Abstract
Four unique isoflavone aglycones (barpisoflavone A (1), 2′-hydroxygenistein (2), 5-methylgenistein (3), and gerontoisoflavone A (4)) whose structures were related to genistein were prepared from the tuber of Apios americana Medik. We examined the estrogen receptor and androgen receptor binding activities, estrogen agonistic activities, antioxidant activities, and α-glucosidase inhibitory activities of 1–4. The results obtained showed that 2 possessed potent and 1, 3, and 4 possessed moderate estrogen partial agonistic activities, 1 and 2 possessed moderate antioxidant activities, and 2 and 3 possessed moderate α-glucosidase inhibitory activities.
Graphical abstract
Four unique isoflavone aglycones (barpisoflavone A (1), 2′-hydroxygenistein (2), 5-methylgenistein (3), and gerontoisoflavone A (4)) were prepared from the tuber of Apios americana Medik, and tested their biological activities.
Groundnut (Apios americana Medik) is a leguminous perennial vine native to North America that generates edible tubers, which were used as an important food by native Americans. These tubers are considered to be very nutritious, especially for women just after childbirth. Previous studies examined the fatty acid,Citation1) amino acid,Citation2) and carbohydrateCitation3) compositions in groundnut, while other studies investigated its secondary metabolites (saponin,Citation4) genistein,Citation5) and genistein glucosidesCitation6)).
In our previous study,Citation7) we identified 7 rare and new isoflavone glucosides other than genistein and genistein glucosides in the tuber of Apios and examined their estrogen receptor (ER) binding activities, androgen receptor (AR) binding activities, and androgen antagonistic activities. Our findings revealed that 2'-OH genistein-7-O-gentibioside and 2′-OH genistein-7-O-glucoside possessed moderate anti-androgenic activities.
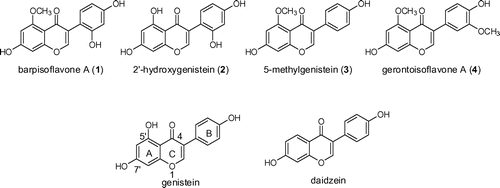
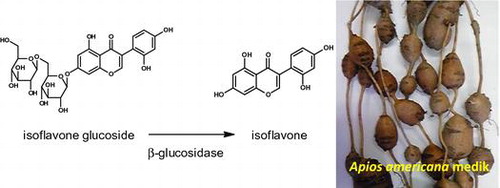
Isoflavone glucosides are hydrolyzed to the corresponding isoflavones (aglycones) by β-glucosidase in the large intestine, and the isoflavones produced are adsorbed into blood.Citation8) Therefore, the biological activities of isoflavones (not isoflavone glucosides) are essential for humans. In the present study, we prepared 4 unique isoflavones (barpisoflavone A (1),Citation9) 2′-hydroxygenistein (2),Citation10) 5-methylgenistein (3),Citation11) and gerontoisoflavone A (4)Citation12) (Fig. )), whose biological activities remain unclear, by digesting isoflavone glucosides with β-glucosidase. We compared their biological activities to the ER and AR binding activities,Citation13) estrogen antagonistic/agonistic activities,Citation13) antioxidant activities,Citation14) and α-glucosidase inhibitory activitiesCitation15) of genistein and daidzein.
Materials and methods
Preparation and isolation of 4 isoflavone aglycones
The tubers of Apios (225.65 g) were freeze dried and powdered in a mixer. The powder (180.89 g) was extracted with MeOH (1 L) by stirring for 1 h at room temperature (x 2). The MeOH solution (2 L) was filtered and the filtrate was concentrated to dryness in vacuo to give a crude isoflavone glucoside fraction (6.91 g). This fraction was suspended in 100 mL β-glucosidase solution (10 mg β-glucosidase (2 units/mg, SIGMA)/1 mL water) and incubated for 48 h at 37 °C with recipro-shaking (120 rpm). In this incubation, all of the isoflavone glucosides were hydrolyzed to the corresponding aglycones.
The solution containing aglycones was concentrated to dryness in vacuo, DMSO (3 mL) was then added, followed by sonication for 5 min. The DMSO soluble fraction containing isoflavone aglycones was recovered by centrifugation for 10 min at 3000 rpm. The DMSO solution was injected into preparative ODS HPLC (column: Developsil C30-UG-5 (10 × 250 mm, Nomura Chemical), solvent: 30% CH3CN, flow rate: 3.0 mL/min, detect 220–500 nm (PDA)), and the peaks eluted at tR 5.4 min (compound 1), tR 11.1 min (compound 2), tR 15.1 min (compound 3), and tR 16.3 min (compound 4) were collected and concentrated to dryness to afford the purified compounds. In this preparative HPLC, genistein was eluted at tR 47.5 min.
Spectroscopic analysis
1H NMR spectra were measured on AVANCE400 (Bruker BioSpin, Karlsruhe, Germany) in DMSO-d6 with the residual solvent peak as an internal standard (δH 2.50 ppm).
Physicochemical data for compounds 1–4
Barpisoflavone A (1). White solid. 1H NMR (DMSO-d6) δ: 3.92 (s, 3H, 5-OCH3), 6.28 (dd, J = 2.4, 8.2 Hz, 1H, H-5′), 6.29 (d, J = 2.4 Hz, 1H, H-3′), 6.32 (d, J = 2.1 Hz, 1H, H-6*), 6.34 (d, J = 2.1 Hz, H-8*), 6.89 (d, J = 8.2 Hz, 1H, H-6′), 7.91 (s, 1H, H-2).
2′-Hydroxygenistein (2). White solid. 1H NMR (DMSO-d6) δ: 6.30 (d, J = 2.0 Hz, 1H, H-6*), 6.12 (d, J = 2.0 Hz, 1H, H-8*), 6.42 (dd, J = 2.4, 8.2 Hz, 1H, H-5′), 6.45 (d, J = 2.4 Hz, 1H, H-3′), 7.02 (d, J = 8.2 Hz, 1H, H-6′), 7.80 (s, 1H, H-2).
5-Methylgenistein (3). White solid. 1H NMR (DMSO-d6) δ: 3.90 (s, 3H, 5-OCH3), 6.39 (d, J = 2.2 Hz, 1H, H-6*), 6.44 (d, J = 2.2 Hz, 1H, H-8*), 6.86 (d, J = 8.7 Hz, 2H, H-3′ and H-5′), 7.34 (d, J = 8.7 Hz, 2H, H-2′ and H-6′), 7.83 (s, 1H, H-2).
Gerontoisoflavone A (4). White solid. 1H NMR (DMSO-d6) δ: 3.90 (s, 3H, 5-OCH3*), 3.91 (s, 3H, 3′-OCH3*), 6.39 (d, J = 2.2 Hz, 1H, H-6**), 6.45 (d, J = 2.2 Hz, 1H, H-8**), 6.87 (d, J = 8.1 Hz, 1H, H-5′), 6.90 (dd, J = 1.6, 8.1 Hz, 1H, H-6′), 7.15 (d, J = 1.6 Hz, 1H, H-2′), 7.84 (s, 1H, H-2). *, **interchangeable.
[3H]Estradiol-ER in vitro binding assay
The gene sequences corresponding to the C-terminus ligand-binding domain (301st a.a.-551st a.a.) of estrogen receptor α (ERC) were subcloned into pMALc-4x, and the maltose-binding protein-fusion estrogen receptor α C-terminus (MBP-ERC) was expressed in E. coli strain DH5 α, and then purified on amylose resin (BioLabs). Thus, the obtained recombinant MBP-ERC (0.5 μg/mL), [3H]-Estradiol (E2, 1 nM), and test samples were incubated at 4 °C for 15 min. [3H]Estradiol-bound MBP-ERC was then precipitated with hydroxyapatite and radioactivity was measured with a liquid scintillation counter. Values are the means of 3 independent determinations.
[3H]DHT-AR in vitro binding assay
This assay was performed according to the method described previously.Citation16) In brief, the gene sequence corresponding to the ligand-binding domain (AR-LBD, 609–919 a.a.) in the C-terminus of AR was expressed in the E. coli strain DH5α as a maltose-binding protein-fused protein (MBP-AR-LBD), followed by purification using amylose resin (BIO-RAD). Thus, the obtained recombinant MBP-AR-LBD (50 μg/mL), [3H]dihydrotestosterone (DHT, 2 nM), and test samples were incubated at 4 °C for 3 h. [3H]DHT-bound MBP-AR-LBD was then precipitated with hydroxyapatite and radioactivity was measured with a liquid scintillation counter. Values are the means of 3 independent determinations.
ER agonist and antagonist activities using MCF-7 cells
Breast cancer MCF-7 cells were plated at 1 × 104cells/well onto 48-well plates and incubated in RPMI 1640 medium supplemented with 10% charcoal-stripped serum for 24 h. The cells were then treated with estradiol (10 nM) in the presence or absence of test compounds for 72 h. Cell proliferation was evaluated by the crystal violet staining method.Citation17) Values are the means of 3 independent determinations.
α-Glucosidase inhibitory assay
α-Glucosidase powder from Yeast (Wako) and 4-nitrophenyl-α-d-glucopyranoside (4-NPG) (Sigma) was dissolved in 50 mM PBS (pH7.0) to 2 units/mL and 1 mM, respectively. The reaction mixture for the assay consisted of 0.05 mL of a sample solution (each sample was dissolved in PBS containing 2% DMSO, and tested at 5, 20, and 100 μM (final concentrations)), 0.1 mL of enzymatic solution, and 0.1 mL of 4-NPG solution. The reaction mixture was kept at 37 °C for 1 h, and the amount of 4-nitrophenol produced by the hydrolysis of 4-NPG was quantified by observing the value of A405. Under the above conditions, A405 of the control (with no inhibitor) reached approximately 0.6. Percentage inhibition was calculated as follows: (1-(T-B)/(C-B)) x 100 (%), in which T, C, and B were the A405 readings of the tested compound, the control (enzymatic action with no reagent), and the zero-time control (no enzymatic action), respectively. Values are the means of 3 independent determinations.
Inhibitory activity against lipid peroxidation in a rat brain homogenate
A rat brain homogenate was prepared according to the method of Kubo et al. with some modifications.Citation18) A frozen rat brain (Wistar, 8 weeks old, male) was purchased from Funakoshi (Japan). After defrosting the brain in ice-cold 100 mM phosphate buffer at pH 7.4, 0.4 g of the brain was immediately mixed for 30 s with 15 mL of the ice-cold phosphate buffer in a Teflon homogenizer. The reaction mixture for the assay consisted of 0.2 mL of the homogenate, 0.6 mL of 100 mM phosphate buffer, 0.1 mL of 1 mM sodium ascorbate (ascorbate enhance lipid peroxidation as a pro-oxidant in this experiment), and 0.05 mL of a sample solution dissolved in methanol. The mixture was incubated at 37 °C for 1 h under reciprocal agitation. Transition metal ion catalyzed peroxidaion proceeded on the lipids in the brain homogenate during the incubation. Thiobarbituric acid reactive substances (TBARS) including malondialdehyde was stoichiometrically formed in the reaction mixture according to the concentration of the lipid peroxides. TBARS thus formed was allowed to react with thiobarbituric acid for spectrophotometric quantification at 532 nm. Percentage inhibition was calculated as follows: (1-(T-B)/(C-B)) x 100 (%), in which T, C, and B were the A532 readings of the treated compound, the control (peroxidation with no reagent), and the zero-time control (no peroxidation), respectively. Values are the means of 3 independent determinations.
Statistical analysis
Data were analyzed using 1-way ANOVA between subjects, and post hoc comparisons were made using Tukey HSD test. In all cases, statistical significance was set at p < 0.05.
Results
Preparation and purification of isoflavones
The MeOH extract of the tuber powder including isoflavone glucosides was treated with α-glucosidase to prepare isoflavones in a mixture, and each isoflavone was purified by preparative ODS HPLC. Barpisoflavone A (1: 5-methyl, 2′-hydroxygenistein) (19.4 mg), 2′-hydroxygenistein (2) (24.9 mg), 5-methylgenistein (3) (18.0 mg), and gerontoisoflavone A (4: 5-methyl, 3′-methoxygenistein) (27.7 mg) were obtained from the tubers of Apios (225.6 g). Genistein (64.4 mg) was also obtained in this preparative HPLC. The structure of each aglycone was confirmed by a 1H NMR spectral analysis. The structures of 1–4 were listed in Fig. . Compounds 1–4 have been detected in other plants,Citation9–12) while few biological activities were reported previously.
AR/ER binding activities of compounds 1–4
The binding activities of 1–4 against ER and AR were tested. The results are shown in Table . Compound 2 showed potent ER binding activity (IC50 5.0 μM) (genistein IC50 0.75 μM) that was superior to that of daidzein (IC50 26 μM). Compounds 1 and 3 also showed moderate ER binding activity (IC50 87.5 and 161 μM, respectively). No compounds showed binding activity against AR less than 200 μM.
Table 1. Estrogen receptor (ER) and androgen receptor (AR) binding activities of 1–4 (IC50 μM).
ER agonistic activity of compounds 1–4
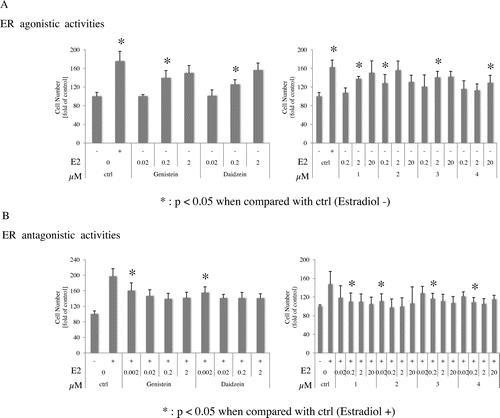
Genistein and daidzein are known to induce transcription and stimulate the growth of MCF-7 (estrogen sensitive human breast cancer cells) as partial agonists.Citation13) Therefore, in order to determine whether compounds 1–4 functioned as ER agonists or antagonists, we examined their effects on MCF-7 growth. The results obtained are shown in Fig. (A) and (B). Compounds 1–4 enhanced the growth of MCF-7 at 0.2 μM (2), 2 μM (1 and 3), or 20 μM (4) (Fig. (A)), and inhibit the growth induced by estradiol at 0.02 μM (2) or 0.2 μM (1, 3, and 4) (Fig. (B)). These results indicated that 1–4 functioned as partial agonists for estrogen like genistein and daidzein.
Compound 2 enhanced the growth of MCF-7 at 2 μM, 1, 3, and 4 enhanced growth at 20 μM (Fig. (A)), and 1–4 inhibit the growth induced by estradiol at 0.02 μM (2) or 0.2 μM (1, 3, and 4) (Fig. (B)). These results indicated that 1–4 functioned as partial agonists for estrogen like genistein and daidzein.Citation13)
Antioxidant activities of compounds 1–4
We evaluated the lipid peroxidation inhibitory activities of 1–4 in a rat brain homogenate assay. The results obtained are shown in Table . Compounds 2 and 4 showed moderate antioxidative activities (IC50 30 μM and 53 μM, respectively), which were almost the same as that of genistein (IC50 44 μM).
Table 2. Antioxidant activities of 1–4 (IC50 μM).
α-Glucosidase inhibitory activities of compounds 1–4
The α-glucosidase inhibitory activities of 1–4 were examined. The results are shown in Table . Compounds 2 and 3 showed potent inhibitory activities (IC50 11 μM and 13 μM, respectively) (genistein: IC50 8.1 μM).
Table 3. α-glucosidase inhibitory activities of 1–4 (IC50 μM).
Discussion
The estrogenic activities,Citation13) antioxidant activities,Citation14) and α-glucosidase inhibitory activitiesCitation15) of genistein and daidzein (soy bean isoflavones) have been reported previously. Therefore, we examined these activities in unique isoflavones 1–4 prepared from the tuber of Apios. Although the estrogenic and antioxidant activities of 2Citation19,20) and 3Citation21,22) have been reported previously, all other activities demonstrated in this study were reported for the first time.
The estrogenic activities of each compound were linked between the ER binding assay and estrogen agonist (antagonist) assay. The estrogenic activity of 2 was evaluated approximately 1/10 to that of genistein, whereas that of 1 and 3 was markedly lower, indicating the weak effect of 2′-OH and stronger effect of methylation at 5-OH on lowering estrogenic activity. Methylation at 5-OH may interfere with the docking of these compounds on the ER. Compound 4 did not exhibit estrogenic activity. These results indicated that the substitution at C-3′ also disturbed binding to the ER. Compound 4 may have exhibited weak estrogen agonistic and antagonistic activities due to weak ER binding activity (37% inhibition at 200 μM, data not shown).
The antioxidant activities of 1–4 were similar to those of genistein and daidzein. Thus, these activities may have been derived from the isoflavone skeleton itself when it does not possess 1,2-diol or 1,4-diol functions in B ring.
The α-glucosidase inhibitory activities of many flavonoids including genistein and daidzein have been reported previouslyCitation23); however, the mode of action has not yet been elucidated in sufficient detail. Previous studies reported enhancements in the activities of 5-OH and 4′-OH functions.Citation24,25) In the present study, OCH3 function at C-5 was found, for the first time, to markedly decrease α-glucosidase inhibitory activity.
A comparison of the estrogenic, antioxidant, and α-glucosidase inhibitory activities of 1–4 showed similar structure-activity relationships between estrogenic activity and α-glucosidase inhibitory activity (we were unable to conclude whether this similarity was coincidental). However, poor relationships were observed between estrogenic activity and antioxidant activity, and between α-glucosidase inhibitory activity and antioxidant activity.
One hundred grams of Apios tuber contains approximately 60 mg of genistein (mainly as genistein diglucosideCitation6,7)) and 20–40 mg of 1–4 (as their glucosides,Citation7)) and 100 g of soy bean contains 80 mg of genistein (mainly as genistin). We usually eat Apios tuber after cooking (boiled or fried), and the reduction of isoflavone glucosides in Apios after cooking was not observed on HPLC analyses (data not shown). Soy bean was reported to possess preventive effects for osteoporosis,Citation26) and to lower blood glucose levels.Citation27) Considering the biological activities of 1–4 in our experiments, similar pharmaceutical activities may be expected for groundnut.
Funding
This work was supported by the Grant-in Aid for Scientific Research [25560064] to K.S. from The Japan Society for the Promotion of Science (JSPS).
Author contributions
K. Shindo conceived and designed the study. H. Kaneta, M. Koda, S. Saito, M. Kawada, Y. Yamazaki, I. Momose performed study. K Shindo and M. Imoto wrote the manuscript. All authors reviewed and approved the final manuscript.
Acknowledgments
We thank Prof. Taiichiro Seki (Nihon Univeristy) for his helpful suggestions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Wilson PW, Gorny JR, Blackmon WJ, et al. Fatty acids in the American groundnut (Apios americana). J. Food Sci. 1986;51:1387–1388.10.1111/jfds.1986.51.issue-5
- Wilson PW, Pichardo FJ, Liuzzo JA, et al. Amino acids in the American groundnut (Apios americana). J. Food Sci. 1987;52:224–225.10.1111/jfds.1987.52.issue-1
- Ogasawara Y, Hidano Y, Kato Y. Study on carbohydrate composition of Apios (Apios americana Medikus) flowers and tubers. Nippon Shokuhin Kagaku Kogaku Kaishi. 2006;53:130–136 . (in Japanese).10.3136/nskkk.53.130
- Okubo K, Yoshiki Y, Okuda Keiko, et al. DDMP-conjugated saponin (soyasaponin β g) Isolated from American Groundnut (Apios americana). Biosci. Biotech. Biochem. 1994;58:2248–2250.10.1271/bbb.58.2248
- Krishman HB. Identification of genistein, an anticarcinogenic compound, in the edible tubers of the American groundnut (Apios americana Medikus). Crop Sci. 1998;38:1052–1056.
- Nara K, Nihei K, Ogasawara Y, et al. Novel isoflavone diglycoside in groundnut (Apios americana Medik). Food Chem. 2011;124:703–710.10.1016/j.foodchem.2010.05.107
- Ichige M, Fukuda E, Miida S, et al. Novel isoflavone glucosides in groundnut (Apios americana Medik) and their antiandrogenic activities. J. Agric. Food Chem. 2013;61:2183–2187.10.1021/jf305233t
- Rowland I, Faughnan M, Hoey L, et al. Bioavailability of phyto-oestrogens. Br. J. Nutr. 2003;89:S45–58.
- Adesanya SA, O’Neill MJ, Roberts MF. Isoflavonoids from Phaseolus coccineus. Phytochemistry. 1985;24:2699–2702.10.1016/S0031-9422(00)80697-4
- Biggs R. Post-infectional compounds From the French bean Phaseolus vulgaris; isolation and identification of genistein and 2′,4′,5,7-tetrahydroxyisoflavone. Aust. J. Chem. 1975;28:1389–1392.10.1071/CH9751389
- Grayer-Barkmeijer RJ, Ingham JL, Dewick PM. 5-O-methylbiochanin A, a new isoflavone from Echinospartum horridum. Phytochemistry. 1978;17:829–830.10.1016/S0031-9422(00)94255-9
- Chang CH, Lin CC, Kadota S, et al. Flavonoids and a prenylated xanthone from Cudrania cochinchinensis var. gerontogea. Phytochemistry. 1995;40:945–947.10.1016/0031-9422(95)00277-E
- Morito K, Hirose T, Kinjo J, et al. Interaction of phytoestrogens with estrogen receptors α and β. Biol. Pharm. Bull. 2001;24:351–356.10.1248/bpb.24.351
- Ian RR, Ivor ED, Jennifer KM. The antioxidant activity of genistein in vitro. J. Nutr. Biochem. 1995;6:481–485.
- Kim JS, Kwon CS, Son KH. Inhibition of α-glucosidase and amylase by luteolin, a flavonoid. Biosci. Biotech. Bioch. 2000;64:2458–2461.10.1271/bbb.64.2458
- Kawamura T, Fujimaki T, Hamanaka N, et al. Isolation and structure elucidation of a novel androgen antagonist, arabilin, produced by Streptomyces sp. MK756-CF1. J. Antibiot. 2010;63:601–605.10.1038/ja.2010.98
- Saotome K, Morita H, Umeda M. Cytotoxicity test with simplified crystal violet staining method using microtitre plates and its application to injection drugs. Toxicol. in vitro. 1989;3:317–321.10.1016/0887-2333(89)90039-8
- Kubo K, Yoshitake I, Kumada Y, et al. Radical scavenging action of flunarizine in rat brain in vitro. Arch. Int. Pharmacod. T. 1984;272:283–295.
- Zhang Z, Yuan W, Wang P, et al. Flavonoids from Lupinus texensis and their free radical scavenging activity. Nat. Prod. Res. 2011;25:1641–1649.10.1080/14786419.2010.523423
- Ahn EM, Nakamura N, Akao T, et al. Estrogenic and antiestrogenic Activities of the roots of Moghania philippinensis and their constituents. Biol. Pharm. Bull. 2004;27:548–553.10.1248/bpb.27.548
- Utkina NK, Kulesh NI. Antioxidant activity of polyphenols and polyphenol complex from the far-eastern tree Maackia amurensis. Pharm. Chem. J-USSR. 2012;46:488–491.10.1007/s11094-012-0831-z
- Garritano S, Pinto B, Giachi I, et al. Assessment of estrogenic activity of flavonoids from Mediterranean plants using an in vitro short-term test. Phytomedicine. 2005;12:143–147.10.1016/j.phymed.2004.01.004
- Kumar S, Narwal S, Kumar V, et al. α-Glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn. Rev. 2011;5:19–29.
- Choi CW, Choi YH, Cha MR, et al. Yeast α-glucosidase inhibition by isoflavones from plants of leguminosae as an in vitro alternative to acarbose. J. Agric. Food Chem. 2010;58:9988–9993.10.1021/jf101926j
- Matsui T, Kobayashi M, Hayashida S, et al. Luteolin, a flavone, does not suppress postprandial glucose absorption through an inhibition of α-glucosidase action. Biosci. Biotech. Biochem. 2002;66:689–692.10.1271/bbb.66.689
- Taku K, Melby MK., Nishi N. Soy isoflavones for osteoporosis: an evidence-based approach. Maturitas. 2011;70:333–338.10.1016/j.maturitas.2011.09.001
- Shim JY, Kim KO, Seo BH, et al. Soybean isoflavone extract improves glucose tolerance and raises the survival rate in streptozotocin-induced diabetic rats. Nutr. Res. Pract. 2007;1:266–272.10.4162/nrp.2007.1.4.266