Abstract
The thermal and rheological history of mayonnaise during freezing and its dispersion stability after the freeze-thaw process were investigated. Mayonnaise was cooled to freeze and stored at −20 to −40 °C while monitoring the temperature; penetration tests were conducted on the mayonnaise, which was sampled at selected times during isothermal storage at −20 °C. Significant increases in the temperature and stress values due to water-phase crystallization and subsequent oil-phase crystallization were observed. The water phase crystallized during the cooling step in all the tested mayonnaise samples. The oil phases of the prepared mayonnaise (with rapeseed oil) and commercial mayonnaise crystallized during isothermal storage after 6 and 4 h, respectively, at −20 °C. The dispersion stability was evaluated from the separation ratio, which was defined as the weight ratio of separated oil after centrifuging to the total amount of oil in the commercial mayonnaise. The separation ratio rapidly increased after 4 h of freezing. This result suggests that crystallization of the oil phase is strongly related to the dispersion stability of mayonnaise.
Graphical abstract
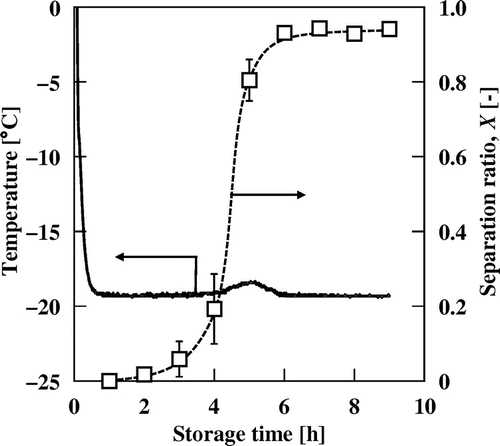
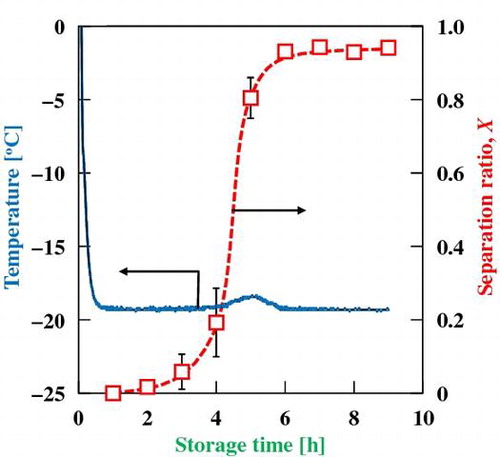
Temperature changes during storage at −20 °C and separation ratio, X, after the freeze-thaw process of commercially available mayonnaise.
Food products are often frozen to prevent microbial growth and chemical reactions. Food emulsions are thermodynamically unstable; thus, the thermal stress generated by freezing potentially diminishes the dispersion stability. In oil-in-water (O/W) emulsions, this destabilization is usually observed as creaming, increased oil-droplet size, oil–water separation, and inversion from an O/W to water-in-oil emulsion.Citation1,2) These phenomena should be suppressed to maintain the quality of the food emulsion. Many factors associated with destabilization of O/W emulsions during freezing have been suggested: (1) Denaturation of the emulsifier and stabilizer because of low-temperature preservation,Citation3,4) (2) change in the amount of emulsifier absorbed or its conformation at the interface,Citation1,5) (3) increase in internal stress caused by volumetric expansion of water into ice,Citation5,6) (4) aggregation of oil droplets due to freeze concentration,Citation5,7) and (5) partial coalescence of oil droplets by bridge-building among fat crystals.Citation8,9) It has been experimentally determined that crystallization of oil is likely the main cause of the destabilization of O/W emulsions,Citation1,11) and oil-rich O/W emulsions are commonly less stable toward freezing. It is therefore likely that destabilization of frozen mayonnaise, which contains more than 65% (w/w) dispersed oil phase, is mainly caused by lipid crystallization.Citation8,10–12)
A relationship between the dispersion stability of mayonnaise after the freeze-thaw process and oil type in the dispersion phase has been reported in several publications; these stated that oil droplets coalesce more easily in mayonnaise made with oil with a high solid-fat content than in one made with oil with a low solid-fat content.Citation6,8,13) However, this result is not always in agreement with the results of differential scanning calorimetry (DSC) analyses on the crystallization points of oils.Citation6) Because the crystallization behavior would depend on the cooling process parameters, such as cooling rate, storage time, specimen size, and so on,Citation14) this discrepancy could be caused by differences in these processing parameters. Attempts to directly observe the crystallization of oil in mayonnaise during freezing using optical microscopy have been reportedCitation15,16) and provided images of the crystallization of the oil and changes in oil-droplet size. Diluted mayonnaise, or mayonnaise with larger oil-droplets, was often used for these observations.Citation6,16) Further investigations are still necessary to fully elucidate the destabilization mechanism in mayonnaise.
In this study, mayonnaise was cooled to freeze and stored at isothermal conditions. The effect of crystallization of the oil on the stability of the mayonnaise was evaluated in terms of thermal and rheological changes.
Materials and methods
Materials
Rapeseed oil, soybean oil, and acetic acid were purchased from Wako Pure Chemical Industries (Osaka, Japan). Freeze-dried egg yolk (Dry egg yolk No.1) was purchased from Kewpie Egg Corp. (Tokyo, Japan). Commercial mayonnaise (Kewpie mayonnaise, Kewpie, Tokyo, Japan) was also employed in this study.
Preparation of mayonnaise
Two types of mayonnaise were prepared using either rapeseed or soybean oil and are denoted as MN–R and MN–S, respectively. The oil, 5% (w/w) aqueous acetic acid solution, and egg yolk solution were mixed at a 70:12:15 ratio (w/w/w). The egg yolk solution was prepared by mixing dry egg yolk and deionized water at a 1:1.25 ratio (w/w). The aqueous phase was prepared by homogenizing the egg yolk and aqueous acetic acid solutions using a mechanical homogenizer (T20SK, Kinematica, Luzern, Switzerland) at 2000 rpm for 30 s. The aqueous solution and oil were cooled in a water bath at 20 °C before and during homogenization to avoid overheating. A quarter of the oil was added to the aqueous phase and homogenized at 12,000 rpm for 30 s; this procedure was repeated four times. About 25 g of mayonnaise was obtained using this protocol.
Oil-droplet size measurement
The oil-droplet size distributions of MN–R, MN–S, and commercial mayonnaise (MN–C) were measured using a laser diffraction scattering-type particle-size distribution analyzer with the complex refractive index of 1.70−0.20i (SALD–2100, Shimadzu, Kyoto, Japan) after dilution to 0.05% (w/v) with deionized water. The droplet size was expressed as volume mean diameter ().
DSC measurements
The thermal characteristics of the aqueous phase, MN–R, MN–S, and rapeseed and soybean oils were analyzed by DSC (DSC7020, Hitachi High–Tech Science Corp., Tokyo, Japan). For these analyses, about 2 mg of the specimen was enclosed in an Alodine-coated aluminum cell. An empty cell was employed as the reference. The specimen was cooled from 20 °C to −80 °C at a rate of 1 °C/min and then heated to 20 °C at a rate of 5 °C/min.
Penetration test
The rheological characteristics were analyzed via penetration tests. The stress during storage at −20 °C for the aqueous phase, MN–R, its oil phase, and MN–S was measured using a creep meter (RE2–3305S, Yamaden, Tokyo, Japan) with a cylindrical-type plunger (ø3 mm) at a rate of 1 mm/s. Three small glass tubes (ø18 mm × 30 mm) were filled with the specimen and connected with Parafilm® to make a tube (ø18 mm × 90 mm); the tube ends were then tightly capped with silicone rubber stoppers. The specimen was pre-heated at 30 °C for about 1 h in an air oven (DO–300FA, As One Corp., Osaka, Japan) and then placed in a thermostat (SU–261, Espec Corp., Osaka, Japan) set at −20 °C. The stress was measured over time for the specimen in the central part of the prepared tube. This small tube was embedded in an aluminum heat exchanger equipped with an external cooling device (FP–87, Julabo Japan, Osaka, Japan) in order to maintain the temperature at −20 °C during the measurement.
Measurement of temperature during storage
Changes in the temperature during storage were measured for the aqueous phase, MN–R, MN–S, their oil phases, and MN–C. A glass tube (ø18 mm × 90 mm) was filled with the specimen, and the tube ends were tightly capped with silicone rubber stoppers. A thermocouple (AD–1214, A&D Company, Tokyo, Japan) was installed at the center of the tube and fixed with a phenol resin. The specimen was pre-heated and placed at −20 °C following the same methods described above, and the temperature was monitored at 20-s interval using a logger (GL–220, Graphtec Corp., Kanagawa, Japan). For MN–R, MN–S, and their oil phases, the changes in temperature during storage at −30 and −40 °C were also measured.
Dispersion stability
A glass tube (ø18 mm × 90 mm) was filled with MN–C, and the tube ends were tightly capped with silicone rubber stoppers. The specimen was pre-heated and then cooled at −20 °C following the protocol described above. The specimen was later removed, placed in a centrifuge tube, thawed in an oven at 30 °C for 30 min, and centrifuged at 5 °C at 8900 rpm for 5 min; the separation ratio of the oil phase, X, was calculated using the following equation:(1)
where WM is the weight of MN–C in the centrifuge tube, WW is the weight of MN–C after the oil separated by centrifugation was removed, and r is 0.7, which is the weight fraction of oil in MN–C.
Results
Characteristics of the mayonnaise
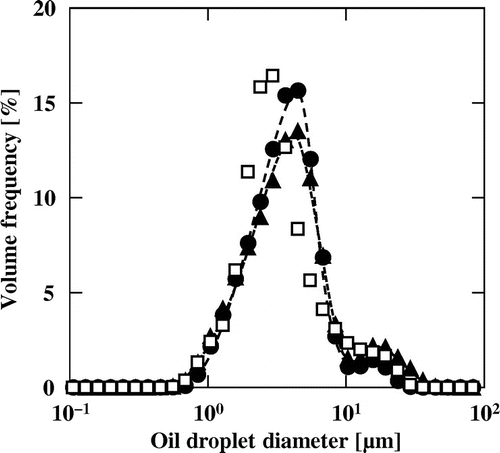
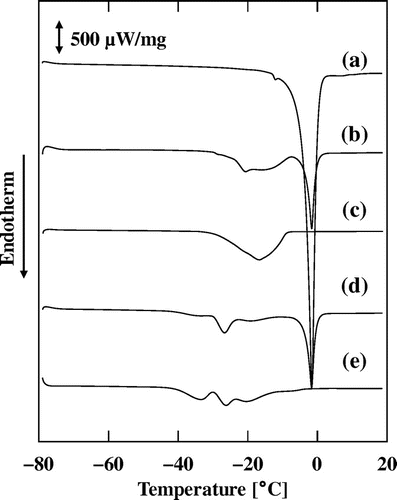
Fig. shows the oil-droplet size distributions of MN–R, MN–S, and MN–C. The obtained size distributions were almost monodisperse with a small shoulder at around 20 μm. The median oil-droplet diameters of MN–R and MN–S were around 3.3 μm, which is slightly larger than that of MN–C of 2.7 μm. The DSC curves during the thawing process of the aqueous phase, MN–R, MN–S, and their oil phases are shown in Fig. . The sharp endothermal peaks at around −2 °C were ascribed to melting of the ice in the aqueous phase of the mayonnaise. The broad endothermal peaks for MN–R, which has an onset temperature of around −30 °C, were ascribed to melting of the rapeseed oil. The three small peaks for MN–S, which has a lowest onset temperature of around −45 °C, were ascribed to melting of the soybean oil.
Penetration tests
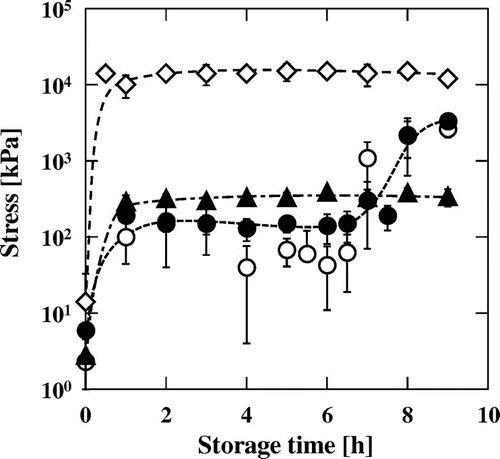
Changes in stress at −20 °C over time for the aqueous phase, MN–R, rapeseed oil, and MN–S are shown in Fig. . The stress value of the aqueous phase rapidly increased to about 10 MPa (i.e. about 103 times higher than the initial value) after 1 h of storage at −20 °C. This increase in stress was simply a consequence of the freezing process. For MN–R and its oil phase, the stress values reached about 0.1 MPa after 1 h, and then these values significantly increased to about 15 MPa (i.e. several hundred times higher than the initial values) after 6 h. This resulted from crystallization of the rapeseed oil, which occurred after about 6 h. Interestingly, a certain period was necessary for the rapeseed oil to crystallize. In contrast, the stress value of MN–S reached 0.1 MPa after 1 h, and further changes were not observed even after storage for more than 10 h.
Temperature change during storage
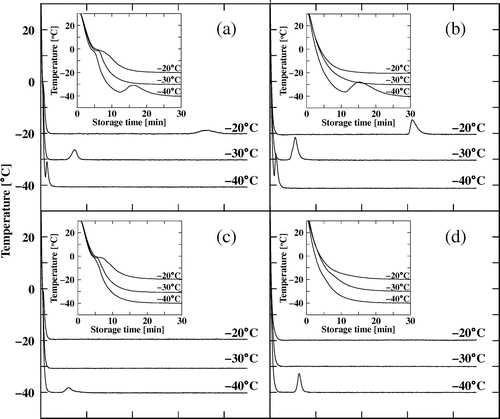
The temperature profiles during storage at −20, −30, and −40 °C for the aqueous phase, MN–R, MN–S, their oil phases, and MN–C are shown in Figs. and . The shoulder peaks of the temperature profiles of the aqueous phase and all the mayonnaise below 0 °C indicate ice formation and coincide with the rise in the stress values shown in Fig. . When stored at −20 °C, the temperature rise for MN–C was observed after about 4 h of storage, whereas those for MN–R and its oil phase occurred after around 6 h. The time required for the temperature rise decreased in MN–R and its oil phase with decreasing storage temperature. For MN–S, the temperature rise was only observed at −40 °C, and more time was required than that required for MN–R.
Dispersion stability of mayonnaise after thawing
The separation ratio of MN–C, X, which is an indicator of dispersion stability after the freeze-thaw process, was plotted as a function of time (Fig. ). The value of X slightly increased after storage for 3 h and greatly increased between 4 and 5 h to reach 0.9. The setting point of the sharp increase in the X value coincided with that of the temperature rise for MN–C.
Discussion
Changes in the viscosities of the aqueous and oil phases reflect the stress values, and crystallization of these phases reflects both the stress value and temperature rise. For MN–R, the increase in the stress value at 1 h, which was caused by crystallization of the aqueous phase and increased viscosity of the rapeseed oil, was much smaller than that at 7 h, which was caused by crystallization of the rapeseed oil. This suggests that the physical characteristics of mayonnaise are largely affected by the oil phase. Interestingly, the stress value of the rapeseed oil slightly decreased between 4 and 6 h of storage (Fig. ).
The peak shapes of the temperature rise were different for MN–R and its oil phase, i.e. that of the oil phase was more apparent and sharper than that of MN–R. The observed shapes of these peaks were the result of a balance between heat generated due to crystallization and heat lost to the environment. Thus, the differences in the shapes of the peaks of MN–R and its oil phase suggest that each would differ in their balance even though the peaks appeared at almost the same times. It has been reported that dispersed oils must be supercooled to crystallize, which differs from the behavior in bulk oil.Citation17) However, this trend was not confirmed, and the results of the current study indicate that crystallization of oil in mayonnaise and bulk oil occurs at a similar time.
After the temperature rise, the rapeseed oil can be regarded as a solid, as evident from the penetration tests. DSC analyses of the rapeseed oil showed that the set point was around −30 °C and the end point was around −15 °C (Fig. ). This result suggests that the rapeseed oil did not completely crystallize at −20 °C. Monitoring the DSC curves of rapeseed oil at −20 °C revealed the presence of an exothermal peak due to fat crystallization after about 6 h as well as in the thermostat, and its melting behavior was different from that shown in Fig. , i.e. the set point was around −17 °C (data not shown). Therefore, the crystallization behavior is largely dependent on the manner of freezing.
The time required for crystallization of the soybean oil was longer than that of the rapeseed oil, and this result agrees with the DSC analyses results shown in Fig. . Therefore, the time required for crystallization of the oil during isothermal freezing depends on the melting point of the oil.
The temperature rise and increased firmness of MN–C were confirmed after 4 h; this time duration differed from those of MN–R and its oil phase. This temperature rise could also be attributed to crystallization of the oil in MN–C. It was confirmed that the time durations for the temperature rises were well reproducible for specimens with the same lot number. However, the duration varied significantly for different lot numbers (data not shown). The product components and/or oil type have a significant impact on crystallization behavior. Destabilization of MN–C is strongly linked to crystallization of the oil phase because a rapid increase in the separation rate, X, coincides with crystallization of the oil in MN–C.
Magnusson et al.Citation6) and Campos et al.Citation14) reported that mayonnaise made with soybean oil is more stable than that made with rapeseed oil after the freeze-thaw process. This result supports our results: More time was required for crystallization of soybean oil than rapeseed oil, and the dispersion stability of mayonnaise depends on crystallization of the oil.
In conclusion, the rheological value and temperature of mayonnaise and its water and oil phases changed during storage because of crystallization of the respective phases. The dispersion stability of commercial mayonnaise is strongly related with crystallization of the oil phase.
Author contribution
All the authors conceived and designed the experiments and discussed the results. Y.M. performed the experiments and analyzed the data in collaboration with T.O. Y.M. wrote the manuscript.
Acknowledgments
This study was carried out during the course of the following project: “The Cereal Science Consortium by Kyoto University, Gifu University and the Nisshin Seifun Group, Inc.”
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Rousseau D. Fat crystals and emulsion stability — a review. Food Res. Int. 2000;33:3–14.10.1016/S0963-9969(00)00017-X
- Thanasukarn P, Pongsawatmanit R, McClements DJ. Influence of emulsifier type on freeze-thaw stability of hydrogenated palm oil-in-water emulsions. Food Hydrocoll. 2004;18:1033–1043.10.1016/j.foodhyd.2004.04.010
- Ghosh S, Cramp GL, Coupland JN. Effect of aqueous composition on the freeze-thaw stability of emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2006;272:85–88.
- Yanagisawa T, Watanuki C, Ariizumi M, et al. Super chilling enhances preservation of the freshness of salted egg yolk during long-term storage. J. Food Sci. 2009;74:E62–E69.10.1111/jfds.2009.74.issue-2
- Ghosh S, Coupland JN. Factors affecting the freeze-thaw stability of emulsions. Food Hydrocoll. 2008;22:105–111.10.1016/j.foodhyd.2007.04.013
- Magnusson E, Rosén C, Nilsson L. Freeze-thaw stability of mayonnaise type oil-in-water emulsions. Food Hydrocoll. 2011;25:707–715.10.1016/j.foodhyd.2010.08.024
- Cornacchia L, Roos YH. Solid–liquid transitions and stability of HPKO-in-water systems emulsified by dairy proteins. Food Biophysics. 2011;6:288–294.10.1007/s11483-010-9203-y
- Boode K, Walstra P. Partial coalescence in oil-in-water emulsions 1. Nature of the aggregation. Colloids Surf. A Physicochem. Eng. Asp. 1993;81:121–137.10.1016/0927-7757(93)80239-B
- Kimura T, Tanaka T, Umedzu T, et al. Latest technology development for investing mayonnaise with functionality. Nihon Shokuhin Kougakkaishi. 2011;12:113–115. ( Japanese).
- Vanapalli SA, Palanuwech J, Coupland JN. Influence of fat crystallization on the stability of flocculated emulsions. J. Agric. Food Chem. 2002;50:5224–5228.10.1021/jf020319y
- Coupland JN. Crystallization in emulsions. Curr. Opin. Colloid Interface Sci. 2002;7:445–450.10.1016/S1359-0294(02)00080-8
- Harada T, Yokomizo K. Demulsification of oil-in-water emulsion under freezing conditions: effect of crystal structure modifier. J. Am. Oil. Chem. Soc. 2000;77:859–864.10.1007/s11746-000-0137-y
- Vanapalli SV, Palanuwech J, Coupland JN. Stability of emulsions to dispersed phase crystallization: effect of oil type, dispersed phase volume fraction, and cooling rate. Colloids Surf. A Physicochem. Eng. Asp. 2002;204:227–237.10.1016/S0927-7757(01)01135-9
- Campos R, Narine SS, Marangoni AG. Effect of cooling rate on the structure and mechanical properties of milk fat and lard. Food Res. Int. 2002;35:971–981.10.1016/S0963-9969(02)00159-X
- Hashioka Y, Watanabe M, Suzuki T. Effect of kinds of vegetable oil on fat crystallization of O/W emulsion at subzero temperature. Cryobiol. Cryotechnol. 2010;56:127–134. ( Japanese).
- Langton M, Jordansson E, Altskär A, et al. Microstructure and image analysis of mayonnaises. Food Hydrocoll. 1999;13:113–125.10.1016/S0268-005X(98)00076-9
- Kloek W, Walstra P, van Vliet T. Nucleation kinetics of emulsified triglyceride mixtures. J. Am. Oil. Chem. Soc. 2000;77:643–652.10.1007/s11746-000-0104-7