Abstract
MicroRNAs are a class of 18–22 nucleotide non-coding RNAs that modulate gene expression by associating with the 3′ untranslated regions of mRNAs. A large number of microRNAs are involved in the regulation of myoblast differentiation, many of which remain undiscovered. In this study, we found that miR-143-3p was upregulated during C2C12 myoblast differentiation and over-expression of miR-143-3p significantly inhibited the relative expression levels of MyoD, MyoG, myf5, and MyHC genes, especially in the later stages of differentiation. In addition, miR-143-3p inhibited expression of genes involved in the endogenous Wnt signaling pathway during C2C12 myoblast differentiation, including Wnt5a, LRP5, Axin2, and β-catenin. These results indicate that miR-143-3p represents a new myogenic differentiation-associated microRNA that can inhibit C2C12 myoblast differentiation, especially in the later stages of differentiation.
Graphical abstract
MiR-143-3p may inhibit C2C12 myoblast differentiation by regulating marker genes related myoblast differentiation and Wnt signaling pathway.
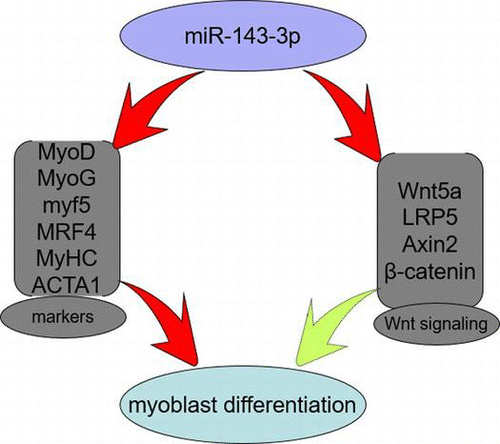
A number of catabolic disease states are characterized by muscle wasting, including sepsis, cancer, acquired immune deficiency syndrome, diabetes mellitus, and heart and renal failure. Skeletal muscle is a major site of metabolic activity and the most abundant tissue in the human body.Citation1,2) Genesis of skeletal muscle is a multistep process that includes the recruitment of myoblasts from myogenic precursors, myoblasts proliferation, cell-cycle arrest, and fusion of myocytes into multinucleated myotubes.Citation3,4) Previous studies have shown that myogenesis is orchestrated by a family of four related, basic helix-loop-helix, myogenic regulatory factors, including Myf5, MyoD, MyoG, and MRF4, and these transcription factors coordinate the expression of genes involved in muscle growth, morphogenesis, differentiation, and contractility.Citation5) As the major muscle structure and function proteins, MyHC and ACTA1 are widely used as marker genes during myoblast differentiation.Citation2,6–8) Additionally, the Wnt signaling pathway plays an important role in the process of muscle development and over-expression of the Wnt antagonist, Sfrp3, blocks myogenic differentiation in mouse somites.Citation9) Wnt signaling regulates myogenic differentiation in the developing avian wing, and canonical Wnt signaling is involved in the switching from cell proliferation to myogenic differentiation of mouse myoblast cells.Citation10,11) A recent study has indicated that MEF2A can modulate Wnt signaling by regulating the Gtl2-Dio3 microRNA mega-cluster during the process of skeletal muscle regeneration.Citation12)
MicroRNAs are a class of approximately 18–22 nucleotide, single-stranded, non-coding small RNA molecules. MicroRNAs normally regulate the expression of protein-coding genes post-transcriptionally by binding to the 3′ untranslated regions of target mRNAs via either promoting degradation or inhibiting translation of the target mRNAs.Citation13–15) MicroRNAs play essential roles in diverse biological processes, including cell proliferation, differentiation, apoptosis, and tumorigenesis.Citation16,17) Recent studies have revealed that miR-143 can not only suppress osteogenic differentiation of smooth muscle cells by targeting osterix, but also regulate their fate and plasticity, as well as modulating their responsiveness to injury.Citation18) Interestingly, miR-143 not only can inhibit Wnt/β-catenin signaling by targeting other positively regulated constituents in the Wnt pathway, but is also able to bind to the target sequences of Frizzled-6 and Frizzled-3 receptor genes in the Wnt/β-catenin signaling pathway.Citation19) However, little is known about whether miR-143-3p plays a role in the process of myogenic differentiation through the Wnt signaling pathway.
In the present study, we showed that miR-143-3p was upregulated during C2C12 myoblast differentiation, and when transfected with miR-143-3p mimics or inhibitors, the differentiation of C2C12 myoblasts was inhibited or enhanced, especially in the later stages of differentiation. Additionally, transfection of miR-143-3p inhibitors promoted the expression of genes involved in the endogenous Wnt signaling pathway. Taken together, miR-143-3p has a negative effect on myoblast differentiation, and may inhibit myoblast differentiation by modulating the Wnt signaling pathway.
Materials and methods
Cell culture
C2C12 myoblasts (Stem Cell Bank, Chinese Academy of Science) were maintained at 37 °C, 5% CO2 in growth medium containing Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum before being induced to differentiate. When cells reached 80% confluence, they were digested with 0.25% trypsin and then seeded in 12-well plates. When cell density reached 70–80%, the medium was switched to differentiation medium containing DMEM and 2% horse serum (Gibco).
Transfection of miR-143-3p mimics, inhibitors, and negative control
When the density of C2C12 myoblasts in 12-well plates reached 70–80%, cells were subjected to serum starvation for 4 h prior to switching the medium to differentiation medium containing DMEM and 2% horse serum. At the same time, miR-143-3p mimics (30 nM; catalog number: miR10000247-1-5), inhibitors (30 nM; catalog number: miR20000247-1-5), or negative control (NC, catalog number: miR04101-1-2) (all purchased from RiboBio, Guangzhou, China) were transfected into the C2C12 myoblasts using Lipofectamine 2000 (Invitrogen). Cell differentiation medium changes and transfections were carried out every 48 h to ensure success. MiR-143-3p mimics sequence: 5′-ugagaugaagcacuguagcuc-3′, 5′-gagcuacagugcuucaucuca-3′; miR-143-3P inhibitor: 5′-gagcuacagugcuucaucuca-3′; negative control: 5′-uuuguacuacacaaaaguacug-3′, 5′- caguacuuuuguguaguacaaa-3′.
RNA isolation, real-time polymerase chain reaction (PCR), and quantitative real-time PCR
Total cellular RNAs (including microRNAs) were extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions and the total RNA quality and concentration were estimated using denatured gel electrophoresis and a spectrophotometer (Thermo, Waltham, MA, USA). Reverse transcription of mRNA and microRNA was performed using a commercial kit (TaKaRa, China), according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) of mRNAs and microRNAs reactions was performed using a SYBR Premix Ex Taq kit (TaKaRa, China) on a Bio-RAD IQ™five system (Bio-Rad, Hercules, CA, USA). For miR-143-3p qRT-PCR, the amplification conditions were: initial denaturation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 40 s, and extension at 72 °C for 30 s. Relative expression levels of mRNAs and microRNAs were calculated using the 2−ΔΔCt method. The primer sequences used for qRT-PCR are listed in Table . Beta-actin was used to normalize the expression levels of individual mRNAs, while microRNA expression was normalized against the expression of U6.
Table 1. The primer sequences used for qRT-PCR.
Immunocytochemical analysis
After transfection and induced myogenic differentiation, C2C12 myoblasts cultured in 12-well plates were washed three times with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde for 15 min. After further PBS washes (and for each step thereafter), cells were then permeabilized with 0.5% Triton X-100 prior to blocking in 2% goat serum (diluted in PBS). After blocking, cells were incubated with an anti-myosin primary antibody at 37 °C for 2 h and then fluorescent secondary antibodies at 37 °C for 1 h. The nuclei were stained with Hoechst (Boster, Wuhan, China) for 10 min. Images were captured using a Nikon TE2000 microscope (Nikon, Tokyo, Japan).
Statistic analysis
Each experiment was carried out in triplicate and all quantitative results are represented as means ± standard error of the mean. SPSS 22.0 software was used for statistical analysis. Comparisons were made using the one-way analysis for the least three parametric groups, and student’s t-test was used for two parametric groups. A value of p < 0.05 indicated a significant difference.
Results and discussion
miR-143 expression during C2C12 myoblast differentiation
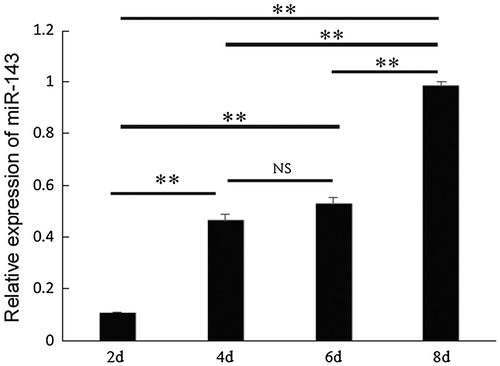
Previous studies have shown that miR-143 is involved in adipocyte differentiation and is essential for cardiac chamber morphogenesis.Citation20–22) However, whether miR-143-3p takes part in myogenic differentiation remains unclear. As shown in Fig. , we detected the temporal expression pattern of miR-143-3p during C2C12 myoblast differentiation. The expression level of miR-143-3p increased up to day 8 of myoblast differentiation, with significant increases between days 2–4 and 6–8 (p < 0.01). These results showed a similar fluctuation with those in previous studies, whereby miR-23a,Citation23) miR-26a,Citation24) miR-199a,Citation25) and miR-27aCitation26,27) were gradually upregulated and found to play important roles in the regulation of myoblast differentiation. We therefore hypothesized that miR-143-3p may also play important roles during C2C12 myoblast differentiation.
Fig. 1. Expression of miR-143 during C2C12 myoblast differentiation.
The role of miR-143-3p during C2C12 myoblast differentiation
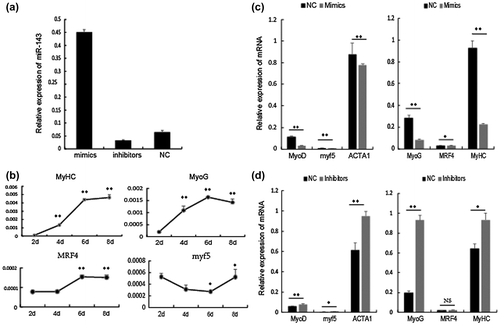
To analyze the roles of miR-143-3p during myogenic differentiation, C2C12 myoblasts were transfected with synthesized miR-143-3p mimics, inhibitors, or NC. As shown in Fig. (a), miR-143-3p was successfully over-expressed or inhibited in C2C12 myoblasts. In previous studies, MyoD, myf5, and ACTA1 have normally been considered early stage markers and MyoG, MRF4, and MyHC as later-stage markers of myogenic differentiation.Citation2,6–8) As shown in Fig. (b), expression of the randomly selected markers, myf5, MyoD, MRF4, and MyHC, were comparable to previous studies. As shown in Fig. (c), expression levels of MyoD, Myf5, and ACTA1 at 96 h and MyoG, MyHC, and MRF4 at 192 h post-transfection were significantly decreased in cells expressing miR-143-3p mimics compared with NC. As shown in Fig. (d), expression of these marker genes was opposite in cells expressing miR-143-3p inhibitors at identical time points.
Fig. 2. The role of miR-143-3p during C2C12 myoblast differentiation.
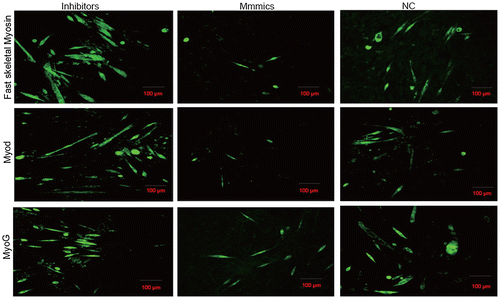
Fusion of individual myocytes to form multinucleated mature myotubes is a unique feature of skeletal myogenic differentiation and myoblast fusion has been shown to be regulated by mechanisms genetically dissociated from other myogenic processes.Citation17,19,20) To further demonstrate the role of miR-143-3p in myogenic differentiation, we performed immunofluorescence for identification of MyoD and fast skeletal myosin. As shown in Fig. , myotubes were significantly decreased or increased in cells expressing miR-143-3p mimics or inhibitors, respectively, compared with the NC cells at 192 h post-transfection. These results indicate that miR-143-3p inhibits C2C12 myoblast differentiation.
Fig. 3. The role of miR-143-3p during C2C12 myoblast differentiation.
The main time point at which miR-143-3p implements its function during C2C12 myoblast differentiation
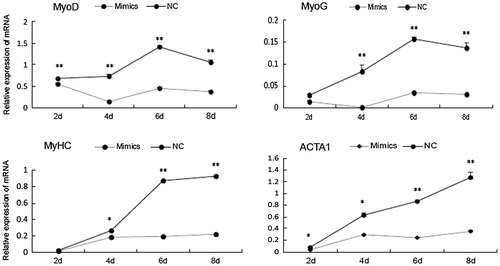
As shown in the present study, miR-143-3p inhibited C2C12 myoblast differentiation. To better understand the main time point at which miR-143 implements its function during C2C12 myoblast differentiation, C2C12 myoblasts were transfected with synthesized miR-143-3p mimics or NC. As shown in Fig. , over-expression of miR-143-3p significantly inhibited expression of MyoD, MyHC, MyoG, and ACTA1 after day 2 of differentiation and to a greater extent after day 4. These results indicate that the main time point at which miR-143-3p implements its function is during the later stages of C2C12 myoblast differentiation.
Fig. 4. Time point at which miR-143-3p implements its function during C2C12 myoblast differentiation.
The effect of miR-143-3p on the main upstream factors of the canonical Wnt pathway
Skeletal muscle development is a complex process involving several signaling pathways. In this study, we aimed to investigate whether miR-143-3p has a negative effect on C2C12 myoblast differentiation through the Wnt signaling pathway. We first analyzed expression of the main upstream components of the canonical Wnt signaling pathway at time points before and after C2C12 cell differentiation. As shown in Fig. (a), accompanied with the differentiation of C2C12, mRNA levels of Wnt5a, LRP5, Axin2, and β-catenin increased gradually. As expected, the temporal expression pattern of these factors was comparable to that of miR-143-3p during C2C12 myoblast differentiation.
Fig. 5. The effect of miR-143-3p on the main upstream components of the canonical Wnt pathway.
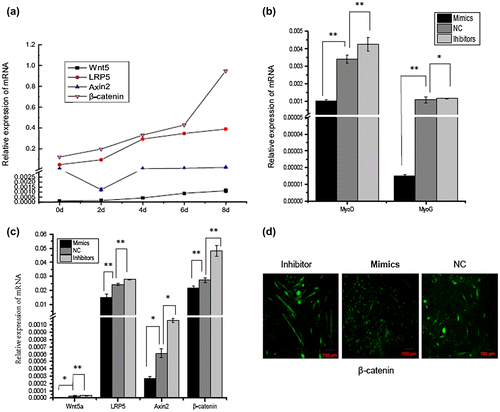
To explore potential mechanisms of how the Wnt signaling pathway associates with miR-143-3p to inhibit C2C12 myoblast differentiation, C2C12 cells were transfected with miR-143-3p mimics, inhibitors, or NC. As shown in Fig (b) and (c), over-expression of miR-143-3p significantly inhibited the expression of MyoG, MyoD, Wnt5a, LRP5, Axin2, and β-catenin. Conversely, expression levels of these factors were significantly upregulated in cells expressing miR-143-3p inhibitors compared with the control. Moreover, immunofluorescence staining for β-catenin (Fig. (d)) found that the protein fluorescence intensity was downregulated or upregulated in cells transfected with miR-143-3p mimics or inhibitors, respectively, compared with the control cells. Previous studies have shown that Wnt7a-expressing cells activate MyoD. Furthermore, Wnt1 expression preferentially activates Myf5 and Wnt4, while Wnt5a and Wnt6 can activate both Myf5 and MyoD.Citation28) In the present study, miR-143-3p inhibited expression of Wnt5a, LRP5, Axin2, and β-catenin during C2C12 myoblast differentiation. However, further experiments are needed to verify whether miR-143-3p inhibits C2C12 myogenic differentiation by modulating the Wnt signaling pathway.
Conclusions
In the present study, we identified miR-143-3p as a novel myoblast differentiation-associated miRNA in C2C12 myoblasts. MiR-143-3p not only had a negative effect on C2C12 myoblast differentiation but inhibited expression of many genes, including Wnt5a, LRP5, Axin2, and β-catenin, which are all involved in the endogenous Wnt signaling pathway during C2C12 myoblast differentiation. These results suggest that miR-143-3p may suppress myotube differentiation and maturation markers through the Wnt pathway and inhibit myotube differentiation. From a clinical point of view, inhibiting miR-143-3p expression may lead to new therapies for treating catabolic diseases characterized by muscle wasting.
Contributions
Jingjing Du, Yi Zhang, and Linyuan Shen led the experiments and designed the analytical strategy. Yihui Liu, Huaigang Lei, and Jia Luo carried out the statistical analysis. Peiwen Zhang, Qiang Pu, Surong Shuai, Qiang Li, Xuewi Li performed cell work. Shunhua Zhang and Li Zhu helped design and contributed to the data interpretation.
Funding
This work was supported by the Sichuan Sci & Tech Support Program [grant number 2013NZ0041]; The earmarked fund for China Agriculture Research System [grant number CARS-36-05B]; The Chinese National Sci & Tech Support Program [grant number 2013BAD20B07]; Program for Changjiang Scholars and Innovative Research Team in University [grant numberIRT13083].
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes
Abbreviations: MyoD, Myogenic differentiation; myf5, myogenic factor 5; ACTA1, Actin-α 1; MyoG, Myogenin; MRF4, Myogenic regulatory factor 4; MyHC, Myosin heavy chain; Wnt5a, Wingless-type family member 5a; LRP5, Low-density lipoprotein receptor-related protein 5; Axin2, Axis formation inhibitor 2; β-catenin, Beta-catenin.
References
- Soares RJ, Cagnin S, Chemello F, et al. Involvement of microRNAs in the regulation of muscle wasting during catabolic conditions. J. Biol. Chem. 2014;289:21909–21925.10.1074/jbc.M114.561845
- Tidyman WE, Moore LA, Bandman E. Expression of fast myosin heavy chain transcripts in developing and dystrophic chicken skeletal muscle. Dev. Dyn. 1997;208:491–504.10.1002/(ISSN)1097-0177
- Kuang S, Kuroda K, Le Grand F, et al. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010.10.1016/j.cell.2007.03.044
- Tapscott SJ. The circuitry of a master switch: myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:268526–268595.
- Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clinical genetics. 2000;57:16–25.
- Kaindl A, Rüschendorf F, Krause S, et al. Missense mutations of ACTA1 cause dominant congenital myopathy with cores. J. Med. Genet. 2004;41:842–848.10.1136/jmg.2004.020271
- Laing NG, Dye DE, Wallgren-Pettersson C, et al. Mutations and polymorphisms of the skeletal muscle α-actin gene (ACTA1). Human Mutation. 2009;30:1267–1277.10.1002/humu.v30:9
- Pereira JASA, Wessels A, Nijtmans L, et al. New method for the accurate characterization of single human skeletal muscle fibres demonstrates a relation between mATPase and MyHC expression in pure and hybrid fibre types. J. Muscle Res. Cell Motil. 1995;16:21–34.
- Borello U, Coletta M, Tajbakhsh S, et al. Transplacental delivery of the Wnt antagonist Frzb1 inhibits development of caudal paraxial mesoderm and skeletal myogenesis in mouse embryos. Development. 1999;126:4247–4255.
- Tanaka S, Terada K, Nohno T. Canonical Wnt signaling is involved in switching from cell proliferation to myogenic differentiation of mouse myoblast cells. J. Mol. Signaling. 2011;6:12.10.1186/1750-2187-6-12
- Topol L, Jiang X, Choi H, et al. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3–independent β-catenin degradation. J. Cell Biol. 2003;162:899–908.10.1083/jcb.200303158
- Snyder CM, Rice AL, Estrella NL, et al. MEF2A regulates the Gtl2-Dio3 microRNA mega-cluster to modulate WNT signaling in skeletal muscle regeneration. Development. 2013;140:31–42.10.1242/dev.081851
- Ke Y, Zhao W, Xiong J, et al. miR-149 inhibits non-small-cell lung cancer cells EMT by targeting FOXM1. Biochem. Res. Int. 2013;2013:1–8.
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev. Cell. 2006;11:441–450.10.1016/j.devcel.2006.09.009
- Lauressergues D, Couzigou J-M, San Clemente H, et al. Primary transcripts of microRNAs encode regulatory peptides. Nature. 2015;520:90–93.
- Zhang N, Wei X, Xu L. miR-150 promotes the proliferation of lung cancer cells by targeting P53. FEBS Lett. 2013;587:2346–2351.10.1016/j.febslet.2013.05.059
- Yang G, Wu D, Zhu J, et al. Upregulation of miR-195 increases the sensitivity of breast cancer cells to Adriamycin treatment through inhibition of Raf-1. Oncol. Rep. 2013;30:877–889.
- Cordes KR, Sheehy NT, White MP, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710.
- Sun X, He Y, Huang C, et al. Distinctive microRNA signature associated of neoplasms with the Wnt/β-catenin signaling pathway. Cell. Signalling. 2013;25:2805–2811.10.1016/j.cellsig.2013.09.006
- Esau C, Kang X, Peralta E, et al. MicroRNA-143 regulates adipocyte differentiation. J. Biol. Chem. 2004;279:52361–52365.10.1074/jbc.C400438200
- Yi C, Xie W, Li F, et al. MiR-143 enhances adipogenic differentiation of 3T3-L1 cells through targeting the coding region of mouse pleiotrophin. FEBS Lett. 2011;585:3303–3309.10.1016/j.febslet.2011.09.015
- Deacon DC, Nevis KR, Cashman TJ, et al. The miR-143-adducin3 pathway is essential for cardiac chamber morphogenesis. Development. 2010;137:1887–1896.10.1242/dev.050526
- Wang L, Chen X, Zheng Y, et al. MiR-23a inhibits myogenic differentiation through down regulation of fast myosin heavy chain isoforms. Exp. Cell. Res. 2012;318:2324–2334.10.1016/j.yexcr.2012.06.018
- Dey BK, Gagan J, Yan Z, et al. miR-26a is required for skeletal muscle differentiation and regeneration in mice. Genes Dev. 2012;26:2180–2191.
- Alexander M, Kawahara G, Motohashi N, et al. MicroRNA-199a is induced in dystrophic muscle and affects WNT signaling, cell proliferation, and myogenic differentiation. Cell Death and Differentiation. 2013;20:1194–1208.
- Chen X, Huang Z, Chen D, et al. Role of microRNA-27a in myoblast differentiation. Cell. Biol. Int. 2014;38:266–271.10.1002/cbin.v38.2
- Huang Z, Chen X, Yu B, et al. MicroRNA-27a promotes myoblast proliferation by targeting myostatin. Biochem. Biophys. Res. Commun. 2012;423:265–269.10.1016/j.bbrc.2012.05.106
- Tajbakhsh S, Borello U, Vivarelli E, et al. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998;125:4155–4162.
