNAs had differential expressions between healthy controls and T2DM patients. These five differential expression miRNAs might be of help for subsequent study in T2DM.
Graphical abstract
This research found five significant disease-special serum miRNAs (hsa-miR-320d, hsa-miR-4534, hsa-miR-3960, hsa-miR-451a, hsa-miR-572) in T2DM compared with healthy control.
Key words:
Diabetes mellitus (DM) has affected over 350 million people worldwide.Citation1) Diabetes is a complex metabolic disorder that is the result of a combination of environmental and genetic factors that induce individuals fail to maintain blood glucose homeostasis.Citation2) T2DM, which accounts for almost 90% of all cases of DM, is characterized by peripheral insulin resistance and β-cell dysfunction. Pancreatic β-cells are required to produce much more insulin to satisfy the increased demand when insulin resistance occurs.Citation3) As one’s age increases, β-cells are damaged by decreased functions, which finally leads to T2DM.Citation4) Multiple chronic macrovascular and microvascular complications (e.g. diabetic nephropathy, cardiomyopathy, neuropathy, and retinopathy) caused by T2DM give rise to considerable global morbidity and mortality rates as a result of strokes, myocardial infractions, blindness, and foot ulcers.Citation5–8) Serious complications can devastate one’s quality of life and life expectancy.Citation6,9) Diabetes has become a major socioeconomic health problem, but there is still no efficient prevention measures or treatments. It is quite essential to explore a novel research direction for T2DM.
MiRNAs were initially identified in early 1990s. They are endogenous, single-stranded RNA molecules consisting of 21–23 nucleotides. It has been suggested that miRNAs regulate gene expression at the post-transcriptional level by binding to the three untranslated regions of the target mRNAs.Citation10–12) Mitchell P. S. and others found that miRNAs detected in serum/plasma were present in a remarkably stable form that was protected from endogenous RNase activity.Citation13–20) Recent studies have suggested that miRNA expressions were potentially disease specific and could be used to identify various types of diseases, including breast cancer, colorectal cancer, large β-cell lymphoma, tissue injury, and non-small cell lung cancer.Citation14, 21–25) These characteristics of circulating miRNAs have great promise as novel, non-invasive biomarkers for diseases.Citation6, 20, 26, 27)
In order to study the relationship between serum miRNAs and T2DM, the expression levels of miRNAs were detected in the serum of T2DM patients and those in the healthy control group. This research investigated 1887 serum microRNA expression profiles with independent validation in a large cohort of 582 participants. The main purpose of the study was to identify the differential expressions of serum miRNAs in T2DM patients.
Materials and methods
Study design and group population
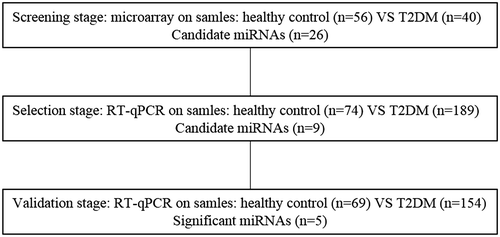
This project was approved by the First Affiliated Hospital of Wenzhou Medical University, and written informed consent was obtained from all volunteers. All serum samples used for the present study were obtained from the First Affiliated Hospital of Wenzhou Medical University between 2012 and 2014. This study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. All of the T2DM patients conformed to the T2DM inclusion and exclusion standard. The T2DM inclusion standards were as follows: symptoms of diabetes and blood glucose ≥11.1 mmol/L (200 mg/dL); fasting blood glucose (FBG) ≥7 mmol/L (126 mg/dL); and glucose tolerance testing 2-h blood glucose ≥11.1 mmol/L (200 mg/dL). The patients included in the T2DM group had to comply with one of the above standards on the day prior to review testing. The T2DM exclusion standards were as follows: patients who had endocrine disease; patients who had abnormal endocrine system-related indicators; patients who had tumor or abnormal tumor markers. All of the people included in the healthy controls group had clinical indicators that were within the medical reference value range and did not have diagnosed diseases, family histories of hereditary diseases, T2DM, or other malignant diseases. A multi-stage, case-control study was designed to identify different expressions of serum miRNAs in order to distinguish T2DM patients from those in the healthy control group (Fig. ). In the screening stage, a total of 96 individuals were categorized accordingly into two groups, T2DM (n = 40) and healthy controls (n = 56), without complications. Serum samples were screened with a microarray platform (Table ). There was no significant difference in the distribution of age and gender in the two groups (healthy and T2DM). The two groups were mixed, respectively, for microarray. Subsequently, individual RT-qPCR was performed in the selection and validation stages to further screen the candidate miRNAs (Table ). There was no significant difference in the distribution of age and gender between the selection and validation stages for the healthy control group and the T2DM group. During the selection stage, 263 samples were tested to selected candidate miRNAs, 74 from T2DM patients and 189 from the healthy control group. In the last stage, 223 more samples were added in order to validate the selected candidate miRNAs, 69 from T2DM patients and 154 from those in the healthy control group.
Table 1. Characteristics of study participants in the screening stages.
Table 2. Characteristics of study participants in the selection and validation stages.
MiRNA isolation
For the study of miRNAs, 10-mL serum tubes with clot activators were collected. After allowing the content of the serum tubes to clot at room temperature for 1 h, the serum was prepared by centrifugation at 1000 × g (2300 rpm) for 15 min at 4 °C. The serum supernatant was slowly removed, leaving 0.5 cm remaining to avoid disturbing the serum–clot interface, and stored at −80 °C. Serum was thawed on ice before use.
The MiRNA extracted from serum using a miRNeasy kit (Qiagen miRNeasy Mini Kit) according to the manufacturer’s recommendations. The extracted miRNAs were stored at −80 °C for microRNA microarrays and qRT-PCR.
MicroRNA microarray
The miRNA samples were spiked by the MicroRNA Spike-In Kit (Agilent Technologies) to help distinguish biological data from processing issues of the labeling and hybridization. 100 ng of miRNA per sample was dephosphorylated by alkaline calf intestine phosphatase incubating the reaction at 37 °C. T4 RNA ligase was used to label the dephosphorylated RNA incorporated with cyanine 3-cytidine biphosphate (miRNA Complete Labeling and Hyb Kit; Agilent Technologies). The labeled miRNA samples were hybridized to human miRNA microarrays (Human miRNA 8 × 60 K V18.0; Agilent Technologies) at 55 °C for at least 20 h. After washing the microarray slides (Gene Expression Wash Buffers; Agilent Technologies), they were dried. Scanning was performed with the Agilent G2565CA Microarray Scanner, and images were extracted using the Feature Extraction 10.7.3.1 Software (Agilent Technologies). All of the steps described were performed according to the manufacturer’s instructions.
qRT-PCR assay
For the testing of candidate miRNAs acquired on microarrays, qRT-PCR was performed using SYBR-Green assays (Applied Biosystems) in a final reaction volume of 20 μl (All-in-One miRNA Q-PCR Detection Kit, GeneCopoeia). The reaction mix was transferred into 96-well plates and run on the qRT-PCR instrument (Applied Biosystems). The amplification curves were analyzed using version 2.1 of the StepOne Software (Applied Biosystems) for both the determination of cycle threshold (Ct) value and for melting curves analysis.
Statistical analyses
For quality control, statistical analysis, miRNA annotation, and visualization purposes, we used Feature Extraction 10.7.3.1 Software and GeneSpring GX11.5.1 (both Agilent Technologies). Cluster analysis was performed using the unweighted pair group method with arithmetic means based on Euclidean distance with hierarchical clustering applied to the normalized data. Absolute fold changes were calculated to identify different expressions between the T2DM and control groups. A T-test was performed between the T2DM and healthy control groups.
Version 2.1 of the StepOne Software (Applied Biosystems) was utilized for instrument control, data acquisition, and raw data analysis. In order to calculate the relative expression levels of the target miRNAs, we performed ΔCt normalization, which was calculated using the following mathematical formula: ΔCt = Ctsample − Ctendogenous. Unpaired T-tests (with significance levels set at p < 0.05) were calculated between the T2DM and healthy control groups.
Statistical analysis was performed using SPSS 17.0, and presented with GraphPad Prism 5.0 software. Results were considered statistically significant when p < 0.05.
Results
Differential microRNA expression based on microarray data
In order to gain an expression profile of serum miRNAs that is specific to T2DM, the microarray was used to identify the differentially expressed miRNAs in those in the healthy control group (n = 56) and those in the T2DM group (n = 40) in the initial screening stage (Supplemental Fig. 1). We applied a stringent filtering approach that compared T2DM with healthy control (absolute fold change > 2.0). Among 1887 miRNAs analyzed, we identified 54 up-regulated and 4 down-regulated miRNAs (Supplemental Table 1). Through the results of the microarray and the relative reportsCitation26, 28) observably differentially expressed microRNAs were selected and identified as candidates for further qRT-PCR. They were the following: hsa-miR-4454, hsa-miR-4497, hsa-miR-4530, hsa-miR-4534, hsa-miR-4713–3p, hsa-miR-4716-3p, hsa-miR-762, hsa-miR-1183, hsa-miR-1305, hsa-miR-134, hsa-miR-3198, hsa-miR-320c, hsa-miR-320d, hsa-miR-3676-5p, hsa-miR-4443, hsa-miR-572, hsa-miR-575, hsa-let-7b-3p, hsa-miR-21-5p, hsa-miR-223-3p, hsa-miR-3960, hsa-miR-4281, hsa-miR-4459, hsa-miR-4514, hsa-miR-5581-5p, and hsa-miR-451a.
qRT-PCR validation
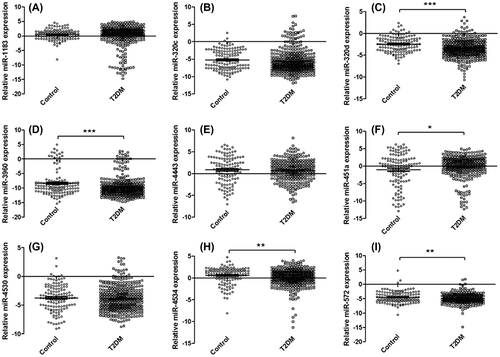
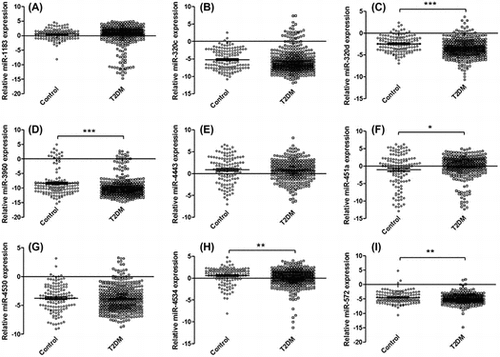
In order to characterize the differential expression of serum miRNAs between those in the T2DM and control groups, the reference gene miR-1228 and the 26 differently expressed miRNAs were detected. The differential expression of the target miRNAs between the T2DM and control groups was summarized (Supplemental Table 2). The expression levels of nine miRNAs (hsa-miR-1183, hsa-miR-320c, hsa-miR-320d, hsa-miR-4530, hsa-miR-4534, hsa-miR-3960, hsa-miR-451a, hsa-miR-4443, and hsa-miR-572) were different in T2DM group compared to the healthy control group.
The assays were further applied on 223 samples for nine candidate miRNAs by qRT-PCR. Finally, five significantly changed serum miRNAs were identified (hsa-miR-320d, hsa-miR-4534, hsa-miR-3960, hsa-miR-451a, and hsa-miR-572) in T2DM patients compared to those from the healthy control group (Fig. ).
Discussion
Diabetes is a complex metabolic disorder disease. This complex disease is based on the consequence of interaction between several factors, including genetics, lifestyle, age, and ethnic and environmental factors. T2DM is characterized by insulin resistance. Enhanced insulin resistance or reduced compensatory insulin secretion bring about decreased glucose tolerance and gradually progress to diabetes. Then, blood glucose levels began to rise. Hie et al. investigated the effects of insulin-dependent DM on osteoblastogenesis and osteoclastogenesis used diabetic rats two weeks after the streptozotocin injection. They found that the level of the receptor activator of NF-κB (RANK) protein increased and the runt-related transcription factor 2 (Runx2) protein decreased in diabetic rats, and the change could be reversed by insulin treatment.Citation29) Subsequently, Hu et al. found miR-3960/miR-2861 promotes osteoblast differentiation by targeting histone deacetylase 5 and increases Runx2 protein production.Citation30) In this study, miR-3960 was found to have a differential expression in the serum of T2DM patients. Based on these studies, it can be seen that miR-3960 might play an important role in diabetes.
This study demonstrated four other differential expressions of serum miRNAs in T2DM patients (miR-451a, miR-320d, miR-4534, and miR-572). Murata K. et al. compared the expression of miR-451 in neutrophils from patients with rheumatoid arthritis (RA) and healthy controls to identify the role of miR-451 in autoimmune arthritis. The research found that miR-451 suppresses neutrophil chemotaxis via p38 MAPK and was a potential target in the treatment of RA.Citation31) Map kinase kinase 3 (MKK3)–p38 MAPK signaling was a pathogenic factor in the progression of diabetic nephropathy in db/db mice.Citation32) Mir-451 might have potential research value in the study of diabetic nephropathy. Zhang et al. found that miR-320d was under-expressed in their research, which might be involved in the regulation of stem cell differentiation,Citation33) but miR-320d was never reported in T2DM. Through deep sequencing, Jima et al. found that miR-4534 has differential expression in normal and malignant human β-cells, but the function of miR-4534 was not detected in this study.Citation28) MiR-572 was reported in a colorectal microRNAome study, but the function of miR-572 was not clear in this research.Citation34)
In conclusion, we identified serum miRNAs that differentiate T2DM patients from healthy individuals with a high degree of accuracy in a large number of participants. Our study demonstrates that serum miRNAs have differential expressions in T2DM patients, and that serum miRNAs dysregulation might be associated with the pathogenesis of diabetes.
Author contributions
Conceived and designed the experiments: Lianjin Zhong and Jianxin Lu.
Analyzed the data: Linchao Ding, Ruihao Wu, and Tao Zhang. Contributed reagents/materials/analysis tools: Li Jing
Performed the experiments and wrote the manuscript: Linchao Ding and Dongdong Ai.
Funding
This work was supported by the Program for Zhejiang Leading Team of S&T Innovation [grant number 2010R50048].
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2015.1107460.
ZhongSupplementalFig1.tif
Download TIFF Image (1.6 MB)ZhongSupplementalTable1.doc
Download MS Word (65.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011;94:311–321.
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820.
- Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384.
- Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53:S16–S21.
- Wadwa RP, Jaiswal M. Vascular measures to detect earlier macrovascular disease. Diabetes Technol. Ther. 2011;13:1269–1270.
- Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013;9:513–521.
- Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289:76–79.
- Guo L, Jiang F, Tang YT, et al. The Association of serum vascular endothelial growth factor and ferritin in diabetic microvascular disease. Diabetes Technol. Ther. 2014;16:224–234.
- Li R, Zhang P, Barker LE, et al. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care. 2010;33:1872–1894.
- Stark A, Brennecke J, Bushati N, et al. Animal microRNAs confer robustness to gene expression and have a significant impact on 3’UTR evolution. Cell. 2005;123:1133–1146.
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev. Cell. 2006;11:441–450.
- Lee RC, Feinbaum RL, Ambros V, et al. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854.
- Chin LJ, Slack FJ. A truth serum for cancer–microRNAs have major potential as cancer biomarkers. Cell Res. 2008;18:983–984.
- Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006.
- Ajit SK. Circulating microRNAs as biomarkers, therapeutic targets, and signaling molecules. Sensors. 2012;12:3359–3369.
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. PNAS. 2008;105:10513–10518.
- Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. PNAS. 2011;108:5003–5008.
- Hunter MP, Ismail N, Zhang X, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS ONE. 2008;3:e3694.
- Gibbings DJ, Ciaudo C, Erhardt M, et al. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Bio. 2009;11:1143–1149.
- Etheridge A, Lee I, Hood L, et al. Extracellular microRNA: a new source of biomarkers. Mutat. Res-Fund Mol. M. 2011;717:85–90.
- Zhu W, Qin W, Atasoy U, et al. Circulating microRNAs in breast cancer and healthy subjects. BMC Res. Notes. 2009;2:89. doi: 10.1186/1756-0500-2-89.
- Lawrie CH, Gal S, Dunlop HM, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. PNAS. 2009;106:4402–4407.
- Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–1381.
- Wang JF, Yu ML, Yu G, et al. Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem. Biophys. Res. Co. 2010;394:184–188.
- Laterza OF, Lim L, Garrett-Engele PW, et al. Plasma microRNAs as sensitive and specific biomarkers of tissue injury. Clin. Chem. 2009;55:1977–1983.
- Whitehead CL, Teh WT, Walker SP, et al. Circulating microRNAs in maternal blood as potential biomarkers for fetal hypoxia in-utero. PLoS ONE. 2013;8:e78487.
- Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012;110:483–495.
- Jima DD, Zhang J, Jacobs C, et al. Deep sequencing of the small RNA transcriptome of normal and malignant human B cells identifies hundreds of novel microRNAs. Blood. 2010;116:e118–e127.
- Hie M, Tsukamoto I. Increased expression of the receptor for activation of NF-κB and decreased runt-related transcription factor 2 expression in bone of rats with streptozotocin-induced diabetes. Int. J. Mol. Med. 2010;26:611–618.
- Hu R, Liu W, Li H, et al. A Runx2/miR-3960/miR-2861 regulatory feedback loop during mouse osteoblast differentiation. J. Biol. Chem. 2011;286:12328–12339.
- Murata K, Yoshitomi H, Furu M, et al. MicroRNA-451 down-regulates neutrophil chemotaxis via p38 MAPK. Arthritis Rheumatol. 2014;66:549–559.
- Lim A, Nikolic-Paterson D, Ma F, et al. Role of MKK3–p38 MAPK signalling in the development of type 2 diabetes and renal injury in obese db/db mice. Diabetologia. 2009;52:347–358.
- Zhang H, Li W, Nan F, et al. MicroRNA expression profile of colon cancer stem-like cells in HT29 adenocarcinoma cell line. Biochem. Biol. Res. Co. 2011;404:273–278.
- Cummins JM, He Y, Leary RJ, et al. The colorectal microRNAome. PNAS. 2006;103:3687–3692.