Abstract
P[(R)-lactate-co-(R)-3-hydroxybutyrate] [P(LA-co-3HB)] was produced in engineered Escherichia coli using lignocellulose-derived hydrolysates from Miscanthus × giganteus (hybrid Miscanthus) and rice straw. Hybrid Miscanthus-derived hydrolysate exhibited no negative effect on polymer production, LA fraction, and molecular weight of the polymer, whereas rice straw-derived hydrolysate reduced LA fraction. These results revealed that P(LA-co-3HB) was successfully produced from hybrid Miscanthus-derived sugars.
Polyhydroxyalkanoates (PHAs) are a group of polyesters that are naturally synthesized by a large number of bacteria and used as bio-based alternatives to petroleum-derived plastics.Citation1) The most typically occurring PHA, poly(3-hydroxybutyrate) [P(3HB)], has, however, opaque and brittle properties that have limited the practical applications of this polymer. This obstacle was overcome by copolymerization technique of PHAs that reduced the crystallinity of the polymer.Citation2) As a good example, poly(lactate-co-3-hydroxybutyrate) [P(LA-co-3HB)] possessed improved flexibility and transparency compared to P(3HB). This copolymer is synthesized using a lactate-polymerizing enzyme (LPE), which is an evolved PHA synthase with acquired LA-polymerizing activity.Citation2,3) It has been demonstrated that this copolymer can be produced from either glucose or xylose as sole carbon source,Citation4) suggesting that lignocellulosic biomass could be a feedstock for P(LA-co-3HB) production.
Therefore, in this study, we attempted to examine the production of P(LA-co-3HB) from lignocellulose-derived sugars. To date, the production of PHAs from lignocellulosic biomass was investigated mainly using tropical and subtropical plants such as sugarcane bagasse Citation5) and rice straw.Citation6) In this study, we focused on a cold-adapted plant Miscanthus × giganteus (hybrid Miscanthus),Citation7) which is a large perennial grass developed by hybridizing M. sacchariflorus and M. sinensis.Citation8) This plant exhibits high biomass production with low fertilizer and has been used as fuel for thermal power stations in European Union,Citation9) and for the production of biofuels and chemicals.Citation10) The goal of this study was to investigate the efficiency of using hybrid Miscanthus-derived hydrolysate in the production of P(LA-co-3HB), and to discuss its side effects on cell growth, by comparing with rice straw-derived hydrolysate and a mixture of pure sugars.
Hybrid Miscanthus and rice straw harvested in the farm of Hokkaido University (Sapporo, Japan) were air dried and crushed using a waring blender (model MX-1200XTS). For delignification, the ground hybrid Miscanthus and rice straw were passed through a sieve of 100 mesh (<0.15 mm), and treated with acidified sodium chlorite (NaClO2) and sodium hydroxide (NaOH), which were known as efficient delignification reagents.Citation11,12) For the acidified NaClO2 treatment, 60 g ground biomass (based on dry weight), 45 g NaClO2, and 3.6 mL glacial acetic acid were added into 3 L deionized water. The mixture was incubated in a water bath at 75 °C under mild agitation for 1 h. Then, 45 g NaClO2 and 3.6 mL acetic acid were added. This step was repeated 3 times. Insoluble fraction was collected by filtration and washed three times with deionized water and two times with absolute ethanol followed by filtration. For the NaOH treatment, 60 g ground biomass (based on dry weight) was added into 15 volumes (v/w) of 1% (w/v) NaOH solution. The reaction was incubated at room temperature for 24 h without shaking. The mixture was filtrated and washed with deionized water until pH became neutral.
The delignified biomass samples were hydrolyzed using the Cellulase complex NS22086 (Novozymes’ Cellulosic Ethanol Enzyme Kit). The sucrose that presented in the enzyme complex as a stabilizer was removed by a Bio-Gel P-2 (Bio-Rad Laboratories, USA) column equilibrated with deionized water. Protein concentration of the enzyme complex was determined by Bradford method (Bio-Rad Laboratories, USA). BSA was used as a standard protein. The reaction mixture for enzymatic hydrolysis containing 10% delignified biomass (as dry weight) and 1% (w/v) of the enzyme complex was incubated at 45 °C for 84 h. Initial pH was adjusted to 5.5 using HCl. After the reaction, the mixtures were boiled for 10 min to inactivate the enzyme complex and subsequently the insoluble fraction was removed by centrifugation at 3,000 rpm for 10 min.
For polymer production, LB medium containing 2% sugar, which was the hydrolysate, or the mixture of pure sugars (1.6% glucose and 0.4% xylose), 10 mM calcium pantothenate, and 100 μg/L ampicillin was used. P(LA-co-3HB) was produced in Escherichia coli BW25113 harboring pTV118NpctC1Ps(STQK)AB, which bears propionyl-CoA transferase gene from Megasphaera elsdenii, Ser325Thr/Gln481Lys mutated PHA synthase (LPE) gene from Pseudomonas sp. 61-3, and 3-ketoacyl-CoA thiolase (PhaA) and acetoacetyl-CoA reductase (PhaB) genes from Ralstonia eutropha.Citation3) The culture was conducted in 10 mL test tubes containing 1.7 mL medium for 48 h at 30 °C. BW25113 harboring pGEMC1(STQK)AB, which bears LPE and PhaA and PhaB genes, was cultured at the same condition for P(3HB) production.Citation13) The sugar concentrations in the medium and the amount of intracellular polymer were determined using HPLC as reported.Citation4) The molecular weight of polymer was determined using gel permeation chromatography as previously described.Citation14)
The delignified biomass was converted into mainly glucose and xylose with trace amount of arabinose by the enzymatic hydrolysis. The acidified NaClO2 treatment resulted in higher sugar yield than the NaOH treatment. However, the hydrolysate obtained via acidified NaClO2 significantly ceased the growth of E. coli cell (data not shown). This can be possibly attributed to the toxicity of residual NaClO2 against the cells; in contrast, no cell inhibition was observed for the NaOH treatment. Considering the advantages of each treatment, the two-step treatment using acidified NaClO2 followed by NaOH was attempted. As the result, hydrolysate was obtained with the highest total sugar yield (0.54 g/g) from hybrid Miscanthus (Table ) and no cell growth inhibition was observed (data not shown). Therefore, the hydrolysates obtained via two-step treatment were used for polymer production.
Table 1. Sugar yield from hybrid Miscanthus and rice straw biomass treated with different delignification methods combined with enzymatic hydrolysis.
P(LA-co-3HB)-producing recombinant E. coli was grown on the hydrolysates of hybrid Miscanthus and rice straw, as well as a mixture of pure sugars (Fig. ). Glucose was consumed within 24 h, and xylose was consumed in the later stage. This time-lag was due to the catabolite repression of glucose.Citation15) There was no significant difference in the sugar consumption among the cultivation conditions using different carbon sources. The polymer was produced along with the consumption of sugars, indicating that hybrid Miscanthus-derived sugars served as a carbon source for P(LA-co-3HB) production.
Fig. 1. Time course for cell growth, sugar consumption and polymer yield during P(LA-co-3HB) synthesis. (A) hybrid Miscanthus; (B) rice straw; and (C) sugar mixture.
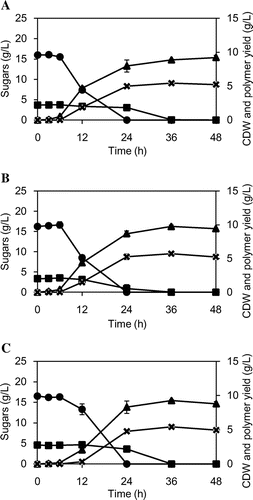
Cell dry weight (CDW) and polymer production (Table ) obtained using lignocellulose hydrolysates were slightly greater than that obtained using mixture of pure sugars. This might be due to the presence of carbon sources in the hydrolysate other than glucose and xylose. The molecular weight of the polymer (Table ) was not significantly altered depending on the carbon source.
Table 2. P(LA-co-3HB) production from hybrid Miscanthus and rice straw hydrolysates.
The LA fraction in the copolymer, however, was affected by the carbon source. The LA fractions of copolymers obtained from the mixture of pure sugars (15.6 mol%) and hybrid Miscanthus hydrolysate (16.9 mol%) were similar, whereas the use of rice straw resulted in a significant reduction of LA fraction in the copolymer (8.7 mol%). To find the reason for this result, P(3HB) was produced using the same carbon sources. As the result, there was no significant difference in the polymer production among the carbon sources (data not shown), indicating that the decrease in LA fraction in the copolymer was probably due to the reduction in lactic acid and/or LA-CoA production rather than the enhancement of 3HB unit production. Currently, the compounds in rice straw hydrolysate contributing to this phenomenon have not yet been identified.
In this study, hybrid Miscanthus-derived hydrolysate was shown to be a good feedstock for the production of P(LA-co-3HB). This is the first report for P(LA-co-3HB) production from lignocellulosic biomass, which also proposes a potent application of hybrid Miscanthus.
Author contribution
Jian Sun, Camila Utsunomia, Ken’ichiro Matsumoto, Toshihiko Ooi, and Seiichi Taguchi conceived and designed the experiments. Jian Sun, Camila Utsunomia, and Shohei Sasaki performed the experiments. Jian Sun, Camila Utsunomia, Ken’ichiro Matsumoto, and Toshihiko Ooi analyzed data. Toshihiko Yamada and Toshihiko Ooi contributed reagents/materials/analysis tools. Jian Sun, Camila Utsunomia, Ken’ichiro Matsumoto, Toshihiko Ooi, and Seiichi Taguchi wrote the paper.
Funding
This study was partially supported by the Japan Society for the Promotion of Science (JSPS) (to J.S.); Research Foundation of Institute for Fermentation, Osaka (IFO) (to S.T.), JST, CREST (to S.T.); JSPS Kakenhi [grant numbers 26660080 to S.T., 26281043 to K.M., and 26420791 to T.O.].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Brigham CJ, Kurosawa K, Rha C, et al. Bacterial carbon storage to value added products. J. Microb. Biochem. Technol. 2011;83:S3–002.
- Yamada M, Matsumoto K, Uramoto S, et al. Lactate fraction dependent mechanical properties of semitransparent poly(lactate-co-3-hydroxybutyrate)s produced by control of lactyl-CoA monomer fluxes in recombinant Escherichia coli. J. Biotechnol. 2011;154:255–260.10.1016/j.jbiotec.2011.05.011
- Taguchi S, Yamada M, Matsumoto K, et al. A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc. Natl. Acad. Sci. USA. 2008;105:17323–17327.10.1073/pnas.0805653105
- Nduko JM, Matsumoto K, Ooi T, et al. Effectiveness of xylose utilization for high yield production of lactate-enriched P(lactate-co-3-hydroxybutyrate) using a lactate-overproducing strain of Escherichia coli and an evolved lactate-polymerizing enzyme. Metab. Eng. 2013;15:159–166.10.1016/j.ymben.2012.11.007
- Moncada J, Matallana LG, Cardona CA. Selection of process pathways for biorefinery design using optimization tools: a colombian case for conversion of sugarcane bagasse to ethanol, poly-3-hydroxybutyrate (PHB), and energy. Ind. Eng. Chem. Res. 2013;52:4132–4145.10.1021/ie3019214
- Sindhu R, Silviya N, Binod P, et al. Pentose-rich hydrolysate from acid pretreated rice straw as a carbon source for the production of poly-3-hydroxybutyrate. Biochem. Eng. J. 2013;78:67–72.10.1016/j.bej.2012.12.015
- Naidu SL, Moose SP, AL-Shoaibi, AK, et al. Cold tolerance of C4 photosynthesis in Miscanthus × giganteus: adaptation in amounts and sequence of C4 photosynthetic enzymes. Plant Physiol. 2003;132:1688–1697.10.1104/pp.103.021790
- Hodkinson TR, Renvoize S. Nomenclature of Miscanthus × giganteus (Poaceae). Kew Bull. 2001;56:759–760.10.2307/4117709
- Christian DG, Riche AB, Yates NE. Growth, yield and mineral content of Miscanthus × giganteus grown as a biofuel for 14 successive harvests. Ind. Crops. Prod. 2008;28:320–327.10.1016/j.indcrop.2008.02.009
- Brosse N, Dufour A, Meng X, et al. Miscanthus: a fast-rowing crop for biofuels and chemicals production. Biofuel, Bioprod. Bior. 2012;6:580–598.10.1002/bbb.1353
- Yoshida M, Liu Y, Uchida S, et al. Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinensis to monosaccharides. Biosci. Biotechnol. Biochem. 2008;72:805–810.10.1271/bbb.70689
- Sun S, Sun S, Cao X, et al. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016;199:49–58. doi:10.1016/j.biortech.2015.08.061.
- Takase K, Taguchi S, Doi Y. Enhanced synthesis of poly(3-hydroxybutyrate) in recombinant Escherichia coli by means of error-prone PCR mutagenesis, saturation mutagenesis, and in vitro recombination of the type II polyhydroxyalkanoate synthase gene. J. Biochem. 2003;133:139–145.10.1093/jb/mvg015
- Kusaka S, Abe H, Lee SY, et al. Molecular mass of poly[(R)-3-hydroxybutyric acid] produced in a recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 1997;47:140–143.10.1007/s002530050902
- Dien BS, Nichols NN, O’Bryan PJ, et al. Development of new ethanologenic Escherichia coli strains for fermentation of lignocellulosic biomass. Appl. Biochem. Biotechnol. 2000;84–86:181–196.10.1385/ABAB:84-86:1-9
