Abstract
Lactococcus lactis subsp. lactis JCM 5805 (JCM5805) has been shown to stimulate plasmacytoid dendritic cells (pDC). Here, we investigated the effect of JCM5805 on NK cells. In vitro studies suggested that JCM5805 activated natural killer (NK) cells via dendritic cells including pDC. Furthermore, the oral administration of JCM5805 enhanced the cytotoxic activity of NK cells
Plasmacytoid dendritic cells (pDC) are an immune cell subset characterized by the production of large amounts of type I interferons (I-IFN).Citation1,2) pDC expresses Toll-like receptor 9 (TLR9) within its endosome, which recognizes non-methylated CpG DNA derived from virus and bacteria.Citation3,4) The recognition of pathogenic DNA by TLR9 activates intracellular signal transduction pathways in pDC to produce large amounts of type I-IFN,Citation5) such as IFN-α which plays a major role in antiviral immunityCitation6) by enhancing the cytotoxic activity of natural killer (NK) cells.Citation7) This is important because strong cytotoxic activity of NK cells induced by IFN-α contributes to host defense against viral infection.Citation8) We previously reported that Lactococcus lactis subsp. lactis JCM 5805 (JCM5805) activates DC including pDC.Citation9) It was also revealed that JCM5805 induces IFN-α from pDC via TLR9-dependent signal transduction. Additionally, the oral administration of JCM5805 prevented viral infection in a murine parainfluenza virus-infected mouse model.Citation10) These studies suggested that JCM5805 activates NK cells, to elicit an antiviral state, via pDC. It is reported that limited conventional species of lactic acid bacteria (LAB) activate NK cells via macrophage or myeloid dendritic cells (mDC).Citation11,12) However, few studies have been performed on Lactococcus species about NK cells activation, and it remains unclear that LAB which stimulates pDC is also able to induce activation of NK cells. To verify this hypothesis, we examined the effect of JCM5805 on NK cells.
NK cells prevent viral infections from expanding by killing virus-infected host cells.Citation13) Upon the recognition of virus-infected cells, NK cells secrete IFN-γ and granzyme B, a family of serine proteases.Citation14,15) IFN-γ and granzyme B have been used as activation markers of NK cells; therefore, we investigated whether the stimulation of NK cells in vitro by JCM5805 would induce the production of IFN-γ and granzyme B. A total of 5 × 106 splenocytes/mL were cultured for 18 h with 10 μg/mL heat-killed JCM5805, and then cells were stimulated for 4.5 h with a cell activation mixture (Leukocyte Activation Cocktail, with BD GolgiPlug, BD Biosciences) to enhance the detectability of cytokine-producing cells by flow cytometric analysis. After stimulation, cells were stained with anti-NK1.1-PE-Cy7 antibody (eBioscience) and anti-CD3-APC-Cy7 antibody (eBioscience) to detect NK cells. Fixation/Permeabilization solution (BD Cytofix/Cytoperm solution, BD Biosciences) was added to stained cells that were then incubated at 4 °C for 20 min. After incubation, cells were stained with anti-IFN-γ-APC antibody (eBioscience) and anti-granzyme B-FITC antibody (eBioscience) to detect intracellular IFN-γ and granzyme B. After staining, cells were analyzed by FACS Canto II (BD Biosciences). NK1.1 positive and CD3 negative cells were defined as NK cells, and the rates of IFN-γ and granzyme B-producing NK cells in total NK cells were analyzed. Culturing splenocytes with JCM5805 significantly increased IFN-γ and granzyme B-producing NK cells in total NK cells compared to the control group (IFN-γ, 1.5-fold increase, granzyme B, 1.4-fold increase) (Fig. (A) and B).
Fig. 1. JCM5805 activates and increases the cytotoxic activity of splenic NK cells. Splenocytes were cultured for 18 h with or without 10 μg/mL heat-killed JCM5805. After 18 h, cultured cells were stimulated for 4.5 h with a cell activation mixture, and then (A) the rate of NK cells expressing intracellular IFN-γ in total NK cells; (B) the rate of NK cells expressing intracellular granzyme B in total NK cells was determined. (C) NK cells cytotoxicity was determined after 18 h culture with or without 10 μg/mL heat-killed JCM5805. Statistical comparisons were performed using the Student’s t-test. Significant differences were compared to the control group, *p < 0.05.
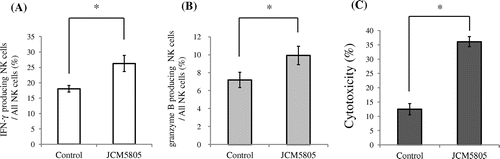
Then, we assessed the cytotoxic activity of NK cells activated by JCM5805. YAC-1 cells, which are targets of NK cells, were stained with 3,3′-Dioctadecyloxacarbocyanine perchlorate (DiO; Sigma). Stained 5 × 104 YAC-1 cells were cultured for 4 h with 5 × 106 splenocytes in a 96-well round-bottomed plate that were previously cultured for 18 h with 10 μg/mL JCM5805. After culture, cells were incubated for 15 min with 20 μL of propidium iodide (PI; 0.5 mg/mL in PBS, Wako) to stain dead cells. Cells were harvested from each well and the rate of dead YAC-1 cells was analyzed by FACS. DiO-stained YAC-1 cells were identified by green fluorescence and PI positive dead cells were identified by red fluorescence. The percentage of effector cell-specific lysis of YAC-1 cells represented the cytotoxic activity of NK cells. When splenocytes were cultured with JCM5805 in advance, the cytotoxic activity of NK cells was significantly enhanced threefold compared to the control group (Fig. (C)).
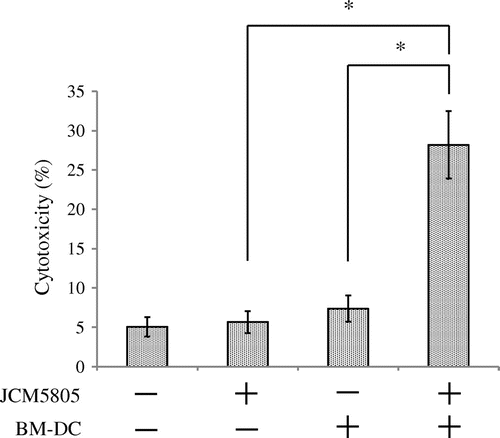
To reveal the mechanism of NK cells activation by JCM5805, we performed a co-culture experiment using NK cells with Flt3L-induced bone marrow-derived DC (BM-DC). BM-DC (mixture of pDC and mDC) were induced by culturing BM isolated from C57BL/6 J mice for 7 days with 100 ng/mL Flt3-L (R&D Systems)Citation16) in RPMI1640 (Sigma) supplemented with 1 mM sodium pyruvate (Life Technologies), 2.5 mM HEPES (Life Technologies), 100 U/mL Penicillin–Streptomycin (Life Technologies), 50 μM 2-Mercaptoethanol (Life Technologies) and 10% fetal calf serum. After 7 days culture, BM-DC were washed three times with RPMI1640 and then incubated with 10 μg/mL JCM5805 for 8 h. Then, 5 × 104 NK cells isolated from splenocytes using NK cell isolation kit II (Miltenyi Biotec), which isolates NK cells by depletion of magnetic labeled non-NK cells, were added to 2.5 × 104 BM-DC cells in a 96-well round-bottomed plate and then incubated for 12 h. Next, 5 × 104 YAC-1 cells were added to each well and incubated for 4 h, and the cytotoxic activity of NK cells was determined. As shown in Fig. , pretreatment of BM-DC with JCM5805 induced a marked increase of NK cell cytotoxic activity. However, the elevation of cytotoxic activity was not observed when NK cells were cultured with JCM5805 only or BM-DC not pretreated with JCM5805.
Fig. 2. NK cells cytotoxicity induced by co-culture with BM-DC stimulated by JCM5805. BM-DC were cultured for 8 h with or without 10 μg/mL heat-killed JCM5805 and then co-cultured for 12 h with NK cells isolated from C57BL/6 J spleens using negative selection by magnetic cell separation. After 12 h co-culturing, YAC-1 cells, a target of NK cells, were added and the rate of dead YAC-1 cells was analyzed by flow cytometry. Statistical comparisons were performed using the Student’s t-test. Significant differences were compared to the control group, *p < 0.05.
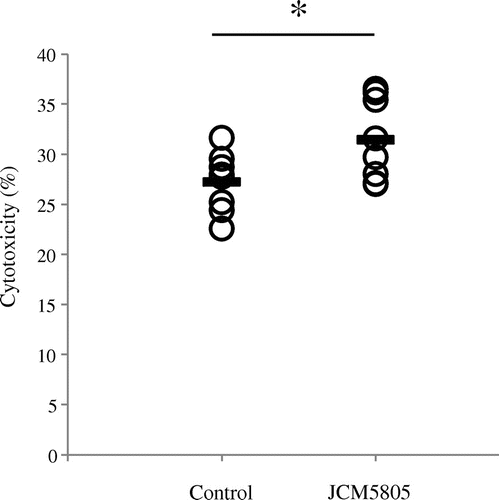
Finally, we evaluated the effect of the oral administration of JCM5805 on NK cells in vivo. Five-week-old female C57BL/6 J mice were obtained from Charles River Japan (Kanagawa, Japan) and acclimatized for 1 week with free access to water and commercial diet, AIN-93G (Oriental Yeast, Tokyo, Japan). Then, mice were divided into two groups of eight based on equal average weight. Control groups were fed with 4 g/day of AIN-93G pellet and test groups were fed with 4 g/day of AIN-93G pellet containing of 1 mg heat-killed JCM5805. Two weeks later, mice were sacrificed, and spleens were collected. This experiment was performed in accordance with the guidelines for the care and use of laboratory animals (Kirin Co, Ltd., Tokyo). Splenocytes were prepared from the collected spleens, and the cytotoxic activity of NK cells was determined using prepared splenocytes. As shown in Fig. , NK cells cytotoxicity in the groups treated with JCM5805 was significantly increased compared to the control groups. We previously reported that orally administered JCM5805 is incorporated in Peyer’s patches (PP) and IFN-α produced from activated pDC in PP might be dispersed and induce expression of IFN-related genes in peripheral tissues, such as lung.Citation10) Therefore, the increase in the cytotoxicity of splenic NK cells in JCM5805 group might be induced by IFN-α produced from pDC in gut-associated lymphoid tissue activated by oral administration of JCM5805.
Fig. 3. Ex vivo effect of the oral administration of JCM5805 on splenic NK cells. Mice (n = 8) were fed with or without heat-killed JCM5805 for 2 weeks. Then, mice were sacrificed and splenocytes were prepared from the collected spleens. Then the cytotoxicity of splenic NK cells was analyzed. Statistical comparisons were performed using the Student’s t-test. Significant differences were compared to the control group, *p < 0.05. Line indicates the mean cytotoxicity %.
The present study investigated the effect of JCM5805 on NK cells. JCM5805 activated NK cells both in vitro and in vivo. Furthermore, the in vitro co-culture study indicated that the effect of JCM5805 on NK cells was dependent upon dendritic cells including pDC. Although we reported that JCM5805 stimulates pDC in mice and humans,Citation9,17) it has not been determined whether JCM5805 affects immune cells, other than pDC. Therefore, this report is the first time to reveal that JCM5805 has also the ability to enhance the activity of NK cells. A number of NK cells activation factors including interleukin (IL)-2, IL-15, and IL-18 have been reported.Citation18–20) Among them, IFN-α is regarded as one of the strongest NK cells activation factors.Citation21) Gerosa et al. reported that IFN-α produced from virus-stimulated pDC markedly increased the cytotoxic activity of NK cells.Citation22) Based on our previous study,Citation9,10) IFN-α produced from pDC stimulated by JCM5805 might contribute largely to the activation of NK cells.
Author contribution
Hiroaki Suzuki designed the experiments; Hiroaki Suzuki and Konomi Ohshio performed the experiments; Hiroaki Suzuki and Daisuke Fujiwara wrote the manuscript.
Notes
Abbreviations: JCM5805, Lactococcus lactis subsp. lactis JCM 5805; pDC, plasmacytoid dendritic cells; I-IFN, Type I Interferones; TLR9, Toll-like Receptor 9; LAB, lactic acid bacteria; mDC, myeloid dendritic cells; BM-DC, bone-marrow derived DC; PP, Peyer’s patches.
References
- Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837.10.1126/science.284.5421.1835
- Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923.
- Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745.
- Bauer S, Kirschning CJ, Hacker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Nat. Acad. Sci. USA. 2001;98:9237–9242.10.1073/pnas.161293498
- Kawai T, Sato S, Ishii kJ, et al. Interferon-α induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 2004;5:1061–1068.10.1038/ni1118
- Gibbert K, Schlaak JF, Yang D, et al. IFN-α subtypes: distinct biological activities in anti-viral therapy. Br. J. Pharmacol. 2013;168:1048–1058.10.1111/bph.12010
- Trinchieri G, Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J. Exp. Med. 1978;147:1314–1333.10.1084/jem.147.5.1314
- Sato K, Hida S, Takayanagi H, et al. Antiviral response by natural killer cells through TRAIL gene induction by IFN-α/β. Eur. J. Immunol. 2001;31:3138–3146.10.1002/(ISSN)1521-4141
- Jounai K, Ikado K, Sugimura T, et al. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9- dependent crosstalk with myeloid dendritic cells. PLoS One. 2012;7:e32588.10.1371/journal.pone.0032588
- Jounai K, Sugimura T, Ohshio K, et al. Oral Administration of Lactococcus lactis Subsp. lactis JCM5805 enhances lung immune response resulting in protection from murine parainfluenza virus infection. PLOS ONE. 2015;10:e0119055.10.1371/journal.pone.0119055
- Iwabuchi N, Yonezawa S, Odamaki T, et al. Immunomodulating and anti-infective effects of a novel strain of Lactobacillus paracasei that strongly induces interleukin-12. FEMS Immunol. Med. Microbiol. 2012;66:230–239.10.1111/j.1574-695X.2012.01003.x
- Kosaka A, Yan H, Ohashi S, et al. Lactococcus lactis subsp. cremoris FC triggers IFN-γ production from NK and T cells via IL-12 and IL-18. Int. J. Immunopharmacol. 2012;14:729–733.10.1016/j.intimp.2012.10.007
- Brandstadter JD, Yang Y. Natural killer cell responses to viral infection. J. Innate. Immun. 2011;3:274–279.10.1159/000324176
- Orange JS, Wang B, Terhorst C, et al. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 1995;182:1045–1056.10.1084/jem.182.4.1045
- Fehniger TA, Cai SF, Cao X, et al. Acquisition of Murine NK Cell Cytotoxicity Requires the Translation of a Pre-existing Pool of Granzyme B and Perforin mRNAs. Immunity. 2007;26:798–811.10.1016/j.immuni.2007.04.010
- Brawand P, Fitzpatrick DR, Greenfield BW, et al. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J. Immunol. 2002;169:6711–6719.10.4049/jimmunol.169.12.6711
- Sugimura T, Jounai K, Ohshio K, et al. Immunomodulatory effect of Lactococcus lactis JCM5805 on human plasmacytoid dendritic cells. Clin. Immunol. 2013;149:509–518.10.1016/j.clim.2013.10.007
- Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-γ production and antiviral defense. J. Immunol. 1996;156:1138–1142.
- Lucas M, Schachterle W, Oberle K, et al. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517.10.1016/j.immuni.2007.03.006
- Gherardi MM, Ramírez JC, Esteban M. IL-12 and IL-18 act in synergy to clear vaccinia virus infection: involvement of innate and adaptive components of the immune system. J. Gen. Virol. 2003;84:1961–1972.10.1099/vir.0.19120-0
- Lee CK, Rao DT, Gertner R, et al. Distinct requirements for IFNs and STAT1 in NK cell function. J. Immunol. 2000;165:3571–3577.10.4049/jimmunol.165.7.3571
- Gerosa F, Gobbi A, Zorzi P, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J. Immunol. 2005;174:727–734.10.4049/jimmunol.174.2.727
