Abstract
Chloroplasts are a significant site for reactive oxygen species production under illumination and, thus, possess a well-organized antioxidant system involving ascorbate. Ascorbate recycling occurs in different manners in this system, including a dehydroascorbate reductase (DHAR) reaction. We herein investigated the physiological significance of DHAR3 in photo-oxidative stress tolerance in Arabidopsis. GFP-fused DHAR3 protein was targeted to chloroplasts in Arabidopsis leaves. A DHAR3 knockout mutant exhibited sensitivity to high light (HL). Under HL, the ascorbate redox states were similar in mutant and wild-type plants, while total ascorbate content was significantly lower in the mutant, suggesting that DHAR3 contributes, at least to some extent, to ascorbate recycling. Activation of monodehydroascorbate reductase occurred in dhar3 mutant, which might compensate for the lack of DHAR3. Interestingly, glutathione oxidation was consistently inhibited in dhar3 mutant. These findings indicate that DHAR3 regulates both ascorbate and glutathione redox states to acclimate to HL.
Graphical abstract
The redox regulation of ascorbate and glutathione by a chloroplastic dehydroascorbate reductase (DHAR3) is essential for photo-oxidative stress tolerance in Arabidopsis.
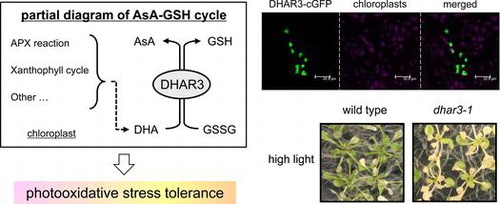
Chloroplasts are one of the most significant production sites of reactive oxygen species (ROS), which are cytotoxic molecules, in plant cells under illumination.Citation1,2) Light is necessary for the photosynthetic electron transport (PET) chain, which converts light-derived energy to biochemical compounds (i.e. ATP and NADPH) and is required for various physiological processes in chloroplasts, particularly CO2 assimilation reactions.Citation1,2) However, the high intensity of light significantly depletes NADP+, a terminal electron acceptor, which lead to the over-reduction of PET, facilitating the photo-reduction of oxygen to produce the superoxide radical (O2−).Citation1,2) Triplet chlorophyll is also formed and reacts with oxygen to produce singlet oxygen.Citation1,2) The over-reduction state of PET occurs not only under strong light, but also under conditions with a normal light intensity, such as drought, salinity, and low temperature, in which the Calvin cycle is inhibited.Citation3–5) In contrast to the oxidative stress theory, ROS have been identified as emergency signals for the activation of a set of defense pathways that allow plants to acclimate to environmental stresses.Citation6–10) A delicate balance between ROS production and scavenging in chloroplasts is critical for plant response to light-evoked oxidative stress (photo-oxidative stress).
Chloroplasts accumulate various antioxidant compounds and enzymes to maintain or modulate ROS levels.Citation1) The ascorbate-glutathione cycle is one of the major antioxidant systems in plant cells.Citation2) In this cycle, ascorbate peroxidase (APX) converts hydrogen peroxide (H2O2) to water using reduced ascorbate (ascorbic acid, AsA) as an electron donor. AsA itself can also detoxify ROS through non-enzymatic reactions. These reactions result in the formation of monodehydroascorbate (MDHA). MDHA can be reduced and recycled back to AsA through a reaction with ferredoxin (in chloroplasts) or a specific reductase (MDAR), otherwise this radical is spontaneously disproportionated into AsA and dehydroascorbate (DHA). As the final stronghold for ascorbate recycling, DHA reductase (DHAR) catalyzes the reduction of DHA to AsA using reduced glutathione (GSH) as an electron donor. Oxidized glutathione (GSSG) produced by the DHAR reaction is reduced back to GSH by glutathione reductase (GR), which uses NAD(P)H as an electron donor. In chloroplasts, the ascorbate-glutathione cycle is directly associated with PET and superoxide dismutase (SOD) reaction, which converts O2− to oxygen and H2O2, and these form the water–water cycle.Citation1) The cycle in chloroplasts is regarded as an indispensable system for plant tolerance against photo-oxidative stress.Citation11,12) Several lines of genetic evidence from Arabidopsis researches have supported this hypothesis; for example, (1) the lethality of a GR knockout mutant,Citation13) (2) the pale green phenotype of mutants lacking chloroplastic Fe-SOD,Citation14) and (3) enhanced oxidative damage in chloroplastic APX mutants.Citation15,16) In addition, ascorbate is required for other photo-protection-related pathways, including the xanthophyll cycle,Citation17) regeneration of tocopherols,Citation18,19) and biosynthesis of flavonoids.Citation20) In the xanthophyll cycle, ascorbate serves as an electron donor for enzymatic reactions catalyzing the de-epoxidation of violaxanthin to antheraxanthin and zeaxanthin, enhancing the dissipation of light energy as a heat under photo-oxidative stress conditions.Citation17)
A multilayered ascorbate recycling pathway (i.e. ferredoxin, MDAR, and DHAR) occurs in chloroplasts, which has led to the speculation that the reduction of oxidized ascorbate is an important step in the photo-protection in the organelle.Citation21) Consistent with this speculation, transgenic tobacco lines overexpressing DHAR within chloroplasts were found to be highly tolerant against chilling, salinity, and oxidative stress.Citation22,23) However, to the best of our knowledge, no information has yet been derived from a knockdown or knockout approach in order to study the function of chloroplastic DHAR. Very recently, Arabidopsis MDAR6, which is dual targeted to both chloroplasts and mitochondria,Citation24) was found to mediate the toxicity of 2,4,6-trinitrotoluene (TNT), a toxic environmental pollutant, in plant cells, and thus, mdar6 mutant was highly insensitive to TNT treatment.Citation25) In contrast, the lack of MDAR6 had no impact on the levels and redox states of ascorbate,Citation25) implying its small contribution to ascorbate recycling. Thus, the mode of action of the ascorbate recycling in chloroplasts and its physiological significance remain largely unclear.
Arabidopsis contains 3 functional DHARs,Citation26) DHAR1 (At1g19750), DHAR2 (At1g75270), and DHAR3 (At5g16710). Among them, only DHAR3 is predicted to be a chloroplastic isoform. We herein confirmed the chloroplastic localization of DHAR3 and analyzed the impact of the lack of this enzyme on the redox cycle and sensitivity to photo-oxidative stress in Arabidopsis. Our results demonstrate that DHAR3 is essential for photo-oxidative stress tolerance.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia (Col-0) was used as the wild-type plant. A T-DNA insertion line for DHAR3 in the Col-0 background, SAIL_435_A09 (dhar3), was obtained from the Arabidopsis Biological Resource Center. The surface-sterilized seeds of the wild-type and mutant plants were cold treated at 4 °C for 2–3 days before germination. Plants were grown on half-strength Murashige & Skoog medium containing 1% sucrose under 23 °C during 16 h of light (100 μmol photons m−2 s−1) and at 20 °C during 8 h of darkness. Two-week-old plants were exposed to excess light. To avoid complexity, the terms of “high light (HL)” and “extreme high light (eHL)” were used to indicate 1000 and 1500 μmol photons m−2 s−1, respectively, in this study. The collection of control (non-stressed) samples and application of stress were performed 4 h after illumination.
Semi-quantitative reverse transcription-PCR
Total RNA was isolated from the leaves of Arabidopsis plants using Sepasol-RNA I super G (Nacalai Tesque, Inc., Kyoto, Japan) according to the manufacturer’s protocol. In order to eliminate any contamination of DNA, 50 μg of total RNA was treated with DNase I (Takara, Shiga, Japan). First strand cDNA was synthesized using reverse transcriptase (ReverTra Ace; Toyobo) with an oligo dT primer. A semi-quantitative RT-PCR analysis was performed according to Ogawa et al.Citation27) Primer sequences were as follows: DHAR3-RT-F (5′-GTGCGGTTCGACTAAACCGG-3′), DHAR3-RT-R (5′-GTGGAAAAGATCTTCGATCC-3′), Actin8-RT-F (5′-GAGATCCACATCTGCTGG-3′), and Actin8-RT-R (5′-GCTGAGAGATTCAGGTGCCC-3′).
Enzyme assay
DHAR activity was measured according to Shigeoka et al.Citation28) with minor modifications. Arabidopsis leaves (0.2 g) were homogenized with 400 μl of 50 mM potassium phosphate (pH 7.5) containing 1 mM EDTA and 10% d-sorbitol. After centrifugation (20,000 × g) for 20 min at 4 °C, the supernatant was used to analyze enzymatic activity. The extract (20 μL) was added to 1 mL of reaction mixture containing 50 mM potassium phosphate (pH 7.5), 2 mM GSH, 1 mM DHA, 0.2 mM NADPH, and 1 unit GR. GSH-dependent DHA reduction was assayed spectrophotometrically at 340 nm. MDAR activity was measured according to Eltayeb et al.Citation29) with minor modifications. Arabidopsis leaves (0.2 g) were homogenized with 400 μL of 50 mM potassium phosphate (pH 7.5) containing 0.2 mM EDTA, 10 mM 2-mercaptoethanol, and 1% d-sorbitol. After centrifugation (20,000 × g) for 20 min at 4 °C, the supernatant was used to analyze enzymatic activity. The extract (20 μL) was added to 1 mL of reaction mixture containing 100 mM potassium phosphate (pH 7.5), 1 mM AsA, and 0.2 mM NADH. The reaction was started by the addition of 0.2 unit AsA oxidase after a pre-incubation for 2 min. The decrease in absorbance at 340 nm was monitored and activity was calculated using an absorbance coefficient of 6.2 mM−1 cm−1. GR activity was measured according to Foyer and Halliwell.Citation30)
Sub-cellular localization of the GFP fusion protein
The cDNA encoding DHAR3 was cloned into the donor vector, pDONR221, and then re-cloned into the destination vector, pGWB505Citation31) using GATEWAY technology, to express a chimeric protein DHAR3-cGFP, in which GFP was fused to C-terminus of DHAR3, under the control of the CaMV 35S promoter. Primer sequences were as follows: DHAR3-attB1 (5′-AAAAAGCAGGCTATGATAAGCCTTAGGTTTCAACCAAGC-3′) and DHAR3-cGFP-attB2 (5′-AGAAAGCTGGGTACCCATAACCTTTGGTCTCCAACC-3′). Italics indicate attB1/B2 sequences.
In order to transiently express the fusion protein and GFP alone, pGWB505/DHAR3 and pGWB506 (empty vector) were absorbed onto tungsten particles (1.0 μm in diameter) following the manufacturer’s instructions (Tanaka, Sapporo, Japan). Rosette leaves were harvested from 4-week-old Arabidopsis wild-type plants grown on soil under growth conditions, and then placed onto a 2% (w/v) agar plate. In each bombardment, the leaves were bombarded with 4 μL of DNA-coated tungsten particles (1 μg of DNA) placed onto the plastic holder using a GIE-III IDERA particle gun (Tanaka) at a helium pressure of 4 kgf cm−2 under a vacuum of 600 mm Hg. Following bombardment, the agar plate was filled with water to prevent desiccation. After being incubated overnight at 22 °C in the dark, the leaves were viewed with a TCS SP5 confocal laser scanning microscope (Leica Microsystems, Wetzlar, Germany) using a HCX PL APO CS 63.0 9 1.20 WATER UV objective lens. The fluorescence of GFP and chlorophyll was detected at 500–530 and 680–700 nm, respectively.
Measurement of oxidants and antioxidants
Ascorbate was measured as described previously.Citation32) AsA and DHA levels were determined spectrophotometrically using ascorbate oxidase. Arabidopsis leaves (0.2 g) frozen in liquid N2 were grounded using a mortar and pestle with 2.5 mL of 6% (v/v) HClO4 and centrifuged at 20,000 × g for 10 min at 4 °C. In order to measure AsA, a 100-μL aliquot of the obtained leaf extract was added directly to 900 μL of a 200 mM succinate buffer (pH 12.7, adjusted with NaOH) in the spectrophotometer. The final pH was very close to 6.0. AsA was determined by a change in absorbance at 265 nm following the addition of 5 units of AsA oxidase. In order to determine total ascorbate, 700 μL of the leaf extract was adjusted to pH 6.0 with K2CO3, followed by the addition of dithiothreitol, dissolved in 50 mM HEPES-KOH buffer (pH 7.5), to a final concentration of 10 mM. The resulting solution was incubated in darkness at 25 °C for 30 min. After centrifugation for 15 min at 20,000 × g (4 °C), the supernatant was assayed as described above. The amount of DHA was determined as the difference between these 2 assays. Glutathione was measured according to Shigeoka et al.Citation33)
The thiobarbituric acid test, which determines the amount of malondialdehyde as an end product of lipid peroxidation, was used to analyze lipid oxidation.Citation34) Arabidopsis leaves (0.2 g) were frozen in liquid nitrogen, grounded in 1 mL of 1% (v/v) trichloroacetic acid, and homogenized with an equal volume of 0.34% (w/v) thiobarbituric acid in 50% (v/v) acetate. The mixtures were heated in a water bath at 95 °C for 60 min and centrifuged 20,000 × g for 10 min at room temperature. The supernatants were mixed with the double equivalent of n-butanol and centrifuged at 4000 × g for 10 min at room temperature. The content of malondialdehyde was estimated by measuring the absorbance of the n-butanol layers from 532 to 600 nm. Absorbance values were converted into nanomoles using an extinction coefficient of 1.56 × 105 M−1 cm−1.Citation35)
Measurement of chlorophyll fluorescence
Chlorophyll fluorescence was measured following Yabuta et al.Citation36) The Arbidopsis plants were kept in darkness for 20 min to determine F0 and Fm, and then exposed to 5 min of actinic light to determine Fm′. Fluorescence parameter was calculated as follows: Fv/Fm = (Fm−F0)/Fm; non-photochemical quenching (NPQ) = (Fm−Fm′)/Fm′. Chlorophyll fluorescence in Arabidopsis leaves was measured at 23 °C with a Closed FluorCam 800MF (Photon Systems Instruments, Brno, Czech Republic). More than 20 independent wild-type and mutant plants were used in this experiment.
Data analysis
The statistical analysis of data was based on Student’s t-tests. Calculations were performed on more than 3 independent biological replicates. In all experiments, except for the measurement of chlorophyll fluorescence, leaves (from >20 seedlings) were pooled and used for 1 biological replicate.
Results and discussion
Sub-cellular localization of DHAR3
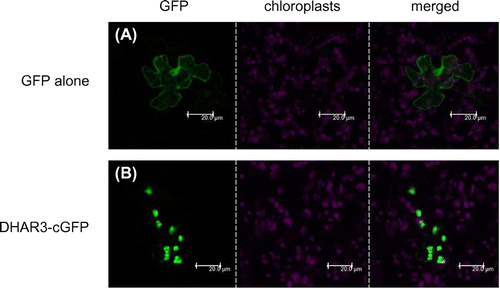
DHAR3 has been suggested to be a chloroplastic isoform in Arabidopsis because it has an N-terminal extension sequence similar to a chloroplast-targeting signal, which is absent in DHAR1 and 2 (Supplemental Fig. 1). We predicted the sub-cellular localization of DHAR3 using iPSORTCitation37,38) (http://ipsort.hgc.jp/), PredotarCitation39) (https://urgi.versailles.inra.fr/predotar/predotar.html), TargetPCitation40) (http://www.cbs.dtu.dk/services/TargetP/), and WoLF PSORTCitation41) (http://www.genscript.com/psort/wolf_psort.html). As a consequence, all programs tested successfully predicted that DHAR3 is a chloroplastic protein. This was supported by a previous proteome analysis using chloroplasts isolated from Arabidopsis leaves.Citation42,43) In order to confirm this in more detail, cDNA was cloned into the pGWB505 vector for the expression of DHAR3-cGFP fusion proteins. When the fusion proteins were transiently expressed in the leaves of 4-week-old wild-type Arabidopsis plants, GFP fluorescence clearly overlapped with the red fluorescence derived from chloroplasts (Fig. ). In contrast, the GFP protein alone was distributed in the cytosol and nucleus. These results demonstrate the chloroplastic localization of DHAR3.
Fig. 1. Sub-cellular localization of DHAR3.
Susceptibility of the dhar3 mutant to eHL
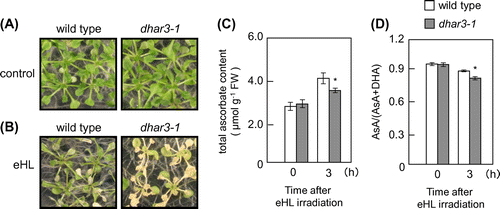
For reverse genetic approach, a T-DNA insertion line (dhar3; SAIL_435_A09) of this gene was obtained. A T-DNA insertion occurred in the third intron of the DHAR3 gene (Fig. (A)). A semi-quantitative RT-PCR analysis revealed that the line was a null mutant (Fig. (B)). Furthermore, total DHAR activity in dhar3 leaves was approximately 74% in wild-type leaves (Fig. (C)). In an attempt to understand the physiological significance of DHAR3 in photo-oxidative stress tolerance, 2-week-old wild-type and dhar3 plants were subjected to eHL at 1500 μmol m−2 s−1 for 15 h. As shown in Fig. , both plants suffered visible damage from this strong stress; however, dhar3 plants were more bleached than the wild-type plants. We then examined the effects of DHAR3 knockout on the redox states of ascorbate. Before eHL irradiation, the dhar3 mutation had no significant effect on the levels or redox states (reduced ascorbate/total) of ascorbate (Fig. (C) and (D)). While the eHL exposure for 3 h did not induce visible damage in both wild type and dhar3, this treatment enhanced ascorbate oxidation especially in the mutant (Fig. (D)). Furthermore, the lack of DHAR3 inhibited the eHL-induced accumulation of ascorbate (Fig. (C)). These findings suggested that chloroplastic DHAR is required for photo-protection through ascorbate recycling in plants under strong photo-oxidative stress conditions.
Fig. 2. Characterization of T-DNA insertion lines lacking DHAR3.
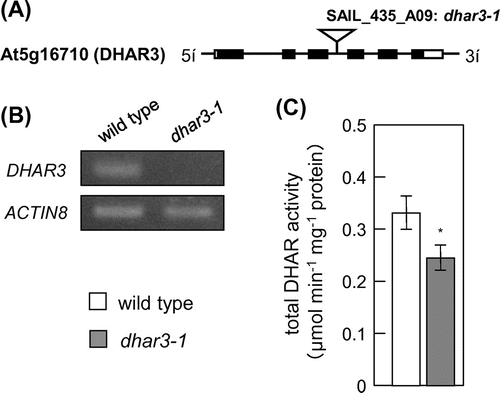
Fig. 3. Sensitivity of dhar3 mutants to photo-oxidative stresses.
Levels and redox states of ascorbate in dhar3 under HL
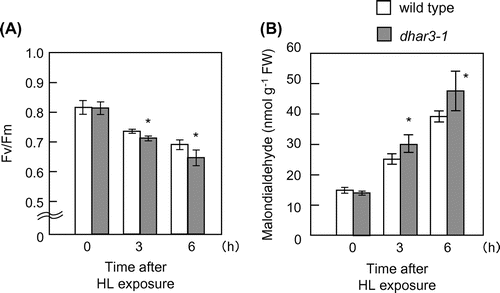
The eHL exposure easily induced visible damage on leaves under the present experimental conditions, making it difficult to understand the cause-and-effect relationship between the ascorbate redox states and dhar3 phenotype. To obtain more detailed data, we used HL at 1000 μmol photons m−2 s−1 in subsequent analyses therefore. We confirmed that dhar3 mutant had lower DHAR activity even under HL (Fig. (A)). No visible oxidative damage was observed in wild type and dhar3 when subjected to this stress for 12 h (data not shown). We evaluated oxidative stress damage in the mutant by measuring Fv/Fm, the maximum quantum yield of photosystem II. The parameter was decreased by HL irradiation in wild-type and dhar3 plants, but was more prominent in the mutant at 6 h (Fig. (A)). Furthermore, this decrease was accompanied by an increase in the levels of malondialdehyde, a marker of lipid oxidation (Fig. (B)). These findings indicated that the dhar3 mutant was more sensitive to this HL than wild type.
Fig. 4. DHAR, MDAR, and GR in dhar3 mutants under high light.
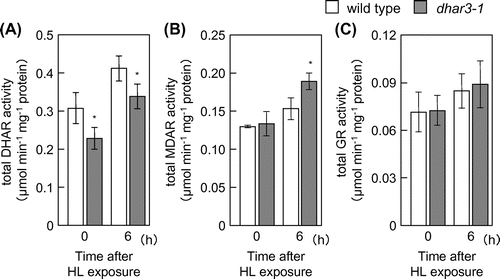
Fig. 5. Oxidative damage in dhar3 mutants under high light.
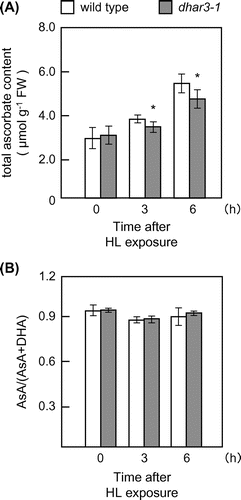
Although the HL-induced accumulation of ascorbate was slightly but significantly suppressed in the dhar3 mutant, the loss of DHAR3 had no detectable effect on the redox states of ascorbate under the stress conditions (Fig. ). At present, we do not have any robust explanation for this discrepancy. One plausible explanation might be that the loss of DHAR3 actually had an impact on the redox states of ascorbate within chloroplasts. We might not be able to detect the impact because of use of whole leaf cells for the ascorbate measurement. We also found that the dhar3 mutation actually facilitated an increase in total MDAR activity under HL (Fig. (B)). These findings suggest that DHAR3 contributed, at least to some extent, to ascorbate recycling under HL, although the lack of DHAR3 could be compensated by other mechanisms, including MDAR.
Fig. 6. Levels and redox states of ascorbate in dhar3 mutants under high light.
As described above, ascorbate is required for the xanthophyll cycle, which is one of the important processes for light acclimation. We measured NPQ to indicate the activity of the cycle in wild type and dhar3 under HL. However, there was no significant difference in this parameter between genotypes before and after HL (Supplemental Fig. 2). This result was probably because of the small difference in ascorbate content between wild type and dhar3, and suggested that DHAR3 does not contribute to the induction of NPQ at least under our experimental conditions.
Levels and redox states of glutathione in dhar3 under milder HL stress
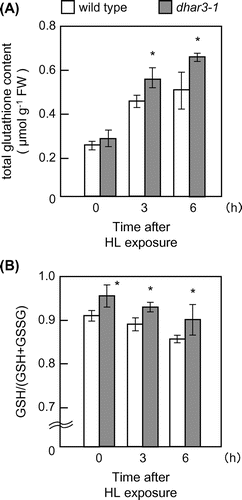
Since DHAR catalyzes the reduction of DHA using GSH as an electron donor, it is possible that DHAR contributes to redox regulation of glutathione through its oxidation. In spite of its negligible effect on ascorbate redox states, the lack of DHAR3 markedly affected the redox states of glutathione. Even in the absence of HL, the redox states of glutathione were more reduced in dhar3 mutant plants than in wild-type plants (Fig. (B)). Furthermore, redox states were decreased in response to HL in wild-type plants, but this decrease was significantly mitigated in dhar3 mutant plants (Fig. (B)). No significant difference was observed in GR activity between wild-type and dhar3 mutant plants (Fig. (C)), suggesting that the high-reduction states of glutathione in the dhar3 mutant were not associated with GR activity. These findings indicate that DHAR3 strongly contributes to the glutathione oxidation event in illuminated leaves. Furthermore, the dhar3 mutation facilitated an increase in the total glutathione content under HL (Fig. (A)). The accumulation of glutathione is a general marker of oxidative stress. We previously found that the lack of chloroplastic APX isoenzymes have the similar impact on glutathione levels under excess light.Citation16) A knockout mutant lacking catalase 2, the predominant enzyme in peroxisomes, also accumulates a large amount of glutathione under normal air conditions.Citation44) Because glutathione and its redox states have been proposed to act as a signal for stress acclimation responses,Citation45,46) the fact that the lack of DHAR3 affected only the glutathione redox states under normal light might indicate that the oxidation of glutathione by DHAR3 is an important process for plant tolerance to photo-oxidative stress.
Fig. 7. Levels and redox states of glutathione in dhar3 mutants under high light.
Conclusion
The present results provide the first genetic evidence for a chloroplastic DHAR3 being essential for photo-oxidative stress tolerance in Arabidopsis plants. However, the mode of action of DHAR3-dependent photo-protection may be complicated due to several reasons. One of the major concerns was the negligible effect of the dhar3 mutation on ascorbate redox states observed under HL. This simply implies that DHAR3 may make a small contribution to ascorbate recycling. MDAR and ferredoxin can reduce MDHA back to ascorbate before its further oxidation to DHACitation21); thus, chloroplasts have acquired multilayered pathways for ascorbate recycling, which may allow the dhar3 mutant to maintain ascorbate redox states even under HL. The physiological significance of ascorbate recycling in chloroplasts needs to be analyzed in more depth using multiple mutants of DHAR3, MDAR6, and ferredoxin in future studies. Interestingly, the levels and redox states of glutathione were largely affected by the lack of DHAR3, in particular under HL. Thus, DHAR3 regulates both ascorbate and glutathione redox states to acclimate to HL. In conclusion, our findings indicate that the redox regulation of both ascorbate and glutathione by DHAR3 in chloroplasts is required for photo-oxidative stress tolerance.
Author contributions
M.N., R.H., and Y.T. carried out the experiments. M.N. drafted the manuscript. N.T. and T.M. edited the manuscript. N.T., T.M., and S.S. conceived of the study and participated in its design and coordination.
Funding
This work was supported by a Grants-in-aid for Scientific Research (A) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan [grant number 22248042: to S.S.], and in part by the Strategic Project to Support the Formation of Research Bases at Private Universities: Matching Fund Subsidy from MEXT, 2011-2015 [grant number S1101035].
Supplemental materials
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2015.1135042.
Disclosure statement
No potential conflict of interest was reported by the authors.
Noshi-supplemental_figure.ppt
Download MS Power Point (94 KB)Supplemental_Fig-cap.docx
Download MS Word (14 KB)Acknowledgments
We are grateful to Prof. Tsuyoshi Nakagawa (Shimane University) for donating the pGWB505 and pGWB506 vector. We are thankful to Dr Kohji Nishimura (Shimane University) for excellent technical help with confocal laser scanning microscope. We are also thankful to Dr Masahiro Tamoi and Dr Takahisa Ogawa (Kinki University) for helpful discussions. We thank Ms. Naoko Okada and Mr. Kensuke Matsuda (Kinki University) for technical assistance.
Notes
Abbreviations: APX, ascorbate peroxidase; AsA, reduced ascorbate; DHA, dehydroascorbate; DHAR, dehydroascorbate reductase; eHL, extreme high light (1500 μmol photons m−2 s−1); GFP, green fluorescent protein; GR, glutathione reductase; HL, high light (1000 μmol photons m−2 s−1); H2O2, hydrogen peroxide; MDHA, monodehydroascorbate; MDAR, monodehydroascorbate reductase; NPQ, non-photochemical quenching; O2−, superoxide radical; PET, photosynthetic electron transport; ROS, reactive oxygen species; SOD, superoxide dismutase.
References
- Asada K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:601–639.10.1146/annurev.arplant.50.1.601
- Foyer CH, Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011;155:93–100.10.1104/pp.110.166181
- Sonoike K. Various aspects of inhibition of photosynthesis under light/chilling stress: “Photoinhibition at chilling temperatures” versus “chilling damage in the light”. J. Plant Res. 1998;111:121–129.10.1007/BF02507158
- Noctor G, Veljovic-Jovanovic S, Driscoll S, et al. Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann. Bot. 2002;89:841–850.10.1093/aob/mcf096
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 2009;103:551–560.
- Mittler R, Vanderauwera S, Gollery M, et al. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498.10.1016/j.tplants.2004.08.009
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875.10.1105/tpc.105.033589
- Mittler R, Vanderauwera S, Suzuki N, et al. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–309.10.1016/j.tplants.2011.03.007
- Schmitt FJ, Renger G, Friedrich T, et al. Reactive oxygen species: re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochim. Biophys. Acta. 2014;1837:835–848.10.1016/j.bbabio.2014.02.005
- Shigeoka S, Maruta T. Cellular redox regulation, signaling, and stress response in plants. Biosci. Biotechnol. Biochem. 2014;78:1457–1470.10.1080/09168451.2014.942254
- Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18.10.1104/pp.110.167569
- Shigeoka S, Ishikawa T, Tamoi M, et al. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002;53:1305–1319.10.1093/jexbot/53.372.1305
- Tzafrir I, Pena-Muralla R, Dickerman A, et al. Identification of genes required for embryo development in Arabidopsis. Plant Physiol. 2004;135:1206–1220.10.1104/pp.104.045179
- Myouga F, Hosoda C, Umezawa T, et al. A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell. 2008;20:3148–3162.10.1105/tpc.108.061341
- Kangasjärvi S, Lepistö A, Hännikäinen K, et al. Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochem. J. 2008;412:275–285.10.1042/BJ20080030
- Maruta T, Tanouchi A, Tamoi M, et al. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 2010;51:190–200.10.1093/pcp/pcp177
- Eskling M, Arvidsson PO, Akerlund HE. The xanthophyll cycle, its regulation and components. Physiol. Plant. 1997;100:806–816.10.1111/ppl.1997.100.issue-4
- Munné-Bosch S, Alegre L. Interplay between ascorbic acid and lipophilic antioxidant defences in chloroplasts of water-stressed Arabidopsis plants. FEBS Lett. 2002;524:145–148.10.1016/S0014-5793(02)03041-7
- Sattler SE, Gilliland LU, Magallanes-Lundback M, et al. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell. 2004;16:1419–1432.10.1105/tpc.021360
- Page M, Sultana N, Paszkiewicz K, et al. The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: further evidence for redox control of anthocyanin synthesis. Plant Cell Environ. 2012;35:388–404.10.1111/j.1365-3040.2011.02369.x
- Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396.10.1104/pp.106.082040
- Kwon SY, Choi SM, Ahn YO, et al. Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J. Plant Physiol. 2003;160:347–353.10.1078/0176-1617-00926
- Le Martret B, Poage M, Shiel K, et al. Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol. J. 2011;9:661–673.10.1111/pbi.2011.9.issue-6
- Chew O, Whelan J, Millar AH. Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual targeting of antioxidant defenses in plants. J. Biol. Chem. 2003;278:46869–46877.10.1074/jbc.M307525200
- Johnston EJ, Rylott EL, Beynon E, et al. Monodehydroascorbate reductase mediates TNT toxicity in plants. Science. 2015;349:1072–1075.10.1126/science.aab3472
- Dixon DP, Davis BG, Edwards R. Functional divergence in the glutathione transferase superfamily in plants. Identification of two classes with putative functions in redox homeostasis in Arabidopsis thaliana. J. Biol. Chem. 2002;277:30859–30869.10.1074/jbc.M202919200
- Ogawa T, Yoshimura K, Miyake H, et al. Molecular characterization of organelle-type nudix hydrolases in Arabidopsis. Plant Physiol. 2008;148:1412–1424.10.1104/pp.108.128413
- Shigeoka S, Nakano Y, Kitaoka S. Metabolism of hydrogen peroxide in Euglena gracilis Z by L-ascorbic acid peroxidase. Biochem. J. 1980;186:377–380.10.1042/bj1860377
- Eltayeb AE, Kawano N, Badawi GH, et al. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta. 2007;225:1255–1264.10.1007/s00425-006-0417-7
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25.10.1007/BF00386001
- Nakagawa T, Kurose T, Hino T, et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007;104:34–41.10.1263/jbb.104.34
- Maruta T, Noshi M, Tanouchi A, et al. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J. Biol. Chem. 2012;287:11717–11729.10.1074/jbc.M111.292847
- Shigeoka S, Onishi T, Nakano Y, et al. Characterization and physiological function of glutathione reductase in Euglena gracilis z. Biochem. J. 1987;242:511–515.10.1042/bj2420511
- Maruta T, Noshi M, Nakamura M, et al. Ferulic acid 5-hydroxylase 1 is essential for expression of anthocyanin biosynthesis-associated genes and anthocyanin accumulation under photooxidative stress in Arabidopsis. Plant Sci. 2014;219–220:61–68.10.1016/j.plantsci.2014.01.003
- Gueta-Dahan Y, Yaniv Z, Zilinskas BA, et al. Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in Citrus. Planta. 1997;203:460–469.10.1007/s004250050215
- Yabuta Y, Mieda T, Rapolu M, et al. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 2007;58:2661–2671.10.1093/jxb/erm124
- Bannai H, Tamada Y, Maruyama O, et al. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics. 2002;18:298–305.10.1093/bioinformatics/18.2.298
- Bannai H, Tamada Y, Maruyama O, et al. Views: fundamental building blocks in the process of knowledge discovery. Proceedings; 2001 May 21–23; Florida (USA): The 14th international FLAIRS conference; 2001.
- Small I, Peeters N, Legeai F, et al. Predotar: a tool for rapidly screening proteomes forN-terminal targeting sequences. Proteomics. 2004;4:1581–1590.10.1002/(ISSN)1615-9861
- Emanuelsson O, Nielsen H, Brunak S, et al. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 2000;300:1005–1016.10.1006/jmbi.2000.3903
- Horton P, Park KJ, Obayashi T, et al. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35:W585–W587.10.1093/nar/gkm259
- Peltier JB, Cai Y, Sun Q, et al. The oligomeric stromal proteome of Arabidopsis thaliana chloroplasts. Mol. Cell. Proteomics. 2006;5:114–133.
- Behrens C, Blume C, Senkler M, et al. The ‘protein complex proteome’ of chloroplasts in Arabidopsis thaliana. J. Proteomics. 2013;91:73–83.10.1016/j.jprot.2013.07.001
- Mhamdi A, Queval G, Chaouch S, et al. Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010;61:4197–4220.10.1093/jxb/erq282
- Tausz M, Šircelj H, Grill D. The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid? J. Exp. Bot. 2004;55:1955–1962.10.1093/jxb/erh194
- Noctor G, Mhamdi A, Chaouch S, et al. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35:454–484.10.1111/j.1365-3040.2011.02400.x
