Abstract
To quantitatively evaluate the therapeutic effects of diosgenin (DG) and investigate the role of IL-4 on skin inflammation, alterations in luciferase-derived signal and general phenotype biomarkers were measured in IL-4/Luc/CNS-1 transgenic mice with phthalic anhydride (PA)-induced skin inflammation after treatment with DG for 4 weeks. High levels of luciferase-derived signal detected in the abdominal region and submandibular lymph node (SL) of the PA treated group was significantly decreased by 67–88% in the PA + DG cotreated group. Furthermore, the weight of the lymph node and spleen, IgE concentration, epidermis thickness, and number of infiltrated mast cells were lower in the PA + DG treated group than the PA + Vehicle treated group. Moreover, expression of IL-6 and vascular endothelial growth factor (VEGF) also decreased in the PA + DG cotreated group. These results suggest that PA-induced skin inflammation could be successfully suppressed by DG treatment in IL-4/Luc/CNS-1 Tg mice through attenuation of IL-4 and IL-6 expression, as well as decreased IgE concentration and mast cells infiltration.
Graphical abstract
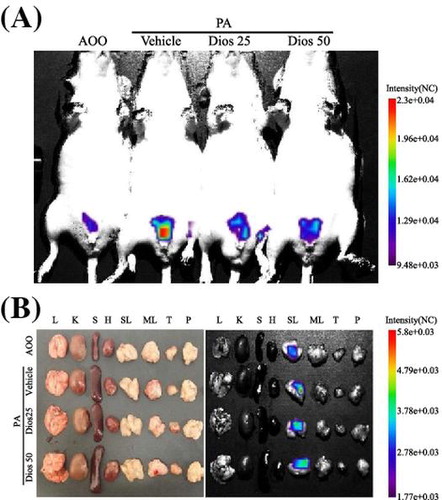
Measurement of luciferase signal in the whole body (A) and each organ (B) of IL-4/Luc/CNS-1 Tg mice.
DG (3β-hydroxy-5-spirostene), a steroid saponin, is found in various plants including the seeds of fenugreek (Trigonella foenum graceum Linn) and the roots of wild yams (Dioscorea villosa Linn), Costus speciosus, and Smilax menispermoidea.Citation1,2) This compound is a well-known precursor of several synthetic steroidal drugs that are widely applied in various pharmaceutical industries.Citation3) DG has been shown to play important pharmacological roles in various diseases including diabetes, cancer, inflammation, hypercholesterolemia, gastrointestinal ailments, and oxidative stress.Citation4) Additionally, many studies have reported that DG plays an important role as an antitumor drug. DG treatment effectively prevented proliferation of breast cancer induced by N-Methyl-N-nitrosurea (NMU) treatment by enhancing the antioxidant defense system.Citation5) DG also inhibited the formation of colonic aberrant crypt foci and putative precancerous lesions of the colon in an rodent colon cancer model induced with azoxymethane (AOM).Citation6) In addition, DG was found to have anti-viral effects in hepatitis C virus infected mammalian cells. A significant reduction in the level of viral RNA and protein, as well as phosphorylation of activator and signal transducer of transcription 3 was observed in DG-treated Ava5 cells and Huh7-derived cell lines.Citation7) Furthermore, DG was found to exert a therapeutic effect in several lipid metabolism-related diseases, including hyperglycemia, hypercholesterolemia, and hypertriacylglycerolemia.Citation8–10)
Interestingly, DG has been reported to exert anti-inflammatory activity in macrophages and several allergic models. Although lipopolysaccharide (LPS)/interferon γ (IFN-γ)-activated murine macrophages showed inhibited NO production, iNOS expression, ROS production, and IL-1/6 expression following pretreatment with DG,Citation4) there are similar reports regarding its effects on ovalbumin (OVA) sensitization in BALB/c mice. A previous study provided evidence that the administration of DG increased serum IgG2 levels and the amount of IFN-γ secreted from splenocytes, whereas the level of serum IgE and IgM and IL-4 expression in the lungs and splenocytes were unaltered.Citation11) Subsequent studies revealed that DG had different effects on the anti-inflammatory activity of OVA-sensitized BALB/c mice. For example, DG treatment markedly attenuated crypt depth, OVA-specific IgE production, and infiltration of mast cells in the duodenal tissue, while the OVA-specific IgG2 level and IL-4 expression increased.Citation12,13) Especially, some differences in the IL-4 expression, an important hallmark of acute allergic skin disease, were observed in different tissues or organs of OVA sensitized mice.Citation14) Therefore, further study was needed to clarify these discrepancies on the systemic role of IL-4 during anti-inflammatory activity of DG because above studies did not provide the correlation between IL-4 and administration of DG. Also, the therapeutic effect of DG was not investigated in the skin inflammation induced by PA treatment.
In this study, we investigated the suppressive effects of long-term DG treatment on PA-induced skin inflammation using IL-4/Luc/CNS-1 Tg mice with luciferase cDNA regulated by human IL-4 promoter and enhancer of IL-4 (CNS-1). The results verified the correlation of IL-4 with suppression of DG in skin inflammation induced by repeated dermal exposure to PA. Specifically, measurement of the luciferase signal and phenotypes revealed that DG effectively attenuated skin inflammation induced by PA treatment in IL-4/Luc/CNS-1 Tg mice.
Materials and methods
Care and use of animals
Eight-week-old IL-4/Luc/CNS-1 Tg mice were kindly provided by the Department of Laboratory Animals Resources at the National Institute of Food and Drug Safety Evaluation (Osong, Korea), and HR1 mice of the same age were purchased from Central Lab Animal Inc. (Seoul, Korea). All mice were provided with ad libitum access to standard irradiated chow diet (Samtako) and autoclaved water throughout the four-week experiment. The diet for mice was composed of crude protein (25.43%), crude fat (6.06%), crude fiber (3.9%), crude ash (5.31%), calcium (1.14%), and phosphorus (0.99%), as well as moisture (12.5%). This diet also contained corn (546 g/kg), vegetable protein (316 g/kg), animal fat and oil (36 g/kg), fish meal (34 g/kg), beet pulp (30 g/kg), calcium/phosphate supplement (13 g/kg), limestone (10 g/kg), salt (5 g/kg), lysine (2 g/kg), choline bitartrate (2 g/kg), 7.17% methionine solution (2 g/kg), mineral mix (2 g/kg), and vitamin mix (2 g/kg). During the four-week experimental period, mice were maintained in a specific pathogen-free state under a strict light cycle (lights on at 08:00 h and off at 20:00 h) at 23 °C ± 2 °C and 50% ± 10% relative humidity. The mice were housed in the Pusan National University-Laboratory Animal Resources Center accredited by the Korea Food and Drug Administration in accordance with the Laboratory Animal Act (Accredited Unit Number-000231) and AAALAC International according to the National Institutes of Health guidelines (Accredited Unit Number; 001525).
Experimental design using IL-4/Luc/CNS-1 Tg mice. IL-4/Luc/CNS-1 used this study were firstly developed to evaluate three types of allergens including a respiratory sensitizer, vaccine additives, and crude extracts of natural allergens in vivo because IL-4 signaling pathway has been identified as a potentially important pathway in the development of allergies.Citation15) This mouse has been considered as an animal model for the evaluation and prediction of the human body response to a variety of allergens originating from the environment as well as the therapeutic effect of anti-allergic substance. Furthermore, this model can be applied to investigate the role of IL-4 during various anti-inflammatory conditions.
The protocols for the animal experiment were carefully reviewed for ethical and scientific care procedures and approved by the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC; Approval Number PNU-2014-0574).
IL-4/Luc/CNS-1 Tg mice (eight-week-old, n = 20) were randomly divided into one of two groups. In the first group (AOO, n = 5), 100 μl of acetone-olive oil (AOO) was spread on the dorsum of the ears three times a week for four weeks as a control. In the second group (PA, n = 15), 100 μl of 15% PA solution in vehicle (4:1 acetone: olive oil, v/v: AOO) was repeatedly spread on the dorsum of the ears three times a week for four weeks. The second group was further divided into three treatment groups, PA + Vehicle, PA + DG25, and PA + DG50, which received PA in combination with a comparable volume of water, 25 mg/kg DG (Sigma-Aldrich Co., St. Louis, MO, USA), or 50 mg/kg of DG, respectively, via oral administration (Fig. (A)).
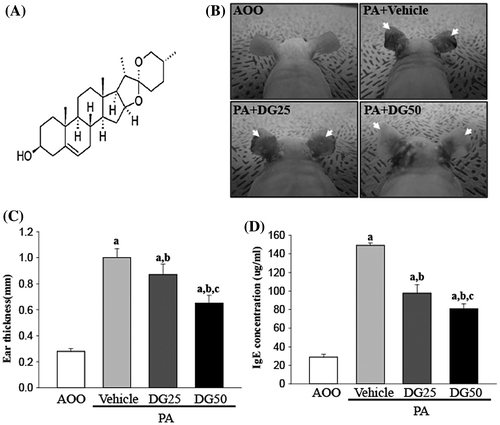
Fig. 1. Chemical structure of DG and ear morphological analysis of IL-4/Luc/CNS-1 Tg mice.
Production and identification of IL-4/Luc/CNS-1 Tg mice
IL-4/Luc/CNS-1 Tg mice for the animal experiment were produced using the breeding method described in previous studies.Citation16) Large numbers of IL-4/Luc/CNS-1 Tg mice and Non-Tg littermates were produced by breeding IL-4/Luc/CNS-1 Tg mice and HR1 mice. Founder mice containing the IL-4/Luc/CNS-1 transgene were then identified by DNA-PCR of tail-derived genomic DNA. For DNA-PCR, 10-pmol each of sense (5′-CTC GCA TGC CAG AGA TCC TA-3′) and antisense (5′-CCA CAA CCT TCG CTT CAA AA-3′) primers were added into the genomic DNA template mixture. Amplification was conducted in a thermal cycler (Perkin-Elmer, Waltham, MA, USA) by subjecting the samples to 25 cycles of 30 s at 94 °C, 30 s at 62 °C, and 45 s at 72 °C. The amplified PCR products were then separated by 1% agarose gel electrophoresis, after which the band patterns were detected using a Kodak Electrophoresis Documentation and Analysis System 120 (Eastman Kodak, Rochester, NY, USA).
Observation of ear morphology and measurement of ear thickness
Changes in ear color, ear vein thickness, and other morphological characteristics were analyzed using photographs. Additionally, ear thickness was measured to determine the degree of skin inflammation induced by PA + Vehicle and PA + DG treatment using a thickness gage (Digimatic Indicator, Matusutoyo Co., Tokyo, Japan).
Measurement of body and organ weight
The body weights of all animals in subset groups were measured using an electronic balance (Mettler Toledo, Greifensee, Switzerland) throughout the experimental period. Additionally, three major immune organs, the lymph node, thymus, and spleen, were collected from scarified mice and weighed using an electronic balance after the final treatment.
Bioluminescence imaging analysis
In vivo imaging analysis to detect luciferase-derived signal was conducted using an IVIS imaging system (Xenogen, Oakland, CA, USA) as previously described.Citation17) Briefly, IL-4/Luc/CNS-1 Tg mice were anesthetized with Zoletil and injected i.p. with 150 mg/kg of D-luciferin (Sigma-Aldrich Co.). Ten minutes after D-luciferin injection, whole body and organ images of mice were taken for 3 min using an IVIS imaging system, after which the photons emitted from specific regions were quantified using the Living Image software (Xenogen). The in vivo luciferase activity was then expressed in photons per second.
Enzyme-linked immunosorbent assay for detection of serum IgE concentration
The serum IgE concentration was measured using an ELISA kit (Shibayagi Inc., Gunma, Japan) according to the manufacturer’s instructions. Briefly, wells coated with antibody were washed three times with washing solution (50-mM Tris, 0.14 M NaCl, 0.05% Tween 20, pH 8.0), after which 50 μl of serum samples and standards diluted 20-fold with dilution solution were added to the wells and the plate was incubated for 2 h at room temperature. Next, the wells were washed with the aforementioned solution, after which 50 μl of biotin-conjugated avidin was added and the samples were incubated for 2 h. Horseradish peroxidase-conjugated detection antibodies were then diluted 5,000 fold with conjugate diluent (50 mM Tris, 0.14 M NaCl, 1% BSA, 0.05% Tween 20, pH 8.0) and transferred to each well. The plates were subsequently incubated at room temperature for 1 h, after which they were washed three times with washing solution. An enzyme reaction was then initiated by adding substrate solution and incubating the plates at room temperature in the dark for 20 min. Finally, the reaction was terminated by adding 2 M H2SO4 solution and the absorbance was measured at 450 nm.
Histological analysis
Ear tissues were removed from Non-Tg and IL-4/Luc/CNS-1 Tg mice, fixed in 10% formalin, embedded in paraffin wax, routinely processed, and then sectioned into 5-μm thick slices. Next, the skin sections were stained with hematoxylin and eosin (H&E), after which they were examined by light microscopy for the presence of immune cell accumulation. The thickness of the epidermis and dermis were also measured using the Leica Application Suite (Leica Microsystems, Wetzlar, Germany). In addition, the infiltration of mast cells into ear tissue was detected by staining with toluidine blue as previously described.Citation18) After deparaffinization and dehydration, ear skin sections were stained with 0.25% toluidine blue (Sigma-Aldrich Co.) and examined by light microscopy for the presence of mast cells. The number of cells per specific area was determined using the Leica Application Suite (Leica Microsystems).
Western blotting
Ear tissues and lymph nodes collected from a subset of the groups were homogenized using a PRO-PREP™ Solution Kit (iNtRON Biotechnology, Sungnam, Korea) supplemented with half of a protein inhibitor cocktail tablet (Roche, Penzberg, Germany), then centrifuged at 10,000×g for 10 min. The prepared proteins were subsequently subjected to 10% SDS-PAGE, after which they were transferred to a nitrocellulose membrane (Amersham Biosciences, Corston, UK) for 2 h at 45 V in transfer buffer (25 mM Trizma-base, 192 mM glycine, and 20% methanol). The efficiency of the transfer and equal protein loading were evaluated by staining the membrane with Amido Black Staining Solution (Sigma-Aldrich Co.) and the gel with Coomassie Blue. Appropriate dilutions of primary antibodies, anti-IL-6 antibody (Santa Cruz Biotechnology, TX, USA), anti-VEGF antibody (Pepro Tech., NJ, USA), and anti-β-actin (Sigma-Aldrich Co.) were added to the membranes and allowed to hybridize overnight at 4°C. After the antibodies were removed, the membrane was washed three times in a solution composed of 10 mM Trizma-base (pH 7.6), 150 mM NaCl, and 0.05% Tween-20 for 10 min. The membrane was subsequently incubated with horseradish peroxidase-conjugated anti-secondary antibody for 1 h at room temperature, after which it was washed again as described above and developed using an enhanced chemiluminescence detection system (Amersham Biosciences). Finally, the results were quantified using the Image Analyzer System (Eastman Kodak 2000MM, Eastman Kodak, Rochester, NY, USA) and expressed as the fold-increase over control values. All results were confirmed by two independent researchers conducting the experiments at least twice.
Statistical analysis
One-way ANOVA was used to identify significant differences between the PA and AOO treated groups (SPSS for Windows, Release 10.10, Standard Version, Chicago, IL, USA). Additionally, differences between the PA + Vehicle treated group and the PA + DG treated group were evaluated by a post hoc test (SPSS for Windows, Release 10.10, Standard Version) of the variance and significance levels. All values were expressed as the means ± SD. A p value of <0.05 was considered significant.
Results
Recovery effect of DG treatment on ear thickness and morphology
To determine if DG treatment can alter the ear phenotypes of PA-induced skin inflammation, the macroscopic morphology and thickness of the ear were observed in IL-4/Luc/CNS-1 Tg mice over 4 weeks. In the PA + Vehicle treated group, ear color changed greatly from a flesh tint to dark brown relative to the AOO treated group, while the outline of the ear vein became cleared or thickened. However, these alterations in ear morphology were rapidly recovered in the PA + DG25 or PA + DG50 cotreated group (Fig. (B)). In addition, ear thickness was significantly increased by three times in the PA + Vehicle treated group relative to the AOO treated group. Both PA + DG25 treated groups showed a lower increase in ear thickness of 17–39%, and the thickness of the PA + DG50 treated group was greater than that of the PA + DG25 treated group (Fig. (C)). Therefore, these findings demonstrate that DG treatment may successfully relieve the increase in ear thickness and severity of alterations in ear morphological features induced by PA treatment.
Suppressive effect of DG treatment on the luciferase signal of IL-4/Luc/CNS-1 Tg mice
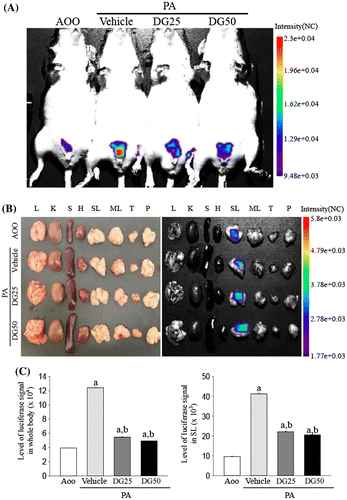
We next investigated whether DG treatment could improve the skin inflammation induced by PA treatment using a luciferase reporter system. To accomplish this, the luciferase signals were measured throughout the body and in eight organs of IL-4/Luc/CNS-1 Tg mice after individual PA + Vehicle or PA + DG treatments using the Living Image software. In the whole body image, high luciferase signals were only detected in the abdominal region of IL-4/Luc/CNS-1 Tg mice treated with PA + Vehicle, while very low levels were measured in the AOO treated group. This signal decreased greatly after PA + DG25 and PA + DG50 treatment, although the difference between groups was very small (Fig. (A) and (C)). In the organ image, high luciferase signals were observed in SL among the eight investigated organs of IL-4/Luc/CNS-1 Tg mice treated with PA, while low levels were observed in the same organs of the AOO treated group. This signal was dramatically decreased in the SL of the PA + DG25 or PA + DG50 cotreated IL-4/Luc/CNS-1 Tg mice, although it was not completely recovered to that of the AOO treated group (Fig. (B) and (C)). Therefore, these results suggest that relief of skin inflammation phenotypes may be induced by DG treatment via suppression of IL-4 promoter activity.
Fig. 2. Measurement of luciferase signal in the whole body (A) and each organ (B) of IL-4/Luc/CNS-1 Tg mice.
Suppressive effect of DG treatment on the weight of immune organs and IgE concentration
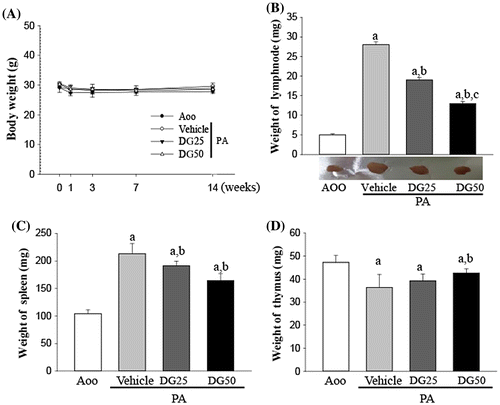
To investigate the suppressive effects of DG on increases in the weight of immune organs after PA treatment, the weight of three immune organs was evaluated in subset groups. The weight of the lymph nodes and spleen increased dramatically in response to topical application of PA, similar to the results observed upon luciferase signal analysis, although the body weight remained at a constant level (Fig. (A)). However, the weight of the lymph nodes and spleen decreased significantly by 33–56% and 14–26%, respectively, in the PA + DG treated group, while those of the AOO treated group remained constant (Fig. (B) and (C)). The opposite pattern was observed for the weight of the thymus. Specifically, the group that received topically applied PA showed a lower thymus weight than the AOO treated group. This level gradually increased in the PA + DG treated group with increasing dose, although a significant increase was only detected in the thymus of the PA + DG50 treated group (Fig. (D)).
Fig. 3. Body and organ weight analysis.
Effect of DG treatment on IgE concentration
Next, alterations in the serum IgE concentrations were measured in the PA + Vehicle and PA + DG treated groups to determine if DG contributed to the suppression of IgE secretion associated with PA-induced skin inflammation. A rapid increase in serum IgE concentrations was detected in IL-4/Luc/CNS-1 Tg mice after repeated topical application of PA solution. However, these levels were significantly decreased by 37 and 41% in the PA + DG25 and PA + DG50 treated groups, respectively (Fig. (D)). These results indicate that DG treatment contributes to decreased IgE concentration in IL-4/Luc/CNS-1 Tg mice.
Effect of DG treatment on histopathological changes in ear tissue
To evaluate the suppressive effects of DG treatment on alterations of ear histology, the histopathological features of ear tissue of IL-4/Luc/CNS-1 Tg mice were evaluated after DG treatment for 4 weeks. Increased thickness of the epidermis and dermis of the ear tissue was observed in the PA + Vehicle treated group relative to the AOO treated group (Fig. ). However, these levels decreased significantly after DG cotreatment (dermis; 44–53%, epidermis; 46–51%), although the effects were not dose dependent in the PA + DG25 or PA + DG50 treated group (Fig. ). Moreover, thick scabs were observed in the skin of the PA + Vehicle treated group, while small thin scabs were detected in the same region of the PA + DG25 treated group and detachment of all scabs was observed in the PA + DG50 treated group. Furthermore, a large number of lymphocytes infiltrated the dermis under the scabs in the PA + Vehicle treated group. After PA + DG50 cotreatment, the infiltrated lymphocytes completely disappeared from these regions, although the level in the PA + DG25 treated group remained nearly the same (Fig. ). Taken together, these results suggest that DG treatment may improve the PA-induced skin inflammation, while DG50 treatment led to complete recovery.
Fig. 4. Histopathological analysis of ear tissue.
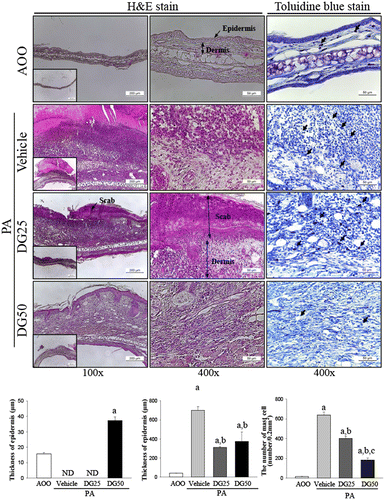
Effects of DG treatment on the infiltration of mast cells
Various inflammatory responses including asthma, eczema, itch, allergic rhinitis, allergic conjunctivitis, and skin inflammation have been shown to be closely correlated with the number of infiltrated mast cells.Citation19) Therefore, tissue sections for ear skin were stained with toluidine blue and the number of infiltrated mast cells was counted to investigate the suppressive effects of DG against infiltration of mast cells. The PA + Vehicle treated group showed a high number of mast cells stained blue in the dermis relative to the AOO treated group; however, the number of stained cells was significantly lower in the PA + DG25 (67%) and PA + DG50 (70%) cotreated groups (Fig. ). Therefore, the present results indicate that DG contributes to the suppression of mast cells infiltration in the dermis of ear skin.
Effects of DG treatment on the expression of inflammatory cytokines
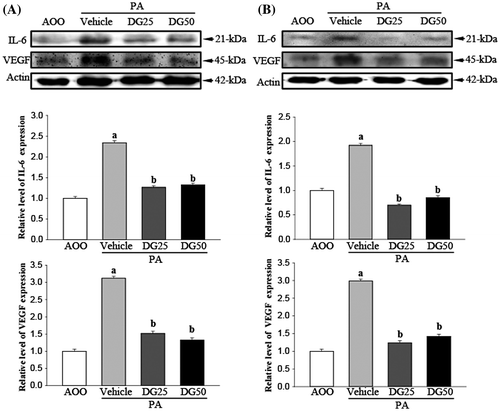
To determine if DG treatment could induce alterations in skin inflammation related cytokines expression, alterations in the expression of IL-6 and VEGF protein were measured in the ear tissue (Fig. (A)) and lymph nodes (Fig. (B)) of subset groups. The pattern of IL-6 and VEGF expression in both groups was similar to the intensity of the luciferase signal, even if the rate of decrease varied among groups. The expression of IL-6 and VEGF protein was higher in the PA + Vehicle treated group than the AOO treated group; however, these levels were dramatically decreased to the level of the AOO treated group in the PA + DG25 and PA + DG50 treated groups (Fig. ). Overall, the above results indicate that regulation of IL-6 and VEGF expression may improve the skin inflammation induced by long periods of DG treatment.
Fig. 5. Analysis of cytokine expression in ear tissues (A) and lymph nodes (B).
Discussion
Saponins have long been considered precursor drugs in the pharmaceutical industry and target molecules for the treatment of diverse chronic diseases because they possess a wide range of beneficial health properties.Citation20,21) Chemically, saponins are classified into two different groups according to the chemical nature of the aglycone, steroids (c-27), and triterpenoid (c-30) glycosides.Citation22) Based on the above structural properties, they include various structural analogs such as dioscine, sarsaponin, ginsenosides, panaxosides, aescin, glycyrrhizin and senegins, which originate from various plants.Citation23) Among these, DG has received a great deal of attention as a novel therapeutic drug candidate for the treatment of various diseases. A number of related studies are currently being conducted to investigate their mechanism of action and novel functions. In an effort to develop drugs for the treatment of inflammatory response, we investigated the suppressive effects of DG in IL-4/Luc/CNS-1 Tg mice after PA treatment. Our results provide important evidence that DG treatment is associated with the suppression of two important cytokines involved in skin inflammation (IL-4 and IL-6).
Generally, a network of secreted cytokines contributes to the regulation of inflammatory and immune response. Cytokines secreted from Th1 cells, including IFN-γ, induce the activation of cellular immunity, whereas Th2-secreted cytokines including IL-4 stimulate humoral immunity.Citation24,25) Although IL-4 plays an important role in the regulation of inflammatory responses, OVA-sensitized BALB/c mice showed different responses to DG treatment. IL-4 secretion from splenocytes and IL-4 mRNA expression in lungs were maintained at constant levels after treatment with 200–400 mg/kg body weight DG for 34 days, while OVA-specific IgG2 levels were enhanced in the serum of these animals.Citation11) However, the expression of IL-4 and GATA-3 proteins was markedly increased in duodenal tissues of OVA-sensitized BALB/c mice after treatment with 100–200 mg/kg body weight DG for 49 days.Citation13) In the present study, the luciferase signal originating from the IL-4/Luc/CNS-1 construct was significantly enhanced in the PA + Vehicle treated group. Following treatment with two concentrations of DG, this signal was markedly decreased in the ventral region of the entire body and SL (Fig. ). These results agreed with those of previous studies in which IL-4 expression in the lung and spleen was not affected by the DG treatment in OVA-sensitized BALB/c mice.Citation11) However, the results of previous studies showing that DG treatment induced IL-4 expression in duodenal tissues were very different from those of the present study, which was likely due to differences in the properties of sensitizing compounds and the specificity of target organs of the inflammatory response. Meanwhile, some difference in the organ type displaying Luc signal in IL-4/Luc/CNS-1 Tg mice after PA + DG treatment. Luc signal in early study was majorly detected in lung, thymus, and SL,Citation15) while this signal in several late studies was observed in thymus, SL, and pancreas.Citation26,27,28) In this study, Luc signal was detected in only SL of IL-4/Luc/CNS-1 Tg mice treated with PA or PA + DG and this pattern was very similar with the results reported by Kwak et al. Citation28). Therefore, the difference between Luc signal showing organs should be considered during the investigation of anti-inflammatory substance using IL-4/Luc/CNS-1 Tg mice although it has difficult to provide exact cause for above difference.
PA applied in this study is a low-molecular-weight organic compound industrially used in the large-scale production of plasticizers, alkyl and polyester paint resin, and as a curing agent for epoxy resins because they are highly reactive and act to cross-link polymer.Citation29,30) However, exposure to PA causes occupational irritation, immunological-mediated allergic diseases including asthma, contact urticarial, rhinitis, conjunctivitis and atopic dermatitis, and direct skin test sensitivity.Citation31,32) Among the organs of human body, the eye, skin, and respiratory tract are irritated and sensitized by PA in the various forms including vapor, fume, and dust.Citation29,33) Especially, PA treatment may tightly implicate with a characteristic feature of type I containing IgE production, infiltration of mast cell, and atopic dermatitis Citation15,34,35) as well as type IV hypersensitivity including activation of Th cells and cytokine secretion.Citation27,36) In our studies, similar responses for PA treatment reported in previous studies were also observed in the various regions of IL-4/Luc/CNS-1 Tg mice exposed with PA although their magnitude was differenced in each study.
It is well known that the hyperproduction of IgE is an indicator of the magnitude of allergic immune response, as well as a characteristic feature of type 1 hypersensitivity.Citation37) However, there are conflicting results regarding the effects of DG treatment on OVA-sensitized BALB/c mice. The serum concentration of OVA-specific IgE was not changed in response to treatment with 200–400 mg/kg BW of DG for 34 days, while OVA-specific IgG2 increased in response to the same conditions.Citation11) Nevertheless, the suppression of IgE production in serum was suppressed in OVA-sensitized BALB/c mice treated with 100–200 mg/kg BW DG for 49 days.Citation12) In a present study, the increased IgE concentration in serum decreased by 37–41% in the PA + DG25 and PA + DG50 treated group. These findings are in agreement with those of previous reports in which DG treatment was found to suppress IgE production. Therefore, the data presented in our study provide additional strong evidence that DG may contribute to attenuation of inflammatory and allergic responses through the regulation of IgE production.
Mast cells have been shown to regulate the protection of immune responses to some parasites and bacteria, as well as allergic disorders and IgE-mediated immediate hypersensitivity.Citation38,39) Therefore, in this study, the number of mast cells in the ear section of IL-4/Luc/CNS-1 Tg mice was measured in the PA + DG treated group. As shown in Fig. , a higher number of mast cells infiltrated into the dermis of the PA + Vehicle treated group than the AOO treated group, but a low number of mast cells was observed in the PA + DG25 and PA + DG50 treated groups. These results are in accordance with those of previous studies that investigated the suppressive effects of DG in the duodenum of OVA-sensitized BALB/c mice. Following the induction of intestinal allergic responses, DG treatment (100–200 mg/kg BW) attenuated the degranulation and infiltration of mast cells in the duodenum.Citation12)
Meanwhile, there are some inconsistencies on the dose-dependent response for ear phenotype, IgE concentration, immune organs and cells, and cytokines level. As show Figs. and , the suppression on the ear thickness, IgE production, and lymph node weight were significantly enhanced with an increase in the DG concentration. But, the suppression effect of DG on IL-4 luciferase signal, IL-6 expression, and VEGF expression was not depend on the concentration of DG (Figs. and ). We think that these the difference on the suppression effects of DG may have correlated with different responses of hypersensitivity induced by PA treatment. Type I hypersensitivity is known as anaphylactic hypersensitivity including IgE production, mast cell degranulation, and release of hydrolytic enzyme from eosinophils,Citation40) while type IV, delayed type hypersensitivity, is characterized by activation of T lymphocytes, monocytes and macrophage, and secretion of cytokines.Citation41) Therefore, we think that minimum effective concentration of DG against PA exposure may differ between characteristic feature of type I and type IV hypersensitivity. However, more studies are needed to understand the molecular mechanism of DG in the response of each type and to determine a effective concentration.
It has been found that action mechanism of DG was investigated in an OVA-induced intestinal allergic model, murine primary splenocytes, and lipopolysaccharide (LPS)-induced acute lung injury model. In intestine of above model, DG successfully enhanced the expression of IFN-γ and IgG2 level through the up-regulation of Th1 cell differentiation which is stimulated by an increase in the T-bet expression.Citation11,13) Also, this function of DG was associated with suppression of IgE production mast cell infiltration and degranulation.Citation12) Furthermore, DG suppressed the cytokine production of IL-2 and IL-10 as well as increased the secretion level of IFN-γ through the modulation of T cell immune responses in primary splenocytes from female BALB/c mice.Citation42) Moreover, in the lung injury model, the histological change of lung and infilteration of inflammatory cells were attenuated by DG treatment via the suppression of the phosphorylation of NF-κB p50/p65 and MAPK/p38, and the expression of iNOS.Citation43) In our study, the similar mechanism reported in previous studies was also observed in PA-induced skin inflammation model although there are few differences on the analysis factors and their magnitude. Therefore, the present results firstly provide additional evidence for the inhibiton mechanism of DG on the PA-induced skin inflammation as well as the role of IL-4 cytokine during anti-inflammatory activity of DG.
Overall, we investigated the suppressive effects of DG in skin inflammation induced by PA treatment using the inflammatory markers and luciferase signal in IL-4/Luc/CNS-1 Tg mice. The luciferase signal and general phenotypes of skin inflammation successfully reflected the therapeutic effects of DG on inflammatory response, and showed a correlation between DG treatment and IL-4 expression. Therefore, the results of the present study indicate that DG should be considered a candidate for the treatment and prevention of inflammatory reactions in the skin.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
We thank Jin Hyang Hwang, the animal technicians, for directing the Animal Facility and Care at the Laboratory Animal Resources Center.
Additional information
Funding
References
- Taylor WG, Elder JL, Chang PR, et al. Microdetermination of diosgenin from fenugreek (Trigonella foenum-graecum) seeds. J. Agric. Food Chem. 2000;48:5206–5210.10.1021/jf000467t
- Marker RE, Krueger J. Sterols. CXII. Sapogenins. XLI. The preparation of Trillin and its conversion to progesterone. J. Am. Chem. Soc. 1940;62:3349–3350.10.1021/ja01869a023
- Raju J, Mehta R. Cancer chemopreventive and therapeutic effects of diosgenin, a food saponin. Nutr. Cancer. 2009;61:27–35.10.1080/01635580802357352
- Jung DH, Park HJ, Byun HE, et al. Diosgenin inhibits macrophage-derived inflammatory mediators through downregulation of CK2, JNK, NF-κB and AP-1 activation. Int. Immunopharmacol. 2010;10:1047–1054.10.1016/j.intimp.2010.06.004
- Jagadeesan J, Nandakumar N, Rengarajan T, et al. Diosgenin, a steroidal saponin, exhibits anticancer activity by attenuating lipid peroxidation via enhancing antioxidant defense system during NMU-induced breast carcinoma. J. Environ. Pathol. Toxicol. Oncol. 2012;31:121–129.10.1615/JEnvironPatholToxicolOncol.v31.i2
- Raju J, Patlolla JM, Swamy MV, et al. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol. Biomarkers Prev. 2004;13:1392–1398.
- Wang YJ, Pan KL, Hsieh TC, et al. Diosgenin, a plant-derived sapogenin, exhibits antiviral activity in vitro against hepatitis C virus. J. Nat. Prod. 2011;74:580–584.10.1021/np100578u
- McAnuff MA, Harding WW, Omoruyi FO, et al. Hypoglycemic effects of steroidal sapogenins isolated from Jamaican bitter yam, Dioscorea polygonoides. Food Chem. Toxicol. 2005;43:1667–1672.10.1016/j.fct.2005.05.008
- Juarez-Oropeza MA, Diaz-Zagoya JC, Rabinowitz JL. In vivo and in vitro studies of hypocholesterolemic effects of diosgenin in rats. Int. J. Biochem. 1987;19:679–683.10.1016/0020-711X(87)90080-2
- Kwon CS, Sohn HY, Kim SH, et al. Anti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodents. Biosci. Biotechnol. Biochem. 2003;67:1451–1456.10.1271/bbb.67.1451
- Jan TR, Wey SP, Kuan CC, et al. Diosgenin, a steroidal sapogenin, enhances antigen-specific IgG 2a and interferon-γ expression in ovalbumin-sensitized BALB/c mice. Planta Med. 2007;73:421–426.10.1055/s-2007-967169
- Huang CH, Ku CY, Jan TR. Diosgenin attenuates allergen-induced intestinal inflammation and IgE production in a murine model of food allergy. Planta Med. 2009;75:1300–1305.10.1055/s-0029-1185578
- Huang CH, Liu DZ, Jan TR. Diosgenin, a plant-derived sapogenin, enhances regulatory T-cell immunity in the intestine of mice with food allergy. J. Nat. Prod. 2010;73:1033–1037.10.1021/np900690z
- Boguniewicz M. Update on atopic dermatitis: insights into pathogenesis and new treatment paradigms. Allergy Asthma Proc. 2004;25:279–282.
- Bae CJ, Lee JW, Bae HS, et al. Detection of allergenic compounds using an IL-4/Luciferase/CNS-1 transgenic mice model. Toxicol. Sci. 2011;120:349–359.10.1093/toxsci/kfr004
- Choi SI, Lee HR, Goo JS, et al. Effects of steaming time and frequency for manufactured red Liriope platyphylla on the insulin secretion ability and insulin receptor signaling pathway. Lab. Anim. Res. 2011;27:117–126.10.5625/lar.2011.27.2.117
- Kim HJ, Kim J, Kim SJ, et al. Anti-inflammatory effect of quercetin on picryl chloride-induced contact dermatitis in BALB/c mice. Lab. Anim. Res. 2010;26:7–13.10.5625/lar.2010.26.1.7
- Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2003;111:486–494.10.1067/mai.2003.120
- Shi J, Arunasalam K, Yeung D, et al. Saponins from edible legumes: chemistry, processing, and health benefits. J. Med. Food. 2004;7:67–78.10.1089/109662004322984734
- Rao AV, Gurfinkel DM. The bioactivity of saponins: triterpenoid and steroidal glycosides. Drug Metab. Drug Interact. 2000;17:211–235.
- Price KR, Johnson IT, Fenwick GR. The chemistry and biological significance of saponins in foods and feeding stuffs. Crit Rev. Food Sci. Nutr. 1987;26:127–135.
- Thakur M, Melzig MF, Fuchs H, et al. Chemistry and pharmacology of saponins: special focus on cytotoxic properties. Botanics: Targets Ther. 2011;1:19-29.
- Oppenheim JJ, Neta R. Pathophysiological roles of cytokines in development, immunity, and inflammation. FASEB J. 1994;8:158–162.
- Ohmori Y, Hamilton TA. STAT6 is required for the anti-inflammatory activity of interleukin-4 in mouse peritoneal macrophages. J. Biol. Chem. 1998;273:29202–29209.10.1074/jbc.273.44.29202
- Gao XK, Nakamura N, Fuseda K, et al. Establishment of allergic dermatitis in NC-Nga mice as a model for severe atopic dermatitis. Biol. Pharm. Bull. 2004;27:1376–1381.10.1248/bpb.27.1376
- Kwak MH, Kim JE, Hwang IS, et al. Quantitative evaluation of therapeutic effect of Liriope platyphylla on phthalic anhydride-induced atopic dermatitis in IL-4/Luc/CNS-1 Tg mice. J. Ethnopharmacol. 2013;148:880–889.10.1016/j.jep.2013.05.036
- Lee YJ, Kim JE, Kwak MH, et al. Quantitative evaluation of the therapeutic effect of fermented soybean products containing a high concentration of GABA on phthalic anhydride-induced atopic dermatitis in IL-4/Luc/CNS-1 Tg mice. Int. J. Mol. Med. 2014;33:1185–1194.
- Kwak MH, Kim JE, Koh J, et al. Characterization of allergic response induced by repeated dermal exposure of IL-4/Luc/CNS-1 transgenic mice to low dose formaldehyde. Lab. Anim. Res. 2014;30:95–103.10.5625/lar.2014.30.3.95
- Dearman RJ, Kimber I. Divergent immune response to respiratory and contact chemical allergens: antibody elicited by phthalic anhydride and oxazolone. Clin. Exp. Allergy. 2014;22:241–250.
- Towae FK, Enke W, Jäckh R, et al. Phthalic acid and derivatives. In: Elvers, B, Hawkins S, Schulz G, editors. Ullmann’s encyclopedia of industrial chemistry, Vol. A20, 5th rev. Ed.. New York (NY), VCH; 1992. p. 181–211.
- Keren RA. Asthma and allergic rhinitis due to sensitization to phthalic anhydride. J. Allergy. 1939;10:164.10.1016/S0021-8707(39)90050-X
- Venables K. Low molecular weight chemicals, hypersensitivity and direct toxicity: acid anhydrides. Br. J. Industrial Med. 1989;46:222–232.
- Ban M, Hettich D. Effect of Th2 cytokine antagonist treatments on chemical-induced allergic response in mice. J. Appl. Toxicol. 2005;25:239–247.10.1002/(ISSN)1099-1263
- Moller DR, Gallagher JS, Bemstein DI, et al. Detection of IgE-mediated respiratory sensitization in workers exposed to hexahydrophthalic anhydride. J. Allergy Clin. Immunol. 1985;75:663–672.10.1016/0091-6749(85)90091-0
- Pakarinen M, Koivuluhta M, Kalkkinen N, et al. Phthalic anhydride allergy: development and characterization of optimized hapten-carrier conjugates for improved diagnosis. Allergy. 2002;57:894-899. 10.1034/j.1398-9995.2002.23579.x
- Mok JY, Jeon IH, Cho JK, et al. Effect of persimmon leaf extract on phthalic anhydride-induced allergic response in mice. Prev. Nutr. Food Sci. 2012;17:14–21.10.3746/pnf.2012.17.1.014
- Dearman RJ, Skinner A, Humphreys NE, et al. Methods for the identification of chemical respiratory allergens in rodents: comparisons of cytokine profiling with induced changes in serum IgE. J. Appl. Toxicol. 2003;23:199–207.10.1002/(ISSN)1099-1263
- Kawakami T, Ando T, Kimura M, et al. Mast cells in atopic dermatitis. Curr. Opin. Hematol. 2009;21:666–678.
- Galli SJ. Mast cells and basophils. Curr. Opin. Hematol. 2000;7:32–39.10.1097/00062752-200001000-00007
- Bannon GA. Hypersensitivity: anaphylactic (Type I). In: Encyclopedia of life sciences (ELS). Chichester: John Wiley & Sons, Ltd; 2005. DOI: 10.1038/npg.els.0003999
- Actor JK, Ampel NM. Hypersensitivity: T Lymphocyte-mediated (Type IV). In: Encyclopedia of life sciences (ELS). Chichester: John Wiley & Sons, Ltd; 2009. DOI: 10.1002/9780470015902.a0001139.pub2
- Ku CM, Lin JY. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013;141:1104–1113.10.1016/j.foodchem.2013.04.044
- Gao M, Chen L, Yu H, et al. Diosgenin down-regulates NF-κB p65/p50 and p38MAPK pathways and attenuates acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2013;15:240–245.10.1016/j.intimp.2012.11.019
