Abstract
Structural and physicochemical properties of oligomeric flavan-3-ols (proanthocyanidins) in aqueous solution were investigated by spectrometric and reversed-phase (RP) HPLC analyses. Circular dichroism and fluorescence spectra of (–)-epicatechin (EC) oligomers linked through C-4 to C-8 interflavan bonds showed that EC oligomers larger than dimers formed a stable secondary structure in water. These EC oligomers are water-soluble hydrophilic compounds, whereas the oligomers were strongly retained by a C8-alkyl stationary phase under conventional RP-HPLC conditions. In a further C8-HPLC study, the hydrophobic interaction between EC oligomers and 1-octanesulfonic acid sodium salt (OSA Na) added to the mobile phase was quantitatively evaluated based on the relationship between the logarithm of the retention factor of the solute and the OSA Na concentration in the mobile phase. The strength values of the hydrophobic interaction of EC oligomers larger than dimers were the highest of 22 tested polyphenolic standards.
Polyphenolic catechins are naturally occurring flavan-3-ols that are present in a wide variety of dietary plants. The greater part of the catechins, containing their isomers and gallates, is constituted by monomer units with the proanthocyanidin (PA) structure. Plant PAs are well known as natural antioxidants and important functional food factors in the prevention of human cancers and other diseases.Citation1–3) Plant PAs are generally a mixture of oligomers consisting chains of flavan-3-ol units linked most commonly through C4–C8 or C6 interflavan bonds (type-B PAs). The condensation of monomeric flavan-3-ol units leads to the formation of a compact geometric PA structure that is helical in aqueous solution.Citation4–6) Based on secondary structure, the hydrophobicity of PA significantly decreases with an increase in its degree of polymerization (DP), and its water solubility significantly increases.Citation7,8) These properties reveal that the molecular surface of PA is covered by a large number of phenolic hydroxyls and that these hydroxyls are hydrated in aqueous solution. On the other hand, in a chromatographic column with organic solvents, plant PAs are strongly retained to polar column packings such as bare silica,Citation9–12) diol-silica,Citation13) amide-silica,Citation8) Sephadex LH-20,Citation14,15) and Toyopearl HW-40Citation16–18) by hydrogen bonding between the phenolic hydroxyls of the solute PAs and proton donors and acceptors on the packing materials. Thus, under most suitable normal-phase HPLC (or hydrophilic interaction chromatography: HILIC) conditions, plant PAs up to decamers have been separated well according to DP.Citation8,10,12,13)
However, this excellent DP-based separation of plant PAs cannot be reproduced by conventional reversed-phase (RP) HPLC using an aliphatic C18 or C8 alkyl column because hydrogen bonding works poorly as a means of retention on alkyl columns with an aqueous mobile phase. Even though RP-HPLC is a useful method for stereoisomeric separation of monomeric flavan-3-ols and small DP oligomers such as dimers and trimers,Citation16,19–22) the elution order of these small compounds is not based on DP. Furthermore, when higher DP oligomers coexist in a sample solution, a broad unresolved hump derived from the higher DP oligomers overlaps the separation profiles of the smaller oligomers; therefore, the separation profile becomes too complex to assign each peak. The main retention mechanisms in common RP-HPLC are as follows: (1) solute partitioning between the alkyl stationary phase and aqueous mobile phase and (2) hydrophobic interaction between the solute in the mobile phase and the alkyl surface of the stationary phase; however, details of retention behavior and the mechanism for the separation of PAs in RP-HPLC remain unknown. In RP-HPLC separation of PAs, the most important observation is that “very hydrophilic PAs having high DP” are strongly retained on aliphatic alkyl stationary phases, as are comparatively hydrophobic monomers (the chromatographic data will be shown in the later Result section of this study). This suggests that the retention mechanism of PAs in RP-HPLC may be different from that of monomeric flavan-3-ols. Furthermore, the unique retention behavior of the hydrophilic PAs in RP-HPLC may be attributed to their unique secondary structure in aqueous solution.
Based on the above speculation, we first examined the secondary structure of (–)-epicatechin (EC) oligomers (up to pentamers) associated with an increase of their DP by circular dichroism and fluorescence spectrometric analyses. Next, the retention behavior of the EC oligomers in C8-HPLC, especially the hydrophobic interaction between the oligomers and a C8-alkyl compound added to aqueous mobile phase, was investigated in detail.
Materials and methods
Reagents
Sodium chloride, potassium nitrate, and 1-octanesulfonic acid sodium salt (OSA·Na) were purchased from Wako Pure Chemical (Osaka, Japan). HPLC-grade acetonitrile and trifluoroacetic acid (TFA) were from Kanto Chemical (Tokyo, Japan). Ultrapure water was produced from distilled water by a Puric-ZII system (Organo; Tokyo, Japan).
Polyphenolic compounds
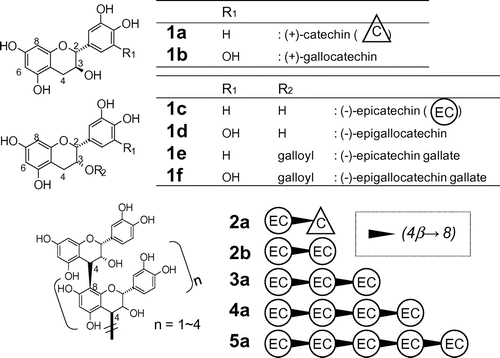
Six types of monomeric flavan-3-ols and five types of oligomeric flavan-3-ols from dimers to pentamers (see Fig. ) were obtained from commercial sources or isolated from a PA-rich fraction obtained from immature apples (apple PA mixture).Citation11,16) Monomeric compounds labeled as 1a ((+)-catechin: C), 1b ((+)-gallocatechin: GC), 1c ((–)-epicatechin: EC), 1d ((–)-epigallocatechin: EGC), 1e ((–)-epicatechin gallate: ECg), and 1f ((–)-epigallocatechin gallate: EGCg) were purchased from Wako. Dimeric compounds labeled as 2a (procyanidin B1: EC-(4β→8)-C) and 2b (procyanidin B2: EC-(4β→8)-EC) were purchased from Funakoshi (Tokyo, Japan). Oligomeric compounds labeled as 3a (procyanidin C1: EC-(4β→8)-EC-(4β→8)-EC), 4a (EC-(4β→8)-EC-(4β→8)-EC-(4β→8)-EC), and 5a (EC-(4β→8)-EC-(4β→8)-EC-(4β→8)-EC-(4β→8)-EC) were isolated from a methyl acetate extract of the apple PA mixture by preparative silica HPLC and preparative ODS HPLC.Citation11,23) Other polyphenolic compounds were purchased from the following manufacturers or distributors: Caffeic acid, chlorogenic acid, and naringenin were from Sigma-Aldrich Japan (Tokyo, Japan); gallic acid, p-hydroxybenzoic acid, and 3,4-dihydroxybenzoic acid were from Tokyo Kasei (Tokyo, Japan); quercetin, hyperoside (quercetin-3-galactoside), and myricetin were from Funakoshi; and rutin (quercetin-3-glucopyranosyl-rhamnoside) and p-coumaric acid were from Wako.
Spectrometric analyses
A J-720 circular dichroism (CD) spectrophotometer (Jasco; Tokyo, Japan) and an RF-5300PC spectrofluorometer (Shimadzu; Kyoto, Japan) were used for analysis of EC (1c) and EC oligomers (2b, 3a, 4a, and 5a). Each test compound was dissolved in water (1 mg/mL), and each solution was diluted to 50 μg/mL for CD analysis or 1 μg/mL for fluorescence (FL) analysis. A CD spectrum of each sample (50 μg/mL) was obtained under the following conditions: optical path length: 2 mm; temperature: 25 °C; scan range: 200–300 nm; sensitivity: 200 mdeg, response: 8 s; band width: 2 nm; resolution: 0.1 nm/data point, scan speed: 20 nm/min; accumulation: 1. The intensity of the CD signal at a certain wavelength was expressed as molar ellipticity ([Ɵ] × 10−3 deg cm2 dmol−1) based on the molar concentration in terms of the EC monomer unit (MW: 291) in each sample containing EC or EC oligomers with different DP. A FL emission spectrum of each sample (1 μg/mL) was obtained under the following measurement conditions: optical path length: 10 mm; excitation wavelength: 281 nm; scan range: 281–400 nm; sensitivity: high; resolution: 1 nm/data point; scan speed: medium.
RP-HPLC
Monomeric and oligomeric flavan-3-ols were separated by RP-HPLC on a Hitachi (Tokyo, Japan) HPLC system, which consisted of an L-2130 pump, a Reodyne 7725i injector, and an L-2400 UV detector. Chromatograms were recorded on a personal computer using D-2000 Elite chromatography data station software (Hitachi). A Chromolith Performance RP-8e analytical column (4.6 mm i.d. × 100 mm; Merck; Darmstadt, Germany) was used for the separation. The retention behavior of the monomeric and oligomeric flavan-3-ols was examined in isocratic elution mode using the following two mobile phase solvents: (A) acetonitrile–water (10:90, v/v) containing 0.1% TFA and (B) solvent A containing OSA·Na (1, 2, 5, 10, or 20 mM). The OSA·Na was added to the mobile phase as hydrophobic interaction competitor (with the C8-alkyl stationary phase), not as ion-pair reagent. The RP-8e (C8-alkyl) column was initially conditioned with the mobile phase solvent at a flow rate of 2.0 mL/min. A 10-μL aliquot of sample (50 μg/mL in mobile phase) was injected and eluted at a flow rate of 2.0 mL/min. The absorbance of the eluate was monitored at 280 nm. The retention factor (k) of each solute was calculated using the equation
where tR is the retention time (in min) of the solute and t0 is the elution time of an unretained compound (potassium nitrate).
Results and discussion
Spectral and structural characteristics of EC oligomers associated with an increase in DP
First, we investigated structural characteristics of PA oligomers by simple spectrometric analyses. Fig. shows the UV and CD spectra in water of monomeric EC (1c) and its oligomers (2b, 3a, 4a, 5a). Their UV spectra (200–300 nm) did not differ much, whereas the CD spectra of oligomers larger than dimers (3a, 4a, 5a) differed significantly in shape from the monomer (1c) and dimer (2b). The oligomers (3a, 4a, 5a) gave strong positive CD signals in the wavelength range from 210 to 250 nm due to asymmetric C-4 carbons on interflavan bonds of their oligomeric structures; the signal shapes and intensities of the tetramer (4a) and the pentamer (5a) appeared similar. In the case of CD of polypeptide and protein, the far-UV CD is generally reflective of the backbone conformation, and different secondary structures in polypeptides and proteins give characteristic far-UV CD spectra.Citation24) Then, the observed strong positive CD signals of EC oligomers (3a, 4a, 5a) would be reflective of their secondary structures.
Fig. 2. CD, UV, and FL (emission spectra excited at 281 nm) spectra of EC (1c) and EC oligomers (2b, 3a, 4a, 5a) in water.
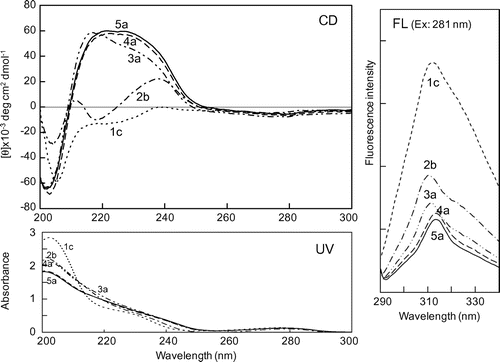
Fig. also shows their FL emission spectra on excitation at their λmax wavelength (281 nm), resulting from aromatic rings in their structures. Monomeric EC (1c) gave a strong FL spectrum (maximum emission wavelength: 310 nm), whereas the FL spectra of oligomers (2b, 3a, 4a, 5a) were quenched, and the quenched FL intensities of the tetramer (4a) and the pentamer (5a) were nearly the same. The observed FL quenching of the oligomers is assumed to be due to a conformational change from the planar configuration of monomeric EC to non-planar one of its oligomeric structure.
On the whole, both the CD and FL spectra in Fig. strongly suggest that EC oligomers larger than dimers (3a, 4a, 5a) form a stable secondary structure in water.
Elution behavior and retention mechanism of monomeric and oligomeric flavan-3-ols in C8-HPLC
We next investigated the elution behavior of several monomeric and oligomeric flavan-3-ols in RP-HPLC. To compare the values of the retention factor k and Po/w (Po/w: partition coefficient in 1-octanol/water two-phase system) for the solutes, the length of the alkyl chain of the separation column was chosen as C8 to match the length of 1-octanol. A typical separation profile of eleven of monomeric (1a–f) and oligomeric (2a, 2b, 3a, 4a, 5a) flavan-3-ols is shown in Fig. . Under isocratic elution in acetonitrile–water (10:90, v/v) containing 0.1% TFA, almost all solutes except 1d and 2a were resolved within 10 min. However, the elution order of the solutes was not based on their DP. Furthermore, the elution order and the retention times of the solutes in Fig. (under the TFA acidic pH condition) were no different from those under the neutral pH 7 condition without TFA (data not shown), because the solute flavan-3-ols are all neutral phenolic compounds in which pKa and Po/w values are no different between the TFA acidic and neutral pH 7 conditions. After the calculation of k for each peak in the chromatogram of Fig. , we examined the relationship between log k and log Po/w of the eleven flavan-3-ols (log Po/w values at pH 7.4 were obtained from our previous data.)Citation8) A scatter plot between log k and log Po/w values (Fig. ) shows the following two relationships: (A) one among six hydrophobic monomers (1a–1f) and (B) another among five EC species (1c, 2b, 3a, 4a, 5a) from monomer to pentamer. The relationship among the six monomers can be expressed as the linear regression formula y = 1.1356 x − 0.1569 (r2 = 0.8457), with log k (= x) values positively correlated with log Po/w (= y) values. The relationship reveals that separation of the monomeric flavan-3-ols was based on a simple RP partitioning mechanism between the C8-alkyl stationary phase and aqueous mobile phase. On the other hand, the relationship among EC and its oligomers can be expressed as y = −3.3331x + 0.793 (r2 = 0.7293), with log k values negatively correlated with log Po/w values. The relationship reveals that the hydrophilic EC oligomers (with negative values for log Po/w) are more strongly retained by the C8-alkyl column according to their DP. It is unusual for such hydrophilic compounds to partition to a C8-alkyl stationary phase from an aqueous mobile phase. We therefore predicted that the oligomers were retained by adsorption based on a hydrophobic interaction between the C8-alkyl surface of the column and hydrophobic regions in the secondary structure of the solute oligomer when in an aqueous mobile phase. To test the prediction, we modified the mobile phase composition of the C8-HPLC and investigated the elution behavior of the flavan-3-ols under the modified conditions.
Fig. 3. Merged chromatogram of eleven flavan-3-ol standards separated by RP C8-HPLC in isocratic elution mode.
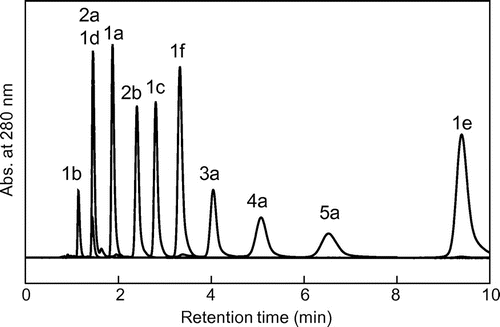
Fig. 4. Relationship between log k values of eleven standards in C8-HPLC and their log Po/w values.
Quantitative evaluation of hydrophobic interaction between flavan-3-ols and C8-alkyl moiety of OSA·Na added to aqueous mobile phase
It is difficult to quantify the strength of hydrophobic interaction between a solute and an alkyl stationary phase of a column in RP-HPLC, because it is not easy to change and estimate the amount of alkyl groups on a silica surface in an LC column. Instead of attempting direct quantification, we dissolved small amounts of 1-octanesulfonic acid sodium salt (OSA·Na) in the aqueous mobile phase used in C8-HPLC, and indirectly evaluated the hydrophobic interaction between the solute flavan-3-ols and the C8-alkyl moiety of the OSA·Na in the mobile phase. OSA·Na has been used as a water-soluble ion-pair reagent for polar cationic solutes in conventional RP-HPLC. In this study, it was used as a hydrophobic interaction competitor with the C8-alkyl stationary phase for nonionic flavan-3-ols in a mobile aqueous phase containing TFA.
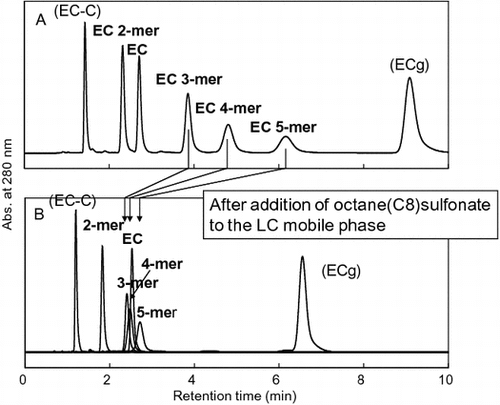
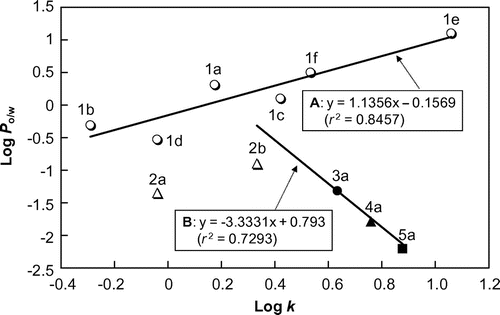
Fig. compares the C8-HPLC profiles of EC oligomers and related flavan-3-ols under the two conditions, with or without 10 mM OSA·Na in the mobile phase. When OSA·Na was added (Fig. (B)), the retention time of monomeric EC (1c) scarcely changed while those of EC oligomers, in particular 3a, 4a, and 5a, were significantly decreased. The decrease in the retention times of the EC oligomers means that the OSA·Na added in the mobile phase formed a complex with the oligomers by hydrophobic interaction and interfered with the retention of the oligomers by the C8-alkyl stationary phase. We expected that any decrease in the retention time of a solute would depend on the OSA·Na concentration. Therefore, further C8-HPLC experiments were carried out under various OSA·Na concentrations (0–20 mM), and the k values of the same flavan-3-ols were calculated from the resulting chromatograms.
Fig. 5. Comparison of C8-HPLC profiles of EC oligomers and related flavan-3-ol standards in different mobile phases.
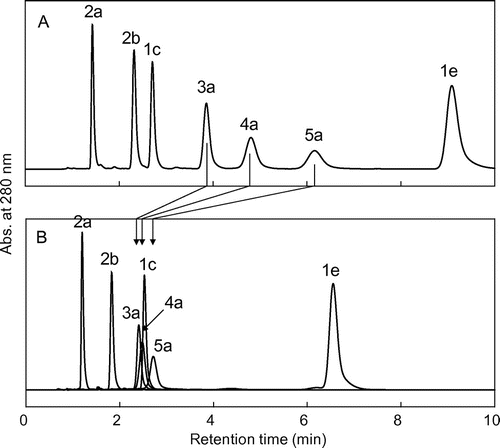
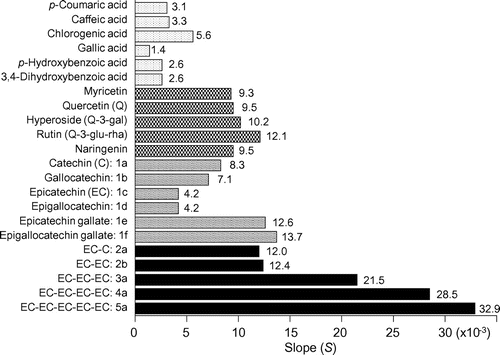
Fig. shows scatter plots for the concentration of OSA·Na added to the mobile phase vs. the log k value of the flavan-3-ols in C8-HPLC for each mobile phase. The log k value of all solutes tended to decrease with increasing OSA·Na concentration in the mobile phase. The OSA·Na concentrations (=x) were negatively correlated with the log k (=y) values of each solute, and all relationships could be expressed as a simple linear regression. For compound 1c: y = −0.0042x + 0.4439 (r2 = 0.9154), 1e: y = −0.0126x + 1.0524 (r2 = 0.9944), 2a: y = −0.012x + 0.0192 (r2 = 0.9796), 2b: y = −0.0124x + 0.3261 (r2 = 0.9926), 3a: y = −0.0215x + 0.5999 (r2 = 0.9899), 4a: y = −0.0285x + 0.6944 (r2 = 0.9816), 5a: y = −0.0329x + 0.8059 (r2 = 0.9154). Each linear regression could be expressed as general expression: y = −Sx + a, and each flavan-3-ol had a different value for the slope (S). A compound with a large S value must more strongly interact hydrophobically with the OSA·Na in the mobile phase. Therefore, we concluded that the S value would be an appropriate index value for the strength of hydrophobic interaction between a solute flavan-3-ol and the C8-alkyl moiety of OSA·Na. The S value of EC monomer (1a: 4.2 × 10−3) was smallest, the values of 1e, 2a, and 2b (12.6 × 10−3, 12.0 × 10−3, 12.4 × 10−3, respectively) were almost the same, and the values of EC oligomers increased with increasing DP (3a: 21.5 × 10−3, 4a: 28.5 × 10−3, 5a: 32.9 × 10−3) (Fig. ). Thus, the hydrophobic interaction between OSA·Na and EC pentamer (5a) was the strongest of the seven flavan-3-ols.
Fig. 6. Relationship between the concentration of OSA·Na added to mobile phase (solvent A) and log k values of seven standards in C8-HPLC in each mobile phase.
In addition to these flavan-3-ols, we measured the S values of four monomeric flavan-3-ols, six phenolic acids, and five flavonols. Each S value was calculated from each scatter plot (as shown in Fig. ) for the concentration of OSA·Na added to the mobile phase vs. the log k of each solute in C8-HPLC for each mobile phase. The comparison data of their S values is shown in Fig. . Of all the polyphenolic compounds tested, the compounds most strongly interacting with the C8-alkyl moiety of OSA·Na were oligomeric PAs larger than dimers (i.e. 3a, 4a, and 5a).
Structural and physiological characteristics of PAs
LC is not only a useful tool for separation, but also for interaction analysis, because the retention behavior of a solute is especially sensitive to intermolecular forces acting in the separation column. In our previous HILIC study,Citation8) water-soluble hydrophilic PAs were strongly retained by an amide-silica column in an organic mobile phase due to hydrogen bonding. On the other hand, in the present RP-HPLC study, PAs were also retained by an aliphatic C8-alkyl column in an aqueous mobile phase by hydrophobic interaction. In summary, PA has a dual nature, i.e. hydrophilicity and hydrophobicity. Presumably, the amphiphilic nature of PA is caused by its high-order oligomeric structure linked through C4–C8 interflavan bonds, suggested by the CD and FL data shown in Fig. . We speculate that higher DP PAs have a helical structure comprising a hydrophilic surface covered by a large number of hydroxyl groups and an internal hydrophobic cavity including stacked rings. Based on these structural characteristics, the PA would interact with biogenic substances such as proteins, peptides, carbohydrates, lipids, and oligonucleotides, resulting in positive (or sometime negative) physiological functions. Under aqueous physiological conditions (for example, in the digestive tract, cell fluid, or blood), nonspecific hydrophobic interaction may predominate between PA and biogenic substances, whereas under physiological conditions resembling organic solvents (for example, in lipid bilayer membranes or in large receptors or enzymes), specific hydrogen bonding may predominate. To clarify such interaction mechanisms in vivo, detailed high-order structural analyses of oligomeric PA are needed.
Author contributions
A.Y. and Y.S. designed the research plan, S.T. and A.Y. performed the experiments, S.T. and A.Y. analyzed the data, A.Y. and Y.S. supervised the research, and A.Y. wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes
Abbreviations: PA, proanthocyanidin; DP, degree of polymerization; RP, reversed-phase; HPLC, high-performance liquid chromatography; OSA·Na, 1-octanesulfonic acid sodium salt; EC, (–)-epicatechin; CD, circular dichroism; FL, fluorescence.
References
- Ouédraogo M, Charles C, Ouédraogo M, et al. An overview of cancer chemopreventive potential and safety of proanthocyanidins. Nutr. Cancer. 2011;63:1163–1173.10.1080/01635581.2011.607549
- de la Iglesia R, Milagro FI, Campión J, et al. Healthy properties of proanthocyanidins. BioFactors. 2010;36:159–168.10.1002/biof.v36:3
- Cos P, Bruyne T, Hermans N, et al. Proanthocyanidins in health care: current and new trends. Curr. Med. Chem. 2004;11:1345–1359.10.2174/0929867043365288
- Muranaka A, Yoshida K, Shoji T, et al. Chiral recognition of apple procyanidins by complexation with oxotitanium phthalocyanine. Org. Lett. 2006;8:2447–2450.10.1021/ol060312s
- Tarascou I, Barathieu K, Simon C, et al. A 3D structural and conformational study of procyanidin dimers in water and hydro-alcoholic media as viewed by NMR and molecular modeling. Magn. Reson. Chem. 2006;44:868–880.10.1002/(ISSN)1097-458X
- Tarascou I, Ducasse M-A, Dufourc EJ, et al. Structural and conformational analysis of two native procyanidin trimers. Magn. Reson. Chem. 2007;45:157–166.10.1002/(ISSN)1097-458X
- Shibusawa Y, Shoji A, Yanagida A, et al. Determination of log Po/w for catechins and their isomers, oligomers, and other organic compounds by stationary phase controlled high‐speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2005;28:2819–2837.10.1080/10826070500225317
- Yanagida A, Murao H, Ohnishi-Kameyama M, et al. Retention behavior of oligomeric proanthocyanidins in hydrophilic interaction chromatography. J. Chromatogr. A. 2007;1143:153–161.10.1016/j.chroma.2007.01.004
- Rigaud J, Escribano-Bailon MT, Prieur C, et al. Normal-phase high-performance liquid chromatographic separation of procyanidins from cacao beans and grape seeds. J. Chromatogr. A. 1993;654:255–260.10.1016/0021-9673(93)83368-3
- Hammerstone JF, Lazarus SA, Mitchell AE, et al. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 1999;47:490–496.10.1021/jf980760h
- Yanagida A, Kanda T, Takahashi T, et al. Fractionation of apple procyanidins according to their degree of polymerization by normal-phase high-performance liquid chromatography. J. Chromatogr. A. 2000;890:251–259.10.1016/S0021-9673(00)00614-2
- Shoji T, Masumoto S, Moriichi N, et al. Apple (Malus pumila) procyanidins fractionated according to the degree of polymerization using normal-phase chromatography and characterized by HPLC-ESI/MS and MALDI-TOF/MS. J. Chromatogr. A. 2006;1102:206–213.10.1016/j.chroma.2005.10.065
- Kelm MA, Johnson JC, Robbins RJ, et al. High-performance liquid chromatography separation and purification of cacao (Theobroma cacao L.) procyanidins according to degree of polymerization using a diol stationary phase. J. Agric. Food Chem. 2006;54:1571–1576.10.1021/jf0525941
- Takahata Y, Ohnishi-Kameyama M, Furuta S, et al. Highly polymerized procyanidins in brown soybean seed coat with a high radical-scavenging activity. J. Agric. Food Chem. 2001;49:5843–5847.10.1021/jf010307x
- Taylor AW, Barofsky E, Kennedy JA, et al. Hop (Humulus lupulus L.) proanthocyanidins characterized by mass spectrometry, acid catalysis, and gel permeation chromatography. J. Agric. Food Chem. 2003;51:4101–4110.10.1021/jf0340409
- Yanagida A, Kanda T, Shoji T, et al. Fractionation of apple procyanidins by size-exclusion chromatography. J. Chromatogr. A. 1999;855:181–190.
- Le Bourvellec C, Picot M, Renard CMGC. Size-exclusion chromatography of procyanidins: comparison between apple and grape procyanidins and application to the characterization of fractions of high degrees of polymerization. Anal. Chim. Acta. 2006;563:33–43.10.1016/j.aca.2005.06.040
- He J, Santos-Buelga C, Mateus N, et al. Isolation and quantification of oligomeric pyranoanthocyanin-flavanol pigments from red wines by combination of column chromatographic techniques. J. Chromatogr. A. 2006;1134:215–225.10.1016/j.chroma.2006.09.011
- Lea AGH. Reversed-phase high-performance liquid chromatography of procyanidins and other phenolics in fresh and oxidising apple juieces using a pH shift technique. J. Chromatogr. 1982;238:253–257.10.1016/S0021-9673(00)82735-1
- Delage E, Bohuon G, Baron A, et al. High-performance liquid chromatography of the phenolic compounds in the juice of some French cider apple varieties. J. Chromatogr. 1991;555:125–136.
- Rohr GE, Meier B, Sticher O. Quantitative reversed-phase high-performance liquid chromatography of procyanidins in Crataegus leaves and flowers. J. Chromatogr. A. 1999;835:59–65.10.1016/S0021-9673(99)00043-6
- Svedstrom U, Vuorela H, Kostiainen R, et al. High-performance liquid chromatographic determination of oligomeric procyanidins from dimers up to the hexamer in hawthorn. J. Chromatogr. A. 2002;968:53–60
- Shoji T, Mutsuga M, Nakamura T, et al. Isolation and structural elucidation of some procyanidins from apple by low-temperature nuclear magnetic resonance. J. Agric. Food Chem. 2003;51:3806–3813.10.1021/jf0300184
- Sreerama N, Woody RW. Circular dichroism of peptides and proteins. In: Berova N, Nakanishi K, Woody RW, editors. Circular dichroism: principles and applications. New York (NY): Wiley-VCH; 2000. p. 601–620.