Abstract
Osteoporosis is a debilitating disease caused by decreased bone density. Compounds with anti-osteoclastic activity, such as bisphosphonates, may help in the prevention and treatment of osteoporosis. Herein, we determined the inhibitory effects of ginger hexane extract (GHE) on receptor activator of nuclear factor kappa-B ligand (RANKL)-induced osteoclastogenesis in RAW264.7 cells. The results showed that GHE (1) suppressed osteoclast differentiation and the formation of actin rings; (2) inhibited the expression of Nfatc1, a master transcriptional factor for osteoclast differentiation, in a dose-dependent manner (10–20 μg/mL); and (3) inhibited other osteoclastogenesis-related genes, such as Oscar, Dc-stamp, Trap, and Mmp9. These findings suggest that GHE may be used to prevent and treat osteoporosis by inhibiting osteoclast differentiation.
Graphical abstract
Ginger hexane extract (GHE) suppresses RANKL-induced osteoclast differentiation.
Osteoporosis is characterized by a low bone mass and deterioration of the bone tissue microarchitecture, leading to enhanced bone fragility and fractures. Several longitudinal studies have indicated that osteoporosis increases the risk of mortality.Citation1–3) Bone homeostasis is maintained through a balance between bone resorption by osteoclasts and bone formation by osteoblasts.Citation4) An imbalance between bone resorption and formation, typically caused by menopause, leads to bone loss and osteoporosis.Citation5,6)
Osteoporosis can be treated pharmacologically by inhibiting osteoclast activation and/or stimulating osteogenesis. Bisphosphonates, the first line of treatment for osteoporosis, selectively bind to bone, are internalized by osteoclasts, and then inhibit bone resorption by disrupting osteoclast function and survival.Citation7,8)
Osteoporosis often progresses undiagnosed until a patient receives a bone scan after a fracture because few subjective symptoms are present. Therefore, maintaining normal bone metabolism and preventing bone loss before bone fragility progress are important. Calcium and vitamin D are important nutrients that help to maintain normal bone metabolism.Citation9,10) Other nutrients such as potassium, magnesium, zinc, copper, iron, vitamin C, and vitamin K may also have beneficial effects.Citation11)
Certain natural plant products have also demonstrated beneficial effects against bone loss in a clinical study and in various experimental models. Isoflavonoids, such as daidzein, prevent bone loss in postmenopausal females.Citation12) β-Cryptoxanthin has also been reported as a valuable compound for anti-osteoporosis because it stimulates osteoblastogenesis and inhibits osteoclastogenesis in vitro.Citation13) Furthermore, a prospective cohort study showed that high levels of serum β-cryptoxanthin were associated with a lower risk for bone loss and osteoporosis in postmenopausal Japanese females.Citation14)
Ginger or ginger root, the rhizome of the plant Zingiber officinale, is used throughout the world as a common cooking spice and has also been used as a natural medicine. Ginger extracts and their components have demonstrated many functions. Ginger has cholagogic,Citation15,16) anti-emetic, and anti-inflammatory activities.Citation17,18) Furthermore, clinical studies indicated ginger has anti-nauseaCitation19) and anti-inflammatory activities.Citation20)
Recently, we succeeded in preparing ginger hexane extract (GHE) at the factory-scale from raw ginger. In the present study, we determined the effects of GHE on receptor activator of nuclear factor kappa-B ligand (RANKL)-induced osteoclast differentiation in RAW264.7 cells by tartrate-resistant acid phosphatase (TRAP) staining, visualization of actin-ring formation, and assessing the expression of genes related to osteoclast differentiation.
Material and methods
Sample preparation
The GHE is made from the residue after squeezing the juice from raw ginger root. First, n-hexane was added to the residue and extraction was carried out at room temperature. After filtration, GHE was obtained by removing the hexane under reduced pressure. The yield ratio of GHE from the raw ginger was approximately 0.01–0.5%. For cell culture, GHE was dissolved in DMSO at the concentrations given below. The final concentration of DMSO in the cell culture medium was 0.1%.
HPLC analysis
The constituents of GHE were analyzed by high-performance liquid chromatography (HPLC) on an HPLC analysis system (Hitachi, Tokyo, Japan) under the following conditions: C18 column (φ 4.6 mm × 250 mm; Wako, Osaka, Japan); UV detector at 228 nm; column temperature of 40 °C; mobile phase of A = H2O and B = CH3CN; gradient: 0–20 min, 30–90% B; 20–45 min 90% B; and flow rate of 0.6 mL/min. Citral, 6-gingerol and 6-shogaol were purchased from WAKO. Phellandrene was purchased from Tokyo Chemical Industry (Tokyo, Japan). Farnecene was purchased from Sigma-Aldrich (MO, USA).
Cell culture, osteoclast differentiation, and GHE treatment
RAW264.7 cells (ECACC, Wiltshire, UK) were cultured in α-minimum essential medium (Life Technologies, Grand Island, NY, USA) with 10% fetal bovine serum (Biowest SAS, Nuaille, France) and 1% Penicillin-Streptomycin Solution (Wako) at 37 °C in 5% CO2. The RAW264.7 cells were cultured until they reached 80% confluence before use in each assay.
To differentiate the RAW264.7 cells into osteoclasts, 3 × 103 cells/cm2 were seeded and cultured for one day in the culture medium. Then, the cells were treated with 0, 10, 20 μg/mL of GHE or 30 μM nobiletin (WAKO). After 24 h, defined as day 0, the medium was changed to osteoclast differentiation medium containing 100 ng/mL of sRANKL (Oriental Yeast, Tokyo, Japan) and 0, 10, 20 μg/mL of GHE or 30 μM of nobiletin. The medium was replaced with fresh medium every 2 days until day 6.
TRAP staining
RAW264.7 cells were differentiated into osteoclasts. At day 6, the cells were fixed with 10% formalin for 5 min, washed with purified water, and stained with TRAP staining solution (COSMO BIO, Tokyo, Japan), according to the manufacturer’ s protocol. The stained cells were imaged using a microscope (CKX41; Olympus, Tokyo, Japan) with an attached camera (DP21; Olympus).
Actin staining
RAW264.7 cells were differentiated into osteoclasts on coverslips (Matsunami Glass, Osaka, Japan). On day 6, the cells were fixed with 4% paraformaldehyde for 10 min and washed with PBS. Next, the cells were permeabilized with 0.5% Triton X-100 in PBS for 10 min and then stained for F-actin with an acti-stain 488 phalloidin (Cytoskeleton, Inc., Denver, CO, USA) for 30 min at room temperature. After staining, the cells were washed with PBS and the coverslips were mounted in Slow Fade Gold Antifade Mount with DAPI (Life Technologies). Actin rings and the cell nuclei were visualized using a fluorescence microscope (Axioplan 2 MOT; Carl Zeiss, Oberkochen, Germany) with a plan apochromat NA 1.4 objective lens equipped with a charge-coupled device camera (MicroMAX; Princeton Instruments, Princeton, NJ, USA).
Western blotting
RAW264.7 cells were differentiated into osteoclasts. Cell lysates, isolated from the cells at day 0, 3 and 6 were separated on a 5–20% gradient SDS-PAGE gel (ATTO, Tokyo, Japan), transferred to a nitrocellulose membrane, and detected with mouse anti-NFATc1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse anti-GAPDH antibody (Life Technologies) as a control. After incubation in HRP-conjugated goat anti-mouse secondary antibody (MBL International, Woburn, MA, USA), the signals were enhanced by chemiluminescence with the ECL system (GE Healthcare, Chalfont St Giles, UK).
Quantitative RT-PCR
Total RNA was isolated from the RAW264.7 cells using TRIzol (Life Technologies). After digestion of genomic DNA with the TURBO DNA-free kit (Life Technologies), 850 ng of RNA was reverse-transcribed to cDNA using the Super Script VILO kit with random primers (Life Technologies). Quantitative PCR was then performed using Fast SYBR Green Master Mix (Life Technologies) in a Step One Plus system (Life Technologies). The following sense and antisense primers were used: Nfatc1, 5′-AAAGGAGAGGTCGGACTCGG-3′ (sense), 5′-AACTGTAGTGTTCTTCCTCGGC-3′ (antisense); Oscar, 5′-CTGGAAGAAGTGACTCCGGC-3′ (sense), 5′-GAGCTGATCCGTTACCAGCAG-3′ (antisense); Dc-stamp, 5′-TACGTGGAGAGAAGCAAGGAA-3′ (sense), 5′-ACACTGAGACGTGGTTTAGGAAT-3′ (antisense); Trap, 5′-CCGCCTCTTCCCAACTCG-3′ (sense), 5′-CATGAATCCATCTTGGCGGTG-3′ (antisense); Mmp9, 5′-GTCCAGACCAAGGGTACAGC-3′ (sense), 5′-ATACAGCGGGTACATGAGCG-3′ (antisense); 18s ribosomal RNA, 5′-CCTGCGGCTTAATTTGACTC-3′ (sense), 5′-AGACAAATCGCTCCACC-3′ (antisense).
Statistics analysis
All data are presented as the mean ± SD. The results were analyzed using Student’s t-tests. p-values less than 0.05 were considered statistically significant.
Results
Composition of ginger hexane extract
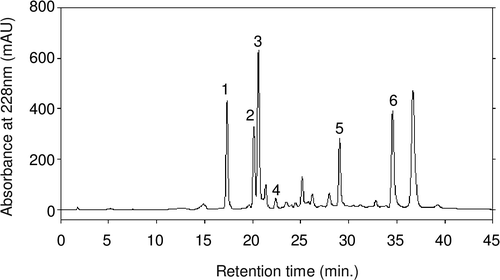
We analyzed the GHE using HPLC by monitoring the UV wavelength at 228 nm and identified its major peaks using reference standards. As shown in Fig. , GHE was found to contain 6-gingerol, phellandrene, farnecene, and two stereoisomers of citral, neral and geranial. The latest major peak is predicted to be zingiberene, a well-known major constituent of ginger oleoresin. In contrast, 6-shogaol, one of the unique components of ginger and formed by heating 6-gingerol,Citation21) was only minimally detected in GHE at 5 mg/g while 110 mg/g 6-gingerol was quantified. These results suggest that GHE preserves the characteristic molecules of raw ginger while minimizing the loss of volatile flavor components and heat-induced chemical changes. Although we conducted LC-MS analysis to identify the unknown components of GHE, we were unable to characterize further compounds because of the abundance of peaks in the MS spectrum (data not shown).
Ginger hexane extract inhibited osteoclast differentiation
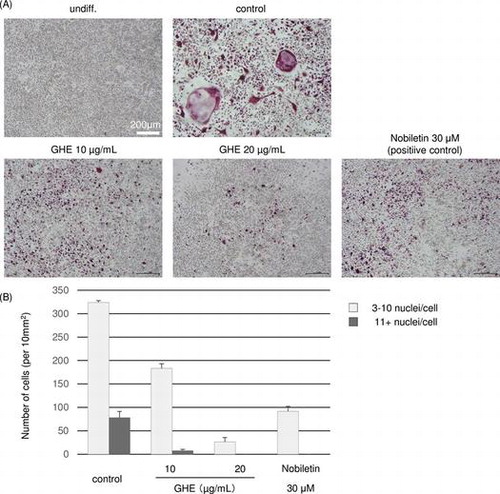
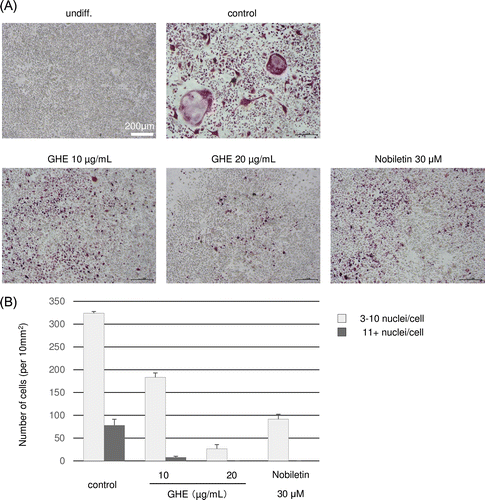
We examined the effects of GHE on RANKL-induced osteoclastogenesis in the murine macrophage cell line RAW264.7. The RAW264.7 cells were cultured in medium with GHE at 0, 10, or 20 μg/mL, or with the positive control 30 μM nobiletin, which is reported to inhibit RANKL-induced osteoclast differentiation at 4–50 μM.Citation22) After 24 h, sRANKL was added to the medium and the cells were cultured for 6 days. Under normal conditions, the RANKL-treated RAW264.7 cells differentiated into multinucleated giant cells through repetitive cell fusion. To evaluate the degree of osteoclast differentiation, we conducted TRAP staining of GHE or nobiletin-treated and non-treated cells, where TRAP-positive multinucleated cells with more than two nuclei were counted as osteoclasts. As shown in Fig. , in control cells, the number of osteoclast cells with 3–10 nuclei/cell and more than 11 nuclei/cell were 324 and 78 cells/10 mm2, respectively, whereas 30 μM nobiletin-treated cells were decreased to 92 cells/10 mm2 and almost none. Similar to nobiletin treatment, GHE inhibited osteoclast differentiation, 20 μg/mL GHE treatment decreased 3–10 nuclei/cell and more than 11 nuclei/cells to 27 cells/10 mm2 and almost none. Additionally, we found that GHE does not cause cell death at the concentrations used (date not shown).
Fig. 2. Effects of GHE on RANKL-induced osteoclast differentiation.
Ginger hexane extract inhibited actin-ring formation
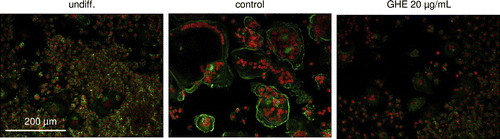
To assess the inhibitory effects of GHE on bone resorption, we stained the cellular actin filaments with phalloidin and observed actin-ring formation. An actin ring is a cytoskeletal structure that is essential for optimal osteoclastic bone resorption.Citation23) RAW264.7 cells cultured with sRANKL for 6 days formed actin rings. In contrast, GHE treatment dramatically inhibited actin-ring formation (Fig. ). These results indicate that GHE inhibits the bone resorption activity of RANKL-treated cells.
Fig. 3. Effect of GHE on actin-ring formation by osteoclast cells.
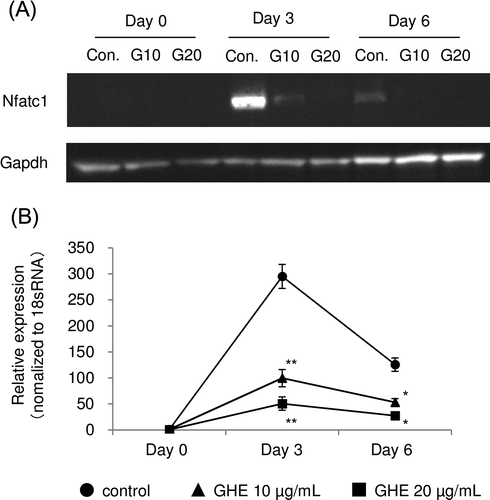
Ginger hexane extract decreased expression of Nfatc1
Next, we determined whether GHE changed the synthesis of nuclear factor of activated T cells (Nfatc) 1 protein. Nfatc1 acts as the master transcriptional factor for osteoclastogenesis during the early stage of osteoclast differentiation and is degraded at later stages.Citation24,25) As shown by the western blots in Fig. (A), the secretion of Nfatc1 protein was significantly decreased by GHE treatment in a dose-dependent manner. Furthermore, we performed quantitative RT-PCR to assess whether the decrease in Nfatc1 protein secretion in the cells treated with GHE was caused by suppression of the Nfatc1 mRNA. As expected, the expression of Nfatc1 mRNA was also reduced in the GHE-treated cells (Fig. (B)).
Fig. 4. Effect of GHE on the secretion and expression of Nfatc1.RAW264.7 cells were cultured in medium with 0, 10, or 20 μg/mL of GHE.
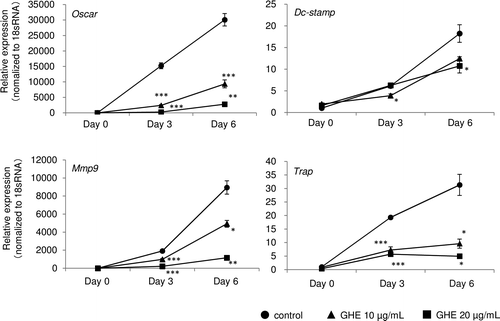
Ginger hexane extract decreased expression of osteoclast differentiation-related genes
The inhibitory effects of GHE on the expression of other osteoclast differentiation related genes including, osteoclast-associated receptor (Oscar), dendritic cell-specific transmembrane protein (Dc-stamp), matrix metalloproteinase 9 (Mmp9), and Trap. GHE significantly decreased the expression of these genes (Fig. ).
Fig. 5. Effect of GHE on genes associated with osteoclast differentiation.
Discussion
Excessive bone resorption is a common cause of the bone loss that occurs in osteoporosis. Therefore, anti-osteoclastic agents are used as one approach for drug therapy. In the present study, we found that treatment with GHE decreased the formation of TRAP-positive and multinucleated RAW264.7 cells in a RANKL-induced osteoclast differentiation model. Actin staining showed that GHE treatment inhibited actin-ring formation. Together, these results suggest that GHE inhibits osteoclast differentiation and bone resorption. Furthermore, we showed that these effects might be attributed to a down-regulation of the expression of Nfatc1 and other osteoclastogenesis-related genes by GHE.
Ginger is a dietary plant that contains rich bioactive components and has been used as a spice and a traditional medicine throughout the world. The World Health Organization has classified ginger as a medicinal plant. Many studies have reported the beneficial effects of ginger as a functional food. Additionally, the wide culinary use of ginger suggests it may be safe for medical use.
GHE was prepared from raw ginger without drying. As shown in Fig. , we identified 6-gingerol, phellandrene, farnesene and citral in GHE by HPLC analysis. Additionally, 6-shogaol, which is a dehydrated product formed by heating 6-gingerol, was only minimally detected. The content of 6-gingerol and 6-shogaol was 110 and 5 mg/g, respectively. GC-MS analysis also showed that GHE contains limonene, pinene, and other volatile components (date not shown). These results suggest that the characteristics of raw ginger are preserved when preparing GHE. Although we attempted to quantify the other identified major peaks using HPLC, we could not achieve it because the guaranteed purity reference standards were unobtainable.
Because little is known about the effects of ginger and its related components on bone remodeling, we assessed the effects of GHE on osteoclastogenesis, a central process in bone resorption. We found that GHE inhibited the RANKL-induced formation of TRAP-positive multinucleated cells in a dose-dependent manner (10–20 μg/mL) (Fig. ). Furthermore, GHE decreased the formation of actin rings, which are essential constructions for bone resorption (Fig. ). The secretion of Nfatc1, a master regulator of osteoclastogenesis, is induced by RANKL during the early phase of osteoclast differentiation and regulates the expression of several genes necessary for osteoclast differentiation.Citation24,25) Therefore, we evaluated the effects of GHE on the secretion and expression of Nfatc1. Quantitative RT-PCR and western blotting analysis showed that GHE dramatically down-regulated the expression of Nfatc1 (Fig. ). Additionally, GHE reduced other osteoclast differentiation-related genes, such as Oscar, Dc-stamp, Mmp9, and Trap probably through the suppression of Nfatc1 (Fig. ). Oscar is an immunoreceptor that is required for RANKL-mediated activation of calcium signaling, which is essential for the autoamplification of Nfatc1, a critical step during osteoclastogenesis.Citation24,26,27) Thus, the suppression of Oscar by GHE may also lead to the inactivation of Nfatc1. Dc-stamp is highly expressed by osteoclasts, but not by macrophages, and acts as a regulator of osteoclast cell fusion.Citation28) Osteoclast multinucleation is required for bone resorption. Mmp9 and Trap are enzymes directly involved in the breakdown of bone matrix.Citation29)
Zerumbone, the main component of the essential volatile oil of tropical ginger Zingiber zerumbet, is reported to inhibit RANKL-induced osteoclastogenesis.Citation30) However, we found that the anti-osteoclastogenesis activity of GHE originates in its non-volatile constituents, separated by distillation, and not in its volatile constituents (data not shown). These results suggest that the volatile constituents of GHE contains little or no zerumbone. In an effort to identify the principal active ingredients of GHE, we fractionated GHE into 10 fractions and evaluated their anti-osteoclastogenesis activity. The fractions containing the identified major peaks, 6-gingerol, phellandrene, farnesene and citral, were less activity (data not shown). Even though citral was reported to inhibit NF-kB activation,Citation31) which plays a key role during osteoclast differentiation, the above findings suggest that citral is not the most effective anti-osteoclastogenesis component, and other compounds in GHE are likely more effective. Although we tried to identify the composition of these active fractions by LC-MS analysis, we were unable achieve this because of the abundance of peaks in the MS spectrum. Further studies are needed to identify the principal active ingredients of GHE Curcumin, derived from Curcuma longa, was shown to suppress RANKL induced NF-κB activation in osteoclast precursors.Citation32) Many reports indicated that ginger contains some compounds structurally similar to curcumin such as diarylheptanoids, 1-dehydrogingergiones and paradols.Citation33,34) Actually, 1-dehydro-[10]-gingergione, a pungent constituent of ginger, is reported to inhibit IKKβ activity of NF-κB activation in LPS-stimulated macrophages.Citation35) Based on these observations, we speculated that some of the minor components of GHE, such as diarylheptanoids and 1-dehydrogingergiones, work together and inhibit osteoclastogenesis.
The results of this study show, for the first time, that GHE inhibits osteoclastogenesis through the suppression of Nfatc1 and other osteoclastogenesis-related genes in RANKL-induced osteoclast differentiation model. Although the active components that are responsible for this function were not identified, these findings may suggest a new application of ginger for enhancing bone health. Studies are currently underway to evaluate the anti-osteoclast effect of ginger in an osteoporosis murine model.
Authors contribution
Suguru Ito, Takeo Yano, Katsuzumi Okumura, Norihiro Nishimura, Kazuhiro Kagontani conceived and designed the experiments. Suguru Ito, Akihiro Ohmi, Akiyo Sakamiya performed the experiments. Suguru Ito wrote the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors thank Kazuto Sugimura for helpful suggestions and Hideaki Kaneoka, Yuko Araki, and Yumi Koda for skilled technical assistance.
References
- Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. J. Am. Geriatr. Soc. 2000;48:241–249.10.1111/jgs.2000.48.issue-3
- Nguyen ND, Center JR, Eisman JA, et al. Bone loss, weight loss, and weight fluctuation predict mortality risk in elderly men and women. J. Bone Miner. Res. 2007;22:1147–1154.10.1359/jbmr.070412
- Suzuki T, Yoshida H. Low bone mineral density at femoral neck is a predictor of increased mortality in elderly Japanese women. Osteoporos. Int. 2010;21:717–719.
- Clarke B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008;3(Suppl 3):S131–S139.10.2215/CJN.04151206
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342.10.1038/nature01658
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell. 2002;2:389–406.10.1016/S1534-5807(02)00157-0
- Rogers MJ, Crockett JC, Coxon FP, et al. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49:34–41.10.1016/j.bone.2010.11.008
- Das S, Crockett JC. Osteoporosis – a current view of pharmacological prevention and treatment. Drug Des. Dev. Ther. 2013;7:435–448.
- Heaney RP. Calcium intake and disease prevention. Arq. Bras. Endocrinol. Metabol. 2006;50:685–693.10.1590/S0004-27302006000400014
- Ooms ME, Roos JC, Bezemer PD, et al. Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J. Clin. Endocrinol. Metab. 1995;80:1052–1058.
- Palacios C. The role of nutrients in bone health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006;46:621–628.10.1080/10408390500466174
- Wei P, Liu M, Chen Y, et al. Systematic review of soy isoflavone supplements on osteoporosis in women. Asian Pac. J. Trop. Med. 2012;5:243–248.10.1016/S1995-7645(12)60033-9
- Yamaguchi M, Uchiyama S. β-Criptoxanthin stimulates bone formation and inhibits bone resorption in tissue culture in vitro. Mol. Cell Biochem. 2004;258:137–144.10.1023/B:MCBI.0000012848.50541.19
- Sugiura M, Nakamura M, Ogawa K, et al. High serum carotenoids associated with lower risk for bone loss and osteoporosis in post-menopausal japanese female subjects: prospective cohort study. PLoS One. 2012;7:e52643.10.1371/journal.pone.0052643
- Yamahara J, Huang QR, Li YH, et al. Gastrointestinal motility enhancing effect of ginger and its active constituents. Chem. Pharm. Bull. (Tokyo). 1990;38:430–431.10.1248/cpb.38.430
- Yamahara J, Miki K, Chisaka T, et al. Cholagogic effect of ginger and its active constituents. J. Ethnopharmacol. 1985;13:217–225.
- Al-Nahain A, Jahan R, Rahmatullah M. Zingiber officinale: a potential plant against rheumatoid arthritis. Arthritis [Internet]. 2014 [cited 2014 May];2014. Available from: http://www.hindawi.com/journals/arthritis/
- Dugasani S, Pichika MR, Nadarajah VD, et al. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010;127:515–520.10.1016/j.jep.2009.10.004
- Schmid R, Schick T, Steffen R, et al. Comparison of seven commonly used agents for prophylaxis of seasickness. J. Travel. Med. 1994;1:203–206.10.1111/j.1708-8305.1994.tb00596.x
- Srivastava KC, Mustafa T. Ginger (Zingiber officinale) in rheumatism and musculoskeletal disorders. Med. Hypotheses. 1992;39:342–348.10.1016/0306-9877(92)90059-L
- Bhattarai S, Tran VH, Duke CC. The stability of gingerol and shogaol in aqueous solutions. J. Pharm. Sci. 2001;90:1658–1664.10.1002/(ISSN)1520-6017
- Murakami A, Song M, Katsumata S, et al. Citrus nobiletin suppresses bone loss in ovariectomized ddY mice and collagen-induced arthritis in DBA/1J mice: Possible involvement of receptor activator of NF-kappaB ligand (RANKL)-induced osteoclastogenesis regulation. BioFactors. 2007;30:179–192.10.1002/biof.v30:3
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508.10.1126/science.289.5484.1504
- Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell. 2002;3:889–901.10.1016/S1534-5807(02)00369-6
- Kim JH, Kim K, Jin HM, et al. Negative feedback control of osteoclast formation through ubiquitin-mediated down-regulation of NFATc1. J. Biol. Chem. 2010;285:5224–5231.10.1074/jbc.M109.042812
- Koga T, Inui M, Inoue K, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763.10.1038/nature02444
- Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 2009;231:241–256.10.1111/imr.2009.231.issue-1
- Xing L, Xiu Y, Boyce BF. Osteoclast fusion and regulation by RANKL-dependent and independent factors. World J. Orthop. 2012;3:212–222.10.5312/wjo.v3.i12.212
- Sundaram K, Nishimura R, Senn J, et al. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp. Cell Res. 2007;313:168–178.10.1016/j.yexcr.2006.10.001
- Sung B1, Murakami A, Oyajobi BO, et al. Zerumbone abolishes RANKL-Induced NF- B activation, inhibits osteoclastogenesis, and suppresses human breast cancer-induced bone loss in athymic nude mice. Cancer Res. 2009;69:1477–1484. 10.1158/0008-5472.CAN-08-3249
- Lee HJ, Jeong HS, Kim DJ, et al. Inhibitory effect of citral on NO production by suppression of iNOS expression and NF-κB activation in RAW264.7 cells. Arch. Pharm. Res. 2008;31:342–349.10.1007/s12272-001-1162-0
- Bharti AC, Takada Y, Aggarwal BB. Curcumin (diferuloylmethane) inhibits receptor activator of NF- B ligand-induced NF- B activation in osteoclast precursors and suppresses osteoclastogenesis. J. Immunol. 2004;172:5940–5947.10.4049/jimmunol.172.10.5940
- Peng F, Tao Q, Wu X, et al. Cytotoxic, cytoprotective and antioxidant effects of isolated phenolic compounds from fresh ginger. Fitoterapia. 2012;83:568–585.10.1016/j.fitote.2011.12.028
- Tanaka K, Arita M, Sakurai H, et al. Analysis of Chemical Properties of Edible and Medicinal Ginger by Metabolomics Approach. Biomed Res Int [Internet]. 2015 [cited 2015 Oct];2015. Available from: http://www.hindawi.com/journals/bmri/.
- Hwa YL, Sun HP, Misoon L, et al. 1-Dehydro-[10]-gingerdione from ginger inhibits IKKβ activity for NF-κB activation and suppresses NF-κB-regulated expression of inflammatory genes. Br. J. Pharmacol. 2012;167:128–140.