Abstract
This study focus on the main factors that affect the antimicrobial capacity of hydroxytyrosol, including the concentration (200, 400, and 1000 μg/mL), target strains, and the culture media (nutrient-rich and less-rich culture media). The potential HT degradation was also evaluated by HPLC-PAD. Kinetic parameters from growth curves showed that HT concentrations produced a doses-dependent shift when compared to the untreated control. In most of the cases, the highest tested dose (1000 μg/mL) was needed to inhibit growth of the selected strains. However, all the strains were able to grow even at the highest HT dose when cultivated in nutrient-rich culture media. It was observed that HT concentrations were reduced by about 15%, except for Escherichia coli 533 and 679 in Muller Hinton broth, where HT was reduced up to 35%. The results showed a limited antimicrobial activity, contrary to information previously published in some research papers.
Hydroxytyrosol (HT) is a secondary plant metabolite derived from oleuropein by enzymatic hydrolysis, which originates during the maturation of the olives, storage of the oil, and preparation of table olives.Citation1) This compound is present in many olive products including olive mill wastewater, a subproduct very rich in HT, which is generated in large amounts by the olive oil industries.Citation2) In fact, by-products from olive oil production constitute a major source of HT.Citation1) In the last decade, HT has received much attention because it has been made responsible for many benefits associated with the ingestion of virgin olive oil including its antioxidant capacity and properties against cardiovascular diseases.Citation3–5)
More recently, several studies focused on the antimicrobial activity of olive extracts rich in HT.Citation4,6–14) However, it was in 1999 when the first study regarding the antimicrobial activity of HT was published showing that very low concentrations of HT (≤8 μg/mL) were effective to inhibit growth of bacterial reference strains.Citation15) Since then, there has been controversy over attributing antimicrobial activity to this phenolic compound. Many researchers have highlighted HT as one of the main antimicrobial substance in olive productsCitation16–18), while other authors have demonstrated that the most powerful antibacterial substance in olive products are glutaraldehyde-like compounds.Citation4,16) In fact, more and more research studies comparing the antimicrobial activity of olive extracts and individual phenolic compounds of olive extracts indicated that HT did not show significant antimicrobial activity.Citation7,9,12,13,19) Additionally, while olive extracts seem to have higher antibacterial activity on Gram positive than on Gram negative bacteria, no differences have been reported for HT.Citation12,18)
To shed light on the controversy regarding the antimicrobial activity of HT, comparison of previously published data is mandatory. However, there is a lack of a standardized experimental approach and the use of different antimicrobial assays (e.g. MIC, counts of viable cells, disk diffusion test), culture media, inoculum sizes, incubation times, and bacterial strains makes comparison of the obtained data very difficult.Citation9,18–22).Additionally, most of the studies usually assess bacterial growth at one time point after overnight incubation, but evaluation of bacterial growth over time is also important to determine the response of the bacteria to an antimicrobial compound.
In view of the contradictory information about the antibacterial effect of HT, the aim of this study was to determine the effect of different concentrations of HT (200, 400, and 1000 μg/mL) on the growth curve kinetic parameters of 4 different Escherichia coli strains (CECT 516, 533 and 4972, and LFMFP 679) using 6 different culture media. Additionally, the potential degradation and/or transformation of HT by E. coli strains during incubation time, which could also explain the observed inconsistencies between different studies, were evaluated.
Materials and methods
Bacterial cultures
The following bacterial reference strains were used in the preliminary study: Erwinia carotovora CECT 225 (Spanish Type Culture Collection), Klebsiella peneumoniae CECT 143, Pseudomonas aeruginosa CECT 110, Yersinia enterocolitica CECT 4315, Salmonella typhimurium NCTC 1203 (England Type Cultures Culture Collection), Aeromonas hydrophila CECT 389, Shigella sonnei CECT 457, Pediococcus acidilactici CECT 98, Kocuria rhizophila 4070, Listeria monocytogenes CECT 940, Staphylococcus aureus CECT 794, and Escherichia coli CECT 4972. Additionally, E. coli CECT 516, E. coli CECT 533, E. coli CECT 4972, and E. coli O157:H7 LFMFP 679 (Laboratory of Food Microbiology and Food Preservation, Ghent University, Belgium) were used in the main experiments. All bacteria strains were maintained in Tryptic Soy Broth, TSB (Scharlab, Barcelona, Spain) containing 20% (v/v) of glycerol and stored at −20 °C until use.
Monitoring E. coli growth in different culture media
Described E. coli strains kept at –20 °C were grown on TSB agar surface at 37 °C during 24 h to obtain isolated colonies. Plates were maintained at 7 °C. Subcultures were freshly prepared before use by inoculation of a single colony into 5-mL TSB and incubation overnight at 37 °C during 18 h. Later on, 4 1/10 serial dilutions in peptone water (0.1%) were prepared from each culture and inoculated. Diluted cultures of each strain grown overnight were inoculated into 96-well plates containing 6 different culture media including: Iso-sensitest broth (ISO) (Oxoid, Basingstoke, Hampshire, England), Luria Bertani (LB) broth according to Miller (Scharlab), Nutrient broth (NB) (Scharlab), TSB, Brain Heart Infusion (BHI) (Scharlab), Muller Hinton broth (MH) (Scharlau). Growth curves of each strain were hourly monitored after shaking by optical density measurement using a wavelength of 600 nm during incubation at 37 °C for 20 h with a microplate reader (TECAN Infinite 200, Salzburg, Austria).
Minimal inhibitory concentrations of hydroxytirosol
The Minimal Inhibitory Concentrations (MICs) of HT were screened by the microdilution technic against 13 human and foodborne pathogenic bacteria, including Gram-negative and Gram-positive bacteria. All bacterial strains were first grown on ISO broth at 30 °C for 24 h in the case of E. carotovora, A. hydrophila, P. acidilactici, and K. rhizophila or 37 °C for 24 h for K. peneumoniae, P. aeruginosa, Y. enterocolitica, S. typhimurium, S. sonnei, P. acidilactici, K. rhizophila, L. monocytogenes, E. coli CECT 4972, and S. aureus. A hydroxytyrosol 2-(3, 4-dihydroxyphenyl-ethanol) (HT) solution (Seproxbiotech, Madrid, Spain) of 10,000 μg/mL was prepared in sterile distilled water. Negative controls containing ISO broth and bacterial strains without HT were prepared. Tubes were incubated at 30 or 37 °C for 24 h as previously indicated. The MIC was defined as the lowest concentration of the extract need to inhibit microbial growth.
Antimicrobial activity of hydroxytyrosol against E. coli strains
Hydroxytyrosol solutions containing 200, 400, and 1000 μg/mL were prepared using the previously described HT solution stock of 10,000 μg/mL. Ninety-six-well microplates were filled with the selected culture media, HT solutions, and E. coli culture. Initial E. coli population in each well was approximately 102–103 CFU/mL. Eight well replicates for each HT concentration were prepared for each culture media and strain. Appropriate culture control (blank) and culture growth control (inoculated culture media) were run in parallel in the same microplate. All the experiments have been independently repeated at least 4 times in different days. Experimental growth data for each bacterial strain were fitted to the Baranyi functionCitation23) using a complementary tool for Microsoft Excel (D-model, J. Baranyi, Institute of Food Research, Norwich, UK). Kinetic parameters including lag time (λ), maximum specific rate (μmax), and maximum absorbance value (A) for each growth curve were calculated from the generated Baranyi values. The coefficient of determination (R2) indicating the goodness-of-fit was also calculated.
pH measurements
The pH value of each media with and without different HT concentrations was measured using a pH-meter (Crison Basic 20+, Crison Instrument S.A., Alella, Barcelona, Spain).
Hydroxytyrosol stability
Tubes of 9 mL containing different culture media and HT at 1000 μg/mL were prepared. One milliliter of diluted cultures of each strain grown overnight was added to the 9-mL tubes and they were incubated at 37 °C for 20 h. After the incubation time, 1-mL aliquots were taken and filtered using Minisart NY25 0.45 μm syringe filters (Sartourius Stedim). The percentage of decrease in HT was analyzed using a HPLC system (VWR-Hitachi, Barcelona, Spain) equipped with an L-2455 UV–vis detector, an L-2130 pump and an L-2200 auto-sampler. The mobile phases were water with 5% formic acid (solvent A) and methanol (solvent B) at flow rate of 1 mL/min. Elution was performed with a gradient starting with 1% B in A to reach 25% B at 20 min, 55% B at 30 min, and 90% B for 5 min and then returning to the initial conditions for 10 min (1% B in A). UV chromatograms of samples were recorded at 280. HT was identified according to their UV spectra and retention times by chromatographic comparisons with authentic standard.
Statistical analysis
Statistical analysis was done by analysis of variance (ANOVA) followed by Tukey’s multiple range test with a significant level of P ≤ 0.05, using the software IBM SPSS version 19 (Chicago, USA). Least significant differences (LSD), at significant level of p < 0.05, are shown in figures. All experiments were performed 4 times.
Results
Antimicrobial activity of hydroxytyrosol
The MIC values of HT tested against all strains are summarized in Table . In most of the cases, the values of MICs were equal or higher to 1000 μg/mL, except for S. aureus and E. coli CECT 4972 which showed a MIC of 400 μg/mL. Therefore, the obtained results did not show differences between Gram-positive and Gram-negative strains. Taking these results into account, as well as the fact that E. coli is the most frequently microorganisms used in previous studies focused on the antimicrobial activity of HT, the main experiments were carried out in 4 different E. coli strains.
Table 1. Minimal inhibition concentrations (MICs) values of HT against bacteria.
Impact of culture media on the E coli growth
Growth curves of E. coli strains (516, 533, 4972, and 679) were affected by the different culture media (Fig. ). Changes in kinetic parameters such as maximum specific growth rate (μmax), time of the lag phase (λ) and maximum absorbance (A) were observed for all the strains when grown in different culture media; although differences were not always significant (Table ). In most of the cases, growth rates (μmax) of E. coli strains were higher when grown in nutrient-rich media, such as TSB and BHI than in the rest of the culture media. As expected, the lowest growth rates for all the E. coli strains were found when grown in MH and NB broth. The time for microbial responses (λ) was significantly lower in NB than in the rest of the culture media, being the ISO media the one showing the highest λ for most of the E. coli strains. Significant differences among strains were also observed when growing in different culture media. For instance, significant differences in the lag phase were observed between the strains 516 and 533 when grown in LB and MH. Additionally, significant differences in λ were observed between the strains 516 and 679 when grown in MH and NB. As it could be expected, maximum absorbance (A) values attained in the stationary phase by the different E. coli strains were in most of the cases higher in BHI and TSB than in the other culture media (Table ). On the other hand, E. coli 533 grown in BHI and TSB showed the highest A value when compared with the rest of the E. coli strains.
Fig. 1. Growth curve (OD vs. time) of 4 strains of E. coli grown in 6 different media. E. coli CECT 516 (A), E. coli CECT 533 (B), E. coli LMFP 679 (C), E. coli CECT 4972 (D). Values are the mean of 4 replicates ± Standard deviation.
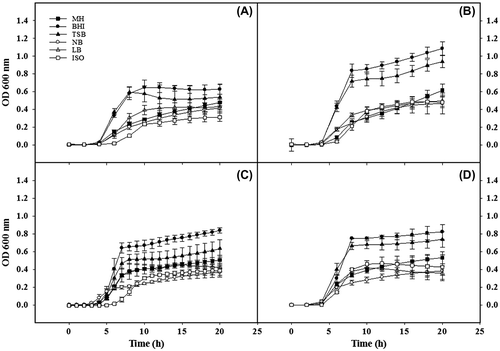
Fig. 2. Growth curve (OD vs. time) of 4 E. coli strains in a nutrient-rich media (BHI) and in basal media (NB) supplemented with different concentrations of HT. E. coli CECT 516 in BHI (A) and in NB (B), E. coli CECT 533 in BHI, (C) and in NB (D), E. coli CECT 679 in BHI (E) and in NB (F), E. coli CECT 4972 in BHI (G) and in NB (H). Values are the mean of 4 replicates ± standard deviation
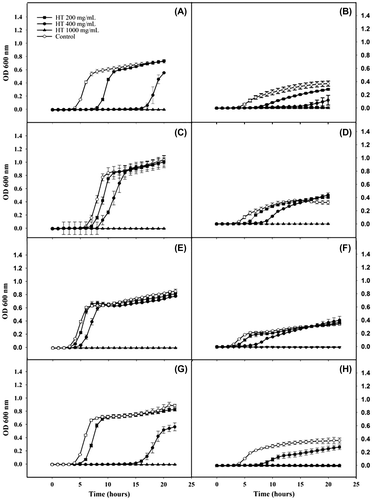
Table 2. Growth kinetic parameters of E. coli CECT 516, 533, 4972, and LMFP 679 grown in 6 different culture media.
Antimicrobial activity of hydroxytyrosol against E coli strains
The potential effect of 3 HT concentrations on the growth curves of 4 E. coli strains was investigated in 6 culture media. The results showed significant changes in the growth kinetic parameters between the 3 HT concentrations. Table represents the differences (∆Y = Y−Y0) in the growth kinetic parameters between the treated samples and the HT control. In most of the cases, a reduction in the growth rate (μmax) and an increment in the lag phase (λ) of manner dose-dependent were observed in all tested media. The highest HT concentration tested (1000 μg/mL) inhibited E. coli growth of all the strains in all culture media except for the LB media in all the E. coli strains and for the Iso-sensitest, in the case of E. coli 4972. However, the addition of a 1000 μg/mL HT concentration to the LB media significantly increased the differences in the lag phase (λ) of all the E. coli strains except for E. coli 533. At HT concentrations of 400 μg/mL, the time for microbial response (λ) of most of the E. coli strains was greatly increased when grown in MH and NB (Table ). It was noticed that E. coli 4972 did not grow in NB medium at HT concentrations ≥400 μg/mL. The obtained results showed that the same strain behaved differently when grown in different culture media supplemented with the same concentrations of HT and conversely, different strains behaved differently to the same concentrations of HT when grown in the same culture media. Therefore, the selection of the bacterial strains and the culture media affected the obtained results regarding the antimicrobial capacity of HT (Table ). For instance, the lag phase (λ) of E. coli 4972 grown in LB supplemented with 1000 μg/mL of HT was able to growth while the growth of the same strain was fully inhibited at 400 and 1000 μg/mL HT concentrations when grown in NB. Additionally, when E. coli 516 was grown without HT in a nutrient-rich media, such as BHI, the value of the lag phase (λ) was not significantly different with the rest of E. coli strains (p < 0.05) (Table ). However, when the same strain was grown in BHI supplemented with a HT concentration of 400 μg/mL of HT, it showed a significantly lower lag phase than the rest of the E. coli strains (Table ).
Table 3. Differences in growth kinetic parameters (∆Y = Y-Y0) of E. coli 516, 533, 679, and 4972 grown in 6 different culture media at different concentrations of hydroxytyrosol.
Hydroxytyrosol stability
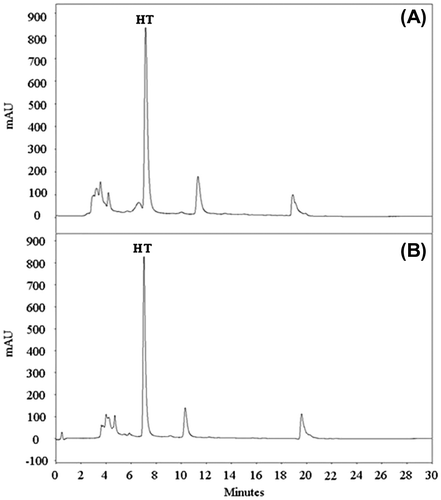
The capacity of E. coli strains to decrease HT concentrations present in the culture media was evaluated. The analysis of HT in the bacterial culture supernatants by HPLC-PAD showed that E. coli strains were able to partly degrade and/or transform HT levels supplemented to the culture media. However, the observed decreases were in most of the cases lower than 15% of the initial HT concentration (Fig. ). The exception was found when E. coli 533 and 679 were grown in MH supplemented with 1000 μg/mL of HT, where HT concentrations were reduced by 35% after 20 h of incubation (Fig. ). However, the HT concentration remaining in the MH broth was enough to inhibit growth of both bacterial strains.
Fig. 3. Percentage of degradation of HT in E. coli strains cultures grown in 6 different media broth supplemented with 1000 μg/mL of HT. E. coli CECT 516 (A), E. coli CECT 533 (B), E. coli LMFP 679 (C), E. coli CECT 4972 (D). Vertical bars represent the standard deviation (n = 3). Bars labeled with different letters indicate significant difference at P < 0.05.
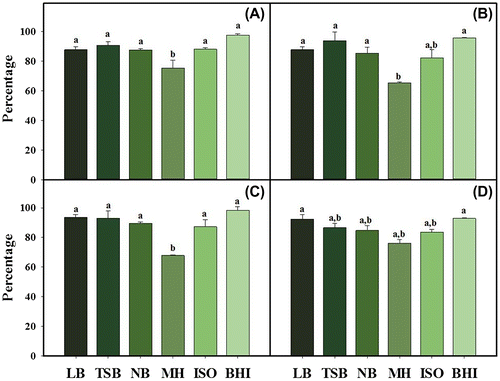
The reductions in HT concentrations observed in the different culture media were only attributed to the bacterial growth because when the different culture media were incubated with different HT concentrations at the same incubation conditions, but without the addition of any bacterial strain, the UV chromatogram revealed that HT was not degraded and/or transformed (Fig. ). HT stability could be also affected by the pH. Our results showed that pH values did not change in any media supplemented with the different HT concentrations. Thus, the pH did not contribute to the HT degradation.
Discussion
The antimicrobial activity of HT and therefore its potential use as a natural preservative have been demonstrated by several researchers; although the MIC described in these studies varies greatly starting as low as 0.24 μg/mL.Citation7,9,15,18,19,22) So far, the antimicrobial capacity of HT has been tested by measuring the inhibition zone diameter, MIC, and bacterial count reduction, where only one strain of each selected bacteria was usually involved and, predominantly, using only one bacterial growth culture media. Table summarizes most of the previously published studies focused on the antimicrobial capacity of HT indicating the selected culture media, microbial strains, HT concentrations, and the tests selected to perform the antimicrobial screening. To our knowledge, studies about the action mode of HT on the growth of bacteria are scarce in general. However, it is important to evaluate the stability of the compound in the different culture media and the potential degradation and/or transformation caused by the microbial growth. In the current study, the main goal was to gain insights into the antimicrobial potential against 4 different E. coli strains when grown in 6 culture media taking into account the stability of HT under the tested conditions.
Table 4. Results obtained by previous studies focused on the antimicrobial activity of olive extracts and hydroxytyrosol.
In general, it was observed that a 400 μg/mL concentration of HT added to the different culture media was able to significantly change the growth curve parameters of the tested E. coli strains respect to the untreated control. Factors such as the bacterial growth culture media and the type of strain affected the bacterial susceptibility to HT. The obtained results show that only the highest HT concentration (1000 μg/mL) was able to inhibit growth of all the bacterial strains under most of the tested conditions. Bacterial survival when 1000 μg/mL of HT were added to the culture media was only observed for specific combinations of bacterial strains and bacterial growth culture media such as E. coli 533, 4972, and 679 grown in LB and E. coli 4972 grown in ISO. Therefore, and based on our results, the antimicrobial capacity of HT can be considered as low.
Previous studies already showed the antimicrobial activity of this compound, although in most of the cases, the reported MIC was much lower than 1000 μg/mL. The first study highlighting the antimicrobial activity of HT was authored by Bisignano et al.Citation15), who reported that HT concentrations as low as 0.24 and 7.85 μg/mL, where effective to inhibit growth of all the tested bacterial reference strains. Friedman et al.Citation22 found an inhibitory effect of HT against E. coli, reporting 0.044% (AB50) as the concentration of the compound able to kill 50% of the initial amount of bacteria under specific test conditions. Tafesh et al.Citation18 reported a bactericidal effect of HT at 400 μg/mL on 4 different bacteria, including E. coli, when grown in TSB. More accordingly to our results, it was reported that HT dissolved in 25% aqueous ethanol at concentrations of 100, 200, and 300 μg/disk did not have inhibitory action against E. coli in MH medium.Citation9) Concentration of 0.5 to 20 mM of HT in contact during 5 min with a bacterial culture was also ineffective against 3 bacterial strains, including E. coli CECT 434, grown in NB.Citation19) Thus, data collected in this study as well as the previously reported studies suggest that the selected culture media and bacterial strains influence the antimicrobial capacity of HT and might contribute to the ongoing controversy.
In general, the type of culture medium used for bacterial growth can determine higher or lower microbial counts.Citation24) Ours results confirm that the growth of E. coli varied depending of the selected broth media. As expected, in nutrient-rich media, such as BHI and TSB, the maximum absorbance and growth rate values were higher than in less rich media such as NB and ISO. These variations in the E. coli growth might be attributed to the different composition of the culture media. Nutrient-rich BHI and TSB contained large quantities of proteins and sugars (for BHI, 460 g of proteins/liter and 2 g of dextrose/liter; for TSB, 23 g of proteins/liter and 2.5 g of dextrose/liter), which might be responsible for an increased bacterial growth rate when compared to nutrient-limiting culture media such as NB and LB. The ISO broth, a culture media designed to minimize the amount of variable nutrients and maximize the defined components, includes iron salts and calcium as antagonists to certain antibiotics, however, this seems to have also a negative impact on the bacterial growth rate.
Differences were not only detected due to the selected culture media. The obtained kinetic parameters varied among the tested E. coli strains when grown in the same culture media. According to this, previous studies have revealed that the antimicrobial activity of a natural product is different between different species and also between strains of the same species.Citation25–27).Bisignano et al.Citation15) studied the effect of HT against several strains per bacteria, such as Haemophilus influenza, Moraxella catarrhalis, Salmonella spp, Vibrio parahaemolyticus, and Staphylococcus aureus, and show that each microbe need a specific range of minimum inhibitory concentration (MIC). The antimicrobial effect of lactoferrin on the growth of E. coli was also strain-dependent and the authors confirm that this specie cannot be seen as one uniform group with regard to the effect of the tested antimicrobial specific compound.Citation28) In agreement with these researches, the antimicrobial activity of HT was also depended on the strain. E. coli 4972 was the only strain inhibited when grown in NB supplemented with 400 μg/mL of HT, while the same strain grown in ISO broth was not inhibited at a HT concentration of 1000 μg/mL. Also growth of E. coli 516, 533, and 4972 was not inhibited when LB was supplemented with 1000 μg/mL of HT. As previously mentioned, beside the culture media and type of strain selected, the physiological state of the Microorganism could be related with the degree of susceptibility of each strain to a natural antimicrobial compound.Citation29) This could be explained because the lag phase is dependent upon the history and physiological state of the bacterial cells.Citation23) In addition, differences among strains or species may be also caused by the ability of some bacteria to transfer plasmids encoding resistance genes to other bacteria.Citation30)
The HT stability during the antimicrobial screening could be also a factor that contributes to the greater or lesser antimicrobial capacity. In the present study, we evaluated the ability of the different bacterial strains to metabolize 2 concentrations of HT when different bacterial growth culture media is used. The obtained results showed that the HT concentration was reduced by 15% in most of the cases, excepting for E. coli 533 and 679 in MH, where HT reductions where above 35% of the initial concentration. A recent study reported the HT instability and potential generation of H2O2 in some cell media due to its rapid oxidation.Citation31) The H2O2 generation must be taken into account when interpreting effects of this phenolic compound on bacteria growth. The stability of HT in aqueous media can be also affected by doses, temperature, and presence of ions. Zafra-Gómez et al.Citation32) reported that HT is more stable in aqueous media at high concentrations as it is rapidly oxidized when present at low concentrations. On the other hand, the presence of Fe could also affect the HT stability in the media Fe(III), due to its chelating properties.Citation33,34) However, only the ISO broth contains Fe in its composition and it did not affect the stability of HT when incubated at 37 °C for 20 h.
In conclusion, the obtained results showed that high and even very high (1000 μg/mL) concentration of HT were needed to inhibit growth of E. coli. Factors such as the bacterial growth culture media and the strain affected the MIC of HT. Therefore, in order to evaluate the antimicrobial capacity of plant extracts or their metabolites factors such as culture media, type of the strains and doses should be considered.
Author contributions
Conceived and designed the experiments: María S. Medina-Martínez, Irene Castro-Ibáñez, Ana Allende. Performed the experiments: María S. Medina-Martínez, Irene Castro-Ibáñez, Pilar Truchado. Statistical analysis, results interpretation, figure and table preparation: Irene Castro-Ibáñez, Pilar Truchado and Ana Allende. Contributed reagents/materials/analysis tools: Ana Allende. Wrote the paper: Pilar Truchado and Ana Allende.
Acknowledgments
This work has been supported by the COST ACTION FA1202 BacFoodNet. P. Truchado holds a post-doctoral fellowship of the Spanish Ministry of Economy and Competitiveness. I. Castro-Ibáñez is the holder of a JAE-PreDoc contract from the CSIC.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Granados-Principal S, Quiles JL, Ramirez-Tortosa CL, et al. Hydroxytyrosol: from laboratory investigations to future clinical trials. Nutr. Rev. 2010;68:191–206.
- Lesage-Meessen L, Navarro D, Maunier S, et al. Simple phenolic content in olive oil residues as a function of extraction systems. Food Chem. 2001;75:501–507.
- Frankel EN. Nutritional and biological properties of extra virgin olive oil. J. Agric. Food Chem. 2011;59:785–792.
- Medina E, de Castro A, Romero C, et al. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: correlation with antimicrobial activity. J. Agric. Food Chem. 2006;54:4954–4961.
- Aldini G, Piccoli A, Beretta G., et al. Antioxidant activity of polyphenols from solid olive residues of c.v. Coratina. Fitoterapia. 2006;77:121–128.
- Medina E, Romero C, Brenes M, et al. Antimicrobial activity of olive oil, vinegar, and various beverages against foodborne pathogens. J. Food Prot. 2007;70:1194–1199.
- Medina E, Romero C, de los Santos B, et al. Antimicrobial activity of olive solutions from stored alpeorujo against plant pathogenic microorganisms. J. Agric. Food Chem. 2011:59;6927–6932.
- Pereira JA, Pereira A, Ferreira I, et al. Table olives from Portugal: phenolic compounds, antioxidant potential, and antimicrobial activity. J. Agric. Food Chem. 2006;54:8425–8431.
- Obied HK, Bedgood DR, Prenzler PD, et al. Bioscreening of Australian olive mill waste extracts: biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem. Toxicol. 2007;45:1238–1248.
- Serra AT, Matias AA, Nunes AVM, et al. In vitro evaluation of olive–and grape–based natural extracts as potential preservatives for food. Innov. Food Sci. Emerg. Technol. 2008;9:311–319.
- Yangui T, Dhouib A, Rhouma A, et al. Potential of hydroxytyrosol-rich composition from olive mill wastewater as a natural disinfectant and its effect on seeds vigour response. Food Chem. 2009;117:1–8.
- Karaosmanoglu H, Soyer F, Ozen F, et al. Antimicrobial and antioxidant activities of Turkish extra virgin olive oils. J. Agric. Food Chem. 2010;58:8232–8245.
- Bubonja-Sonje M, Giacometti J, Abram M. Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chem. 2011;127:1821–1827.
- Brenes M, García A, de los Santos B, et al. Olive glutaraldehyde-like compounds against plant pathogenic bacteria and fungi. Food Chem. 2011;125:1262–1266.
- Bisignano G, Tomaino A, Lo Cascio R, et al. On the in vitro antimicrobial activity of oleuropein of hydroxytyrosol. J. Pharm. Pharmacol. 1999;51:971–974.
- Furneri P, Piperno A, Sajia A, et al. Antimycoplasmal activity of hydroxytyrosol. Antimicrob. Agents Chemother. 2004;48:4892–4894.
- Friedman M, Rasooly R, Do P, et al. The olive compound 4-hydroxytyrosol inactivates staphylococcus aureus bacteria and staphylococcal enterotoxin A (SEA). J. Food Sci. 2011;76:M558–M563.
- Tafesh A, Najami N, Jadoun J, et al. Synergistic antibacterial effects of polyphenolic compounds from olive mill wastewater. Evid. based Complement Altern. Med. 2011;2011:1–9.
- Medina E, Brenes M, García A, et al. Bactericidal activity of glutaraldehyde-like compounds from olive products. J. Food Prot. 2009;72:2611–2614.
- Hadacek F, Greger H. Testing of antifungal natural products: methodologies, comparability of results and assay choice. Phytochem. Anal. 2000;11:137–147.
- Nostro A, Germano MP, D’Angelo V, et al. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett. Appl. Microbiol. 2000;30:379–384.
- Friedman M, Henika P, Levine C. Bactericidal activities of health-promoting, food-derived powders against the foodborne pathogens Escherichia coli, Listeria monocytogenes, Salmonella enterica, and Staphylococcus aureus. J. Food Sci. 2012;78:M270–M275.
- Baranyi J, Robinson T, Kaloti A, et al. Predicting growth of Brochothrix thermosphacta at changing temperature. Int. J. Food Microbiol. 1995;27:61–75.
- Vieira FCS, Nahas C. Comparison of microbial numbers in soils by using various culture media and temperatures. Microb. Res. 2005;160:97–202.
- Hefnawy Y, Moustafa S, Marth F. Sensitivity of Listeria monocytogenes to selected species. J. Food Prot. 1993;56:876–878.
- Delaquis P, Stanich K, Toivonen P. Effect of pH on the inhibition of Listeria spp. by vanillin and vanillic acid. J. Food Prot. 2005;68:1472–1476.
- Senhaji OS, Faid M, Kalalou I. Inactivation of Escherichia coli O157:H7 by essential oil from Cinnamomum zeylanicum. Braz. J. Infect. Dis. 2007;11:234–236.
- Sekse C, Bohlin J, Skjerve E, et al. Growth comparison of several Escherichia coli strains exposed to various concentrations of lactoferrin using linear spline regression. Microb. Inform. Exp. 2012;2:1–12.
- Gill AO, Delaquis P, Russo P, et al. Evaluation of antilisterial action of cilantro oil on vacuum packed ham. Int. J. Food Microbiol. 2002;73:83–92.
- Gómez-López VM. Essential oils for the treatment of fruit and vegetables. In: Barry-Ryan C, Bourke P, editors. Decontamination of fresh and minimally processed produce. Wiley-Blackwell: Oxford; 2012. p. 226–246.10.1002/9781118229187
- Long LH, Hoi A, Halliwell B. Instability of, and generation of hydrogen peroxide by, phenolic compounds in cell culture media. Arch. Biochem. Biophys. 2010;501:162–169.
- Zafra-Gómez A, Luzón-Toro B, Capel-Cuevas S, et al. Stability of hydroxytyrosol in aqueous solutions at different concentration, temperature and with different ionic content: a study using UPLC-MS. Food Nutr. Sci. 2011;02:1114–1120.
- Paiva-Martins F, Gordon M. Effect of pH and ferric ions on the antioxidant activity of olive polyphenols in oil-in-water emulsions. J. AOCS. 2002;79:571–576.
- Paiva-Martins F, Gordon M. Interactions of ferric ions with olive oil phenolic compounds. J. Agric. Food Chem. 2005;53:2704–2709.
- Moore KL, Patel J, Jaroni D, et al. Antimicrobial activity of apple, hibiscus, olive, and hydrogen peroxide formulations against Salmonella enterica on organic leafy greens. J. Food Protec. 2011;74:1676–1683.