Abstract
Murine contact hypersensitivity (CHS) is one of the most frequently used animal models of human allergic contact dermatitis. We investigated the inhibitory effects of soybean and soy isoflavone (SI) diets on 2,4-dinitrofluorobenzene- (DNFB) induced CHS in mice. The DNFB-induced ear swelling was inhibited in the soy- and SI-treated groups. Histopathological investigations revealed that oral feeding of soybean and SI attenuated ear tissue edema and reduced the number of Gr-1+ cell infiltrations into ear tissues. DNA microarray analysis showed that the expression of Ccl24, Xcl1, Ifng, and Ccl17 in the ear tissues was lower in the soy-treated mice than in the positive controls. In addition, CCL24 mRNA and protein expression in the ear tissues were more highly suppressed in the soy- and SI-treated groups. These results suggest that soybean and SI consumption downregulated the gene and protein expression of CCL24, thereby affording protection against CHS in mice.
Graphical abstract
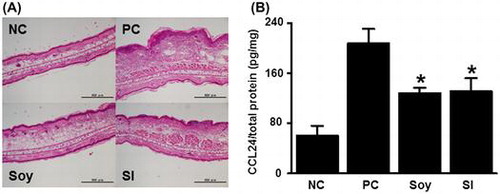
Contact hypersensitivity (A, ear tissue) and CCL24 production (B) were reduced in soybean- (Soy) and soy isoflavone- (SI) fed mice compared to that in the positive control (PC).
Soybean has been a staple of Asian diets for thousands of years and contains several phytochemicals such as soy isoflavones (SIs), saponins, sterols, and lignans. The reduced prevalence of several chronic diseases has been attributed to the consumption of SI-rich soy food.Citation1,2) Recent clinical studies have found that soy food and SIs have significant health benefits in subjects with certain types of cancers,Citation3) cardiovascular diseases,Citation4) osteoporosis,Citation5) and menopausal symptoms.Citation6)
Soybean contains 12 different SI isomers: three aglycones (genistein, daidzein, and glycitein), their respective β-glycosides (genistin, daidzin, and glycitin), and each of these three β-glycosides esterified with either malonic acid (malonyl genistin, malonyl daidzin, and malonyl glycitin) or acetic acid (acetyl genistin, acetyl daidzin, and acetyl glycitin). Genistein, daidzein, and glycitein account for ~50, 40, and 10%, respectively, of the total SI content when all forms of the individual SIs are considered aglycones.Citation7) SI aglycones are the bioactive forms, whereas the β-glycoside forms are predominant in soybean. Because these β-glycoside forms have a higher molecular weight and hydrophilicity than SI aglycones, they must be hydrolyzed to be absorbed across the enterocyte of healthy adults.Citation8)
Previous studies using in vivo models showed that isoflavones mostly exhibit immunosuppressive functions.Citation9–11) Genistein injection has been shown to suppress delayed-type hypersensitivity (DHS) response to oxazoloneCitation12) and 4-hydroxy-3-nitrophenyl acetyl succinimide (NP-O-SU).Citation13) High intake of soy food and SIs is also associated with reduced prevalence of allergic rhinitisCitation14) and chronic obstructive pulmonary disease.Citation15) Oral administration of SIs and genistein significantly reduces ovalbumin-induced airway hyper-responsiveness and inhibits eosinophil infiltration in mouse models of allergic asthma.Citation16) In patients with asthma, dietary SI supplementation has been shown to reduce eosinophil leukotriene C4 (LTC4) synthesis and eosinophilic airway inflammation.Citation17)
Other studies in mouse models have shown that oral administration of genistein suppresses ovalbumin-specific immune responses,Citation18) a fermented soy product reduces peanut-induced allergy symptoms,Citation19) dietary genistein and daidzein protect mice from peanut-induced allergy,Citation20) and a daidzein-rich isoflavone aglycone preparation protects mice from dextran sulfate sodium-induced colitis.Citation21) Similarly, genistein is also known to decrease 2,4,6-trinitrobenzenesulfonic acid-induced acute inflammation in rats.Citation22) Nevertheless, studies on the utilization of soybean and SIs in human immune dysfunctions are very limited and are as yet restricted to animal models.Citation9)
Allergic contact dermatitis (ACD) is one of the most common skin diseases caused by DHS responses to antigens that come into contact with the skin. Contact hypersensitivity (CHS) is an experimental animal model for human ACD.Citation23–25) The MF® diet (Oriental Yeast) is one of the most widely used commercial chows and formulated with soy protein. Studies have shown that most commercial rodent diets contain high amount of SIs because of the presence of soy proteins.Citation26) We hypothesized that the MF diet could show inhibitory effects on CHS because of its high SI content. In the present study, therefore, we used the MF diet and a commercial isoflavone, and investigated the inhibitory effects and mechanisms of action of soybean and SI on CHS.
Materials and methods
Materials
Two kinds of chows, MF and F2PLD1, and Soyaflavone HG were obtained from Oriental Yeast (Tokyo, Japan) and Fuji Oil (Izumisano, Japan), respectively.
Reagents
2,4-Dinitrofluorobenzene (DNFB) was purchased from Kanto Chemical, Tokyo, Japan. Anti-Mouse Ly-6G (Gr-1) Purified (14-5831) and Biotin Mouse Anti-Rat IgG2b (550327) were obtained from eBioscience (San Diego, CA, USA) and BD Biosciences (Bedford, MA, USA), respectively. TaqMan gene expression assays were purchased from Applied Biosystems (Waltham, MA, USA) for the following genes: chemokine (C-C motif) ligand 24 (Ccl24, Mm00444701_m1), chemokine (C motif) ligand 1 (Xcl1, Mm00434772_m1), and β-actin (Actb, Mm00607939_s1). All other chemicals were reagent grade and used without further purification.
SI contents
SI contents were determined using a previously reported method.Citation27)
Animals
Female BALB/c mice (6 weeks old; 15–20 g body weight) were purchased from Japan SLC (Hamamatsu, Japan). The mice were housed in a room with a 12-h light and dark cycle and maintained at 25 ± 2°C. The experimental design was in accordance with the guidelines for animal experimentation and was approved by the Animal Experimental Committee of Kawasaki University of Medical Welfare (authorization number 13-011) and Kawasaki Medical School (authorization number 14-019).
Contact hypersensitivity test
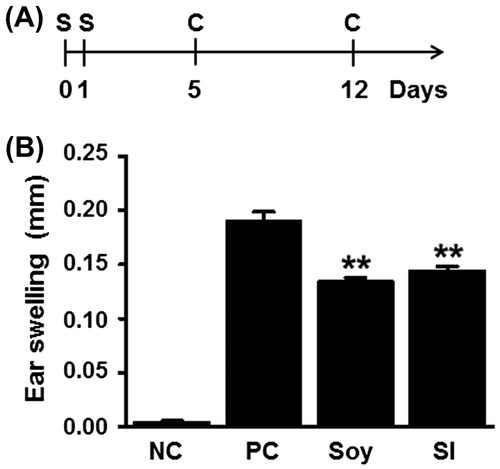
The mice were divided into four groups, which were fed F2PLD1 and acidified water (negative control group, n = 3, and positive control group, n = 6), MF and acidified water (soy-treated group, n = 6), or F2PLD1 and 0.1% Soyaflavone HG (SI-treated group, n = 6). The mice were sensitized on days 0 and 1 by applying 50 μL of 0.5% DNFB diluted in acetone/olive oil (4:1) on the shaved abdominal skin. The animals were then challenged by the application of 20 μL of 0.15% DNFB on the dorsal side of both ears on days 5 and 12. The negative control mice were sensitized and challenged with vehicle (without DNFB). The ear thickness of the mice was measured using a soft touch micrometer (CLM1-15QM, Mitutoyo, Tokyo, Japan). Ear swelling was calculated as the difference in thickness before and after challenge. All animal experimental procedures were performed under anesthesia with sevoflurane (Maruishi Pharmaceutical, Osaka, Japan).
Histological analysis
Ear tissues of mice were harvested 24 h after the second challenge with DNFB and fixed with IHC zinc fixative (BD Biosciences) for 24 h. Paraffin-embedded ear tissues were stained with hematoxylin and eosin and subjected to immunohistochemistry (IHC) analyses conducted using Ventana Discovery® XT (Ventana Medical Systems, Tucson, AZ, USA). The antibodies used were anti-mouse Gr-1 (dilution, 1:25) and secondary biotinylated mouse anti-rat IgG2b (dilution, 1:25). Images were acquired using a microscope (Axio Photo, Carl Zeiss AG, Oberkochen, Germany).
Gene expression assay
Ear tissues of mice were collected 24 h after the second challenge with DNFB. Total RNA was extracted from the ear tissues using an RNeasy Fibrous Tissue Mini Kit (Qiagen, Valencia, CA). DNA microarray analysis was performed using a GeneChip® Mouse Gene 2.0 ST Array (Affymetrix, Santa Clara, CA, USA) by Filgen (Nagoya, Japan). Total RNA was reverse transcribed using the ReverTra Ace® qPCR RT Master Mix with gDNA remover (Toyobo, Osaka, Japan). Ccl24 and Xcl1 mRNA levels were determined by real-time PCR on StepOnePlus™ (Applied Biosystems) according to the manufacturer’s protocol using TaqMan gene expression assays and THUNDERBIRD® probe qPCR mix (Toyobo). β-Actin was used as a control for normalization, and the assays were performed in triplicate. Fluorescence data were analyzed using the StepOnePlus™ software and expressed as Ct, the number of cycles needed to generate a fluorescent signal above a predefined threshold.
CCL24 enzyme-linked immunosorbent assay
Ear tissues of mice were collected 24 h after the second challenge with DNFB. CCL24 expression was determined using a mouse CCL24/Eotaxin-2 DuoSet® ELISA development system (R&D Systems, Minneapolis, MN) according to the manufacturer’s instruction. Protein concentration was determined using the Pierce™ BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). These assays were performed in duplicate.
Statistical analysis
The data were represented as the mean ± standard error of the mean (SEM). Statistical significance was calculated by one-way analysis of variance (ANOVA), followed by Tukey’s post hoc tests, using the Origin 8.5 software (OriginLab, Northampton, MA, USA). The data were considered significant at p < 0.05.
Results
Isoflavone content and the concentration of Soyaflavone HG for SI-treated mice
The isoflavone content in MF was 51.2 mg/100 g (0.0512%); however, it was not detected in F2PLD1. In contrast, Soyaflavone HG contained 54.4% SI. The water intake by the mice was greater than their diet intake. Thus, we chose 0.1% as the concentration of Soyaflavone HG solution for SI-treated mice.
The suppressive effects of dietary soybean and SI on DNFB-induced ear swelling
Generally, a CHS response is induced by application of DNFB to the ear of DNFB-sensitized mice. The degree of ear swelling correlates with the intensity of effector response, and the swelling peaks 24–48 h after the challenge.Citation24) Thus, we examined the ear swelling induced by DNFB challenge to evaluate the suppressive effects of soybean and SI on CHS responses. The sensitized mice were challenged with 0.15% DNFB on days 5 and 12. The ear swelling was evaluated 24 h after the second challenge (Fig. ). Compared to that in the positive control group (0.190 ± 0.009 mm), the soy-treated (0.134 ± 0.004 mm) and SI-treated (0.144 ± 0.005 mm) groups showed reduced (29.5 and 24.2%, respectively) ear swelling (p < 0.01 vs. the positive control). These results indicated that CHS responses were suppressed by oral feeding of soybean or SI.
Fig. 1. Feeding mice with soybean and SI attenuates ear swelling due to contact allergy.
Dietary soybean and SI inhibit edema and immune cell infiltration in contact allergic ear tissue
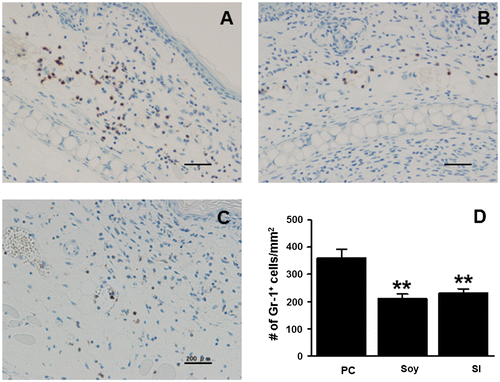
We conducted histopathological analysis of the inflamed ear tissues 24 h after the second challenge with DNFB (Fig. ). Prominent tissue edema and increased numbers of infiltrating immune cells were observed in the positive control mice compared to that in the negative control mice. The ear swelling in the soy- and SI-treated mice was lower than that in the positive control mice. IHC analyses were conducted by staining for Gr-1, a marker expressed by granulocytes and monocytes, to determine the infiltration of myeloid immune cells, which were recruited in large numbers to the site of allergen challenge during the effector phase (Fig. ). The number of infiltrated Gr-1-positive cells in contact allergic ear tissues decreased to a greater extent in the soy-treated (212 ± 18 cells/mm2) and SI-treated (230 ± 18 cells/mm2) groups than in the positive control group (360 ± 33 cells/mm2). The number of Gr-1-positive cells infiltrated into contact allergic ear tissues in the soy- and SI-treated groups was decreased with 41.1 and 36.1%, respectively, compared to that in the positive control group. These results pointed toward an anti-inflammatory role for soybean and SI in CHS.
Fig. 2. Histopathological images of DNFB-challenged mouse ears.
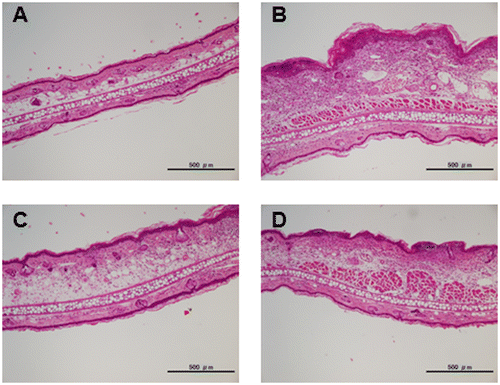
Fig. 3. Feeding mice with soybean and SI inhibits the infiltration of myeloid immune cells.
Attenuating effects of dietary soybean and SI on the secretion of cytokines
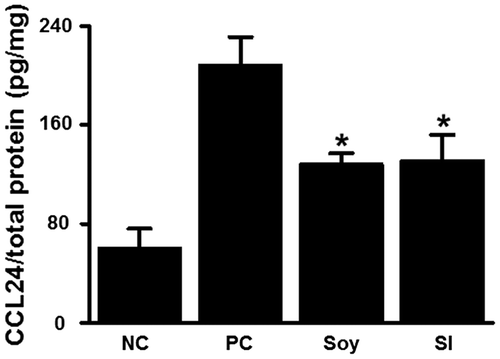
To clarify the mechanisms underlying the attenuation of CHS in mice fed with soybean and SI, we used DNA microarray analyses to examine the differences in gene expression in the inflamed ear tissues 24 h after the second challenge with DNFB in the soy-treated and positive control mice. The comparison between the soy-treated and positive control mice showed that the cytokine genes Ccl24, Xcl1, Ifng, and Ccl17 were most downregulated (Table ). The gene expression of Ccl24 and Xcl1 was determined by real-time PCR (Fig. ). The gene expression of Ccl24 and Xcl1 in the soy- and SI-treated groups was lower than that in the positive control group, even though the gene expression of Xcl1 was not significantly different between the soy-treated and positive control groups. We further examined CCL24 protein production in the ear tissues via ELISA (Fig. ). CCL24 levels were significantly suppressed in the soy- and SI-treated groups compared to those in the positive control group. These results demonstrated that oral feeding of soybean or SI reduced CCL24 secretion in contact allergic ear tissues.
Table 1. Cytokine genes downregulated in the soy-treated mouse compared with the positive control as determined by DNA microarray analysis.
Fig. 4. Effect of feeding mice with soybean and SI on Ccl24 and Xcl1 mRNA expression.
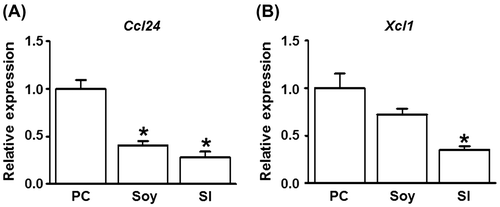
Fig. 5. Feeding mice with soybean and SI suppresses CCL24 production in ear tissues.
Discussion
The results of this study clearly demonstrated that oral feeding of soybean or SI suppressed CHS. Histopathological investigations of inflamed ear revealed that soy and SI attenuated tissue edema and reduced the number of infiltrating Gr-1-positive immune cells. Our observations are in line with those of reports showing that genistein suppressed DHS response to hapten in mouse models.Citation12,13) Verdrengh et al. demonstrated that DHS response to oxazolone was more significantly suppressed in genistein-treated mice than in the control mice. Genistein treatment also led to a decrease in the levels of oxazolone-specific IgG.Citation12) In another study, Yellayi et al. reported that genistein suppressed DHS response to NP-O-SU in ovariectomized mice.Citation13) There was a 46–67% decrease in the DHS response in the footpads of mice injected 8–80 mg/kg genistein compared to that in the controls. Histopathological examination of their feet revealed lower cell infiltration in genistein-treated mice than in the controls, together with a reduction in the numbers of CD4+ and CD8+ T cells in the popliteal lymph nodes. The effects of genistein are mediated through both estrogen receptor (ER) and non-ER pathways.Citation13) In this study, however, we used a mixture of SIs and not genistein. High total isoflavone serum concentrations were observed in adult mice that were fed commercial rodent diets formulated with soy protein (mean values: 1,212–2,388 ng/mL). The bacterially derived metabolite equol was the major isoflavone identified in the serum of these mice, and accounted for 90–97% of the total serum isoflavones.Citation26) In addition, soybean contains several other phytochemicals such as saponins, sterols, and lignans.Citation28) Therefore, further investigation of SI isomers and other soybean phytochemicals is warranted.
Some of the mechanisms underlying the immune-regulatory effects of soy and SI could be attributed to the attenuation of cytokines and chemokines. In this study, DNA microarray analysis showed that the gene expression of Ccl24, Xcl1, Ifng, and Ccl17 was downregulated to a higher extent in the soy-treated group than in the positive control group. In addition, CCL24 mRNA and protein expression were more highly suppressed in the soy- and SI-treated groups than in the positive control group. CCL24 (eotaxin-2) is a cytokine belonging to the CC chemokine family. CCL24 interacts with chemokine receptor CCR3 to induce chemotaxis in eosinophils.Citation30) CCL24 has a dominant role in promoting airway eosinophilia, even though recruitment of eosinophils to the lungs of mice during an allergic response is regulated by both CCL11 (eotaxin) and CCL24.Citation30) Transgenic co-expression of CCL24 and IL-5 within the airway leads to chronic eosinophil-associated lung damage as observed in severe asthma.Citation31) During airway responses or upon IL-4 overexpression, CCL24 is significantly upregulated.Citation32) In contrast, when the expression of chemokine genes at the elicitation site of trinitrochlorobenzene-induced CHS was examined in BALB/c mice, of the 33 genes analyzed, including Ccl11 and Ccl24, Ccl11 and Ccl24 were not among the 11 genes showing altered expression levels.Citation33) In the present study, however, the gene expression of Ccl24 was detected in DNA microarray analysis. CCL24 mRNA and protein production were also more highly suppressed in soy- and SI-treated groups than in the positive control group. Therefore, unlike in mouse models of allergic asthma, the role of CCL24 in CHS remains unknown.
Eosinophils are key effector cells in the pathogenesis of allergic inflammation.Citation29) In this study, feeding mice with soybean and SI reduced the number of infiltrating Gr-1-positive immune cells. Several reports have documented the effects of SI on eosinophils. For example, oral administration of SI and genistein significantly reduced ovalbumin-induced airway hyper-responsiveness (AHR) and inhibited eosinophil infiltration in mouse models of allergic asthma. The potency of 100 mg/kg SI on AHR inhibition was similar to that of genistein at a dose of 30 mg/kg. Furthermore, SI and genistein suppressed ovalbumin-induced mRNA expression of Ccl11.Citation16) In another study, genistein inhibited eosinophil LTC4 synthesis in ex vivo cultures of eosinophils derived from patients with allergic asthma by blocking the activation of 5-lipoxygenase. In patients with asthma, dietary SI supplementation reduced eosinophil LTC4 synthesis and eosinophilic airway inflammation.Citation17)
Most commercial rodent diets contain high amount of SIs because of the inclusion of soy protein in the formulations. Brown and Setchell reported an average of 39.4–82.9 mg/100 g total SI in widely used commercial chows.Citation26) In this study, we used 2 commercial chows: MF and F2PLD1. The MF diet is one of the most widely used commercial chows formulated with soy protein. While the total SI concentration of MF was 51.2 mg/100 g, SI was not detected in F2PLD1. The measured average daily food intake in our study was 1.9 g per mouse; hence, 0.97 mg of SI was consumed each day via the MF diet. Consequently, each mouse was exposed to approximately 49 mg SI/kg body weight/day. For comparison, SI intake, although variable across populations, ranges from 30 to 40 mg/day in Asian adults (samples collected from various parts of Japan and Shanghai).Citation34) These results indicate that the SI intake by the mice used in the present study was relatively high, but was not physiologically relevant when compared to human SI intake. However, we showed that soybean in MF suppressed CHS and that this could mainly be attributed to its SI content. Currently, we are investigating the inhibitory effects of SI on CHS at lower doses.
In summary, our data provide evidence that feeding mice with soybean and SI attenuates CHS and downregulates CCL24 mRNA and protein production. These results suggest that SI consumption may have a therapeutic effect in ACD. Further research will be directed toward testing the beneficial effects of SI on ACD in human subjects. Development of new therapeutic approaches for ACD is of significant importance to public health, and our results could have wide implications for the development of future treatment strategies of ACD.
Authors contributions
TN and KT designed the experiments; TN, WW, and HYY performed the experiments; TN, WW, TK, and KH analyzed the data; TN wrote the manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science KAKENHI [grant number 25350112]; Fuji Foundation for Protein Research; Kawasaki University of Medical Welfare.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
The authors thank the staff of the Medical BioResource Research Unit, BioImaging Research Unit, and Molecular Cell Biology Research Unit in the Central Research Institute of Kawasaki Medical School.
Notes
Abbreviations: SI, soy isoflavone; CHS, contact hypersensitivity; DNFB, 2,4-dinitrofluorobenzene; ACD, allergic contact dermatitis; DHS, delayed-type hypersensitivity; IHC, immunohistochemistry; CCL24, chemokine (C-C motif) ligand 24; XCL1, chemokine (C motif) ligand 1; ELISA, enzyme-linked immunosorbent assay.
References
- Setchell KDR, Cassidy A. Dietary isoflavones: Biological effects and relevance to human health. J. Nutr. 1999;129:758S–767S.
- Messina M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014;100:423S–430S.10.3945/ajcn.113.071464
- Nagata C, Mizoue T, Tanaka K, et al. Soy intake and breast cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2014;44:282–295.10.1093/jjco/hyt203
- Li SH, Liu XX, Bai YY, et al. Effect of oral isoflavone supplementation on vascular endothelial function in postmenopausal women: a meta-analysis of randomized placebo-controlled trials. Am. J. Clin. Nutr. 2010;91:480–486.10.3945/ajcn.2009.28203
- Alekel DL, Van Loan MD, Koehler kJ, et al. The soy isoflavones for reducing bone loss (SIRBL) study: a 3-y randomized controlled trial in postmenopausal women. Am. J. Clin. Nutr. 2010;91:218–230.10.3945/ajcn.2009.28306
- Taku K, Melby MK, Kronenberg F, et al. Extracted or synthesized soybean isoflavones reduce menopausal hot flash frequency and severity. Menopause. 2012;19:776–790.10.1097/gme.0b013e3182410159
- Murphy PA, Barua K, Hauck CC. Solvent extraction selection in the determination of isoflavones in soy foods. J. Chromatog. B. 2002;777:129–138.10.1016/S1570-0232(02)00342-2
- Izumi T, Piskula MK, Osawa S, et al. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000;130:1695–1699.
- Masilamani M, Wei J, Sampson HA. Regulation of the immune response by soybean isoflavones. Immunol. Res. 2012;54:95–110.10.1007/s12026-012-8331-5
- Sakai T, Kogiso M. Soy isoflavones and immunity. J Med. Invest. 2008;55:167–173.10.2152/jmi.55.167
- Cooke PS, Selvaraj V, Yellayi S. Genistein, etrogen receptors, and the acquired immune response. J. Nutr. 2006;136:704–708.
- Verdrengh M, Jonsson IM, Holmdahl R, et al. Genistein as an anti-inflammatory agent. Inflamm. Res. 2003;52:341–346.10.1007/s00011-003-1182-8
- Yellayi S, Zakroczymski MA, Selvaraj V, et al. The phytoestrogen genistein suppresses cell-mediated immunity in mice. J. Endocrinol. 2003;176:267–274.10.1677/joe.0.1760267
- Miyake Y, Sasaki S, Ohya Y, et al. Soy, isoflavones, and prevalence of allergic rhinitis in Japanese women: The Osaka Maternal and Child Health Study. J. Allergy Clin. Immunol. 2005;115:1176–1183.10.1016/j.jaci.2005.02.016
- Hirayama F, Lee AH, Binns CW, et al. Dietary intake of isoflavones and polyunsaturated fatty acids associated with lung function, breathlessness and the prevalence of chronic obstructive pulmonary disease: possible protective effect of traditional Japanese diet. Mol. Nutr. Food Res. 2010;54:909–917.10.1002/mnfr.v54:7
- Bao ZS, Hong L, Guan Y, et al. Inhibition of airway inflammation, hyperresponsiveness and remodeling by soy isoflavone in a murine model of allergic asthma. Int. Immunopharmacol. 2011;11:899–906.10.1016/j.intimp.2011.02.001
- Kalhan R, Smith LJ, Nlend MC, et al. A mechanism of benefit of soy genistein in asthma: inhibition of eosinophil p38-dependent leukotriene synthesis. Clin. Exp. Allergy. 2008;38:103–112.
- Kogiso M, Sakai T, Mitsuya K, et al. Genistein suppresses antigen-specific immune responses through competition with 17β-estradiol for estrogen receptors in ovalbumin-immunized BALB/c mice. Nutrition. 2006;22:802–809.10.1016/j.nut.2006.04.003
- Zhang T, Pan W, Takebe M, et al. Therapeutic effects of a fermented soy product on peanut hypersensitivity is associated with modulation of T-helper type 1 and T-helper type 2 responses. Clin. Exp. Allergy. 2008;38:1365–2222.
- Masilamani M, Wei J, Bhatt S, et al. Soybean isoflavones regulate dendritic cell function and suppress allergic sensitization to peanut. J. Allergy Clin. Immunol. 2011;128:e1.
- Morimoto M, Watanabe T, Yamori M, et al. Isoflavones regulate innate immunity and inhibit experimental colitis. J. Gastroenterol. Hepatol. 2009;24:1123–1129.10.1111/jgh.2009.24.issue-6
- Seibel J, Molzberger A, Hertrampf T, et al. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur. J. Nutr. 2009;48:1436–6207.
- Inagaki N, Nagai H. Analysis of the mechanism for the development of allergic skin inflammation and the application for its treatment: mouse models for the development of remedies for human allergic dermatitis. J. Pharmacol. Sci. 2009;110:251–259.10.1254/jphs.09R01FM
- Kaplan DH, Igyártó BZ, Gaspari AA. Early immune events in the induction of allergic contact dermatitis. Nature Rev. Immunol. 2012;12:114–124.
- Honda T, Egawa G, Grabbe S, et al. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J. Invest. Dermatol. 2013;133:303–315.10.1038/jid.2012.284
- Brown NM, Setchell KDR. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab. Invest. 2001;81:735–747.10.1038/labinvest.3780282
- Kudou S, Fleury Y, Welti D, et al. Malonyl isoflavone glycosides in soybean seeds (Glycine max Merrill). Agric. Biol. Chem. 1991;55:2227–2233.10.1271/bbb1961.55.2227
- Kang J, Badger TM, Ronis MJJ, et al. Non-isoflavone phytochemicals in soy and their health effects. J. Agric. Food Chem. 2010;58:8119–8133.10.1021/jf100901b
- Pope SM, Zimmermann N, Stringer KF, et al. The eotaxin chemokines and CCR3 Are fundamental regulators of allergen-induced pulmonary eosinophilia. J. Immunol. 2005;175:5341–5350.10.4049/jimmunol.175.8.5341
- Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J. Allergy Clin. Immunol. 2007;119:1303–1310.10.1016/j.jaci.2007.03.048
- Ochkur SI, Jacobsen EA, Protheroe CA, et al. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J. Immunol. 2007;178:7879–7889.10.4049/jimmunol.178.12.7879
- Zimmermann N, Hogan SP, Mishra A, et al. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J. Immunol. 2000;165:5839–5846.10.4049/jimmunol.165.10.5839
- Mitsui G, Mitsui K, Hirano T, et al. Kinetic profiles of sequential gene expressions for chemokines in mice with contact hypersensitivity. Immunol. Lett. 2003;86:191–197.10.1016/S0165-2478(03)00017-8
- Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer. 2006;55:1–12.10.1207/s15327914nc5501_1
