Abstract
Pterocarpus indicus Willd has been widely used as a traditional medicine to treat edema, cancer, and hyperlipidemia, but its antiallergic properties and underlying mechanisms have not yet been studied. The purpose of this study was to evaluate the antiallergic activity of Pterocarpus indicus Willd water extract (PIW) using activated mast cells and an atopic dermatitis (AD)-like mouse model. PIW decreased IgE/Ag-induced mast cell degranulation and the phosphorylation of Syk and downstream signaling molecules such as PLC-γ, Akt, Erk 1/2, JNK compared to stimulated mast cells. In DNCB-induced AD-like mice, PIW reduced IgE level in serum, as well as AD-associated scratching behavior and skin severity score. These results indicate that PIW inhibits the allergic response by reducing mast cell activation and may have clinical potential as an antiallergic agent for disorders such as AD.
Graphical abstract
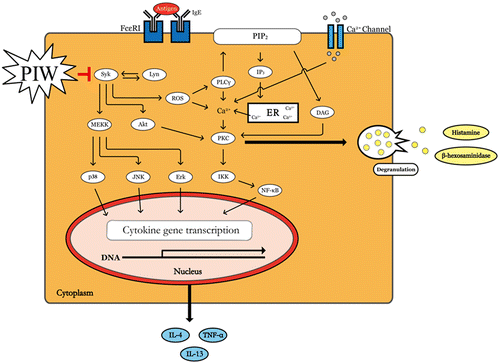
PIW suppresses IgE/Ag-stimulated degranulation via blocking the phoshorylation of Syk and its downstream signal molecules.
Mast cells play a pivotal role in allergic and inflammatory disorders, including anaphylaxis, asthma, and atopic dermatitis (AD), by undergoing rapid degranulation and releasing inflammatory cytokines.Citation1,2) Mast cells express a high-affinity receptor for immunoglobulin E (IgE), which is generated by differentiated plasma B cells.Citation3,4) Stimulation of mast cells through cross-linking of IgE-bound Fc epsilon receptor 1 (FcεRI) with antigen (Ag) leads not only to the activation of Src family tyrosine kinases, including Lyn,Citation5,6) but also to elevated intracellular levels of reactive oxygen species (ROS) that regulate degranulation and cytokine production.Citation7) Activated Lyn then phosphorylates immunoreceptor tyrosine-based activation motifs (ITAMs) of both β and γ subunits of adjacent FcεRI, and the phosphorylated β- and γ-ITAMs recruit Lyn and spleen tyrosine kinase (Syk), respectively.Citation6,8) Recruitment and activation of Syk by phosphorylated ITAM result in activation of downstream signaling pathways that lead to degranulation and release of histamines, leukotrienes, and other preformed chemical mediators.Citation8) In addition, Syk is a critical signaling molecule that activates many downstream signaling molecules such as phospholipase C-gamma (PLC-γ) and Akt. PLC-γ regulates classical protein kinase C activation through diacylglycerol generation and calcium responses, and the phosphatidylinositol-3-kinases-Akt pathway is a signal transduction pathway that promotes survival and growth in response to extracellular signals.Citation9) Further, 3 major subfamilies of mitogen-activated protein kinases (MAPKs), extracellular-signal-regulated kinases (Erks), c-Jun NH2-terminal kinases, and p38, are activated upon FcεRI cross-linking and under the control of Syk.Citation10) As a result, multiple cytokines are newly synthesized and released, triggering allergic inflammation.Citation11)
AD is a chronic and allergic inflammatory skin disease caused by a complex correlation among genetic, environmental, and skin barrier dysfunction factors.Citation12,13) AD is associated with the classic features of allergic T helper (Th) 2 cell-mediated disease, which is characterized by mast cell activation, abnormal IgE production, and upregulation of Th2 and Th1 cytokines in chronically affected skin tissue.Citation14,15) Itching is a significant problem in AD patients because scratching exacerbates dermatitis.Citation16,17) In fact, reduction of itching-associated scratching is the most effective therapeutic strategy for improving the quality of life for patients with AD.
Pterocarpus indicus Willd is an excellent source of timber in southern Asia and has also been reported to have antitumor and antibacterial activity.Citation18) However, the antiallergic properties of Pterocarpus indicus Willd water extract (PIW) and its intrinsic mechanisms have not yet been studied. Thus, in the present study, we investigated the effect of PIW on allergic responses in IgE/Ag-induced mast cells, an anaphylactic mouse model, and a DNCB-induced AD-like mouse model. Our results indicate that PIW may be a good candidate agent for the prevention and treatment of allergic diseases through inhibition of mast cell activation.
Materials and methods
Preparation of PIW
P. indicus Willd was cultivated and harvested in Eumseong (GPS: E 128° 62′ N 36° 56′) according to good agricultural practices prescribed by the Korea Rural Development Administration. For sample preparation, the dried PIW was extracted 3 times with 2 L of water for 1 day. The resulting extracts were filtered through Whatman No. 1 paper (GE Healthcare, Buckinghamshire, UK), combined, and concentrated using a rotary evaporator (EYELA, Tokyo, Japan) at 40 °C. The obtained dried extracts were lyophilized and then powdered. Finally, the obtained PIW was dissolved in distilled water. And then, the dissolved PIW was added to minimal essential medium (MEM: WelGENE, Inc., Daegu, Korea) for using the experiments.
Cell culture
The RBL-2H3 mast cell line was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were grown in MEM containing 10% (v/v) fetal bovine serum (FBS) and 100 units/mL penicillin–streptomycin (Lonza Walkersville, Inc., Walkersville, MD, USA) at 37 °C in a humidified 5% CO2 atmosphere.
Animals
Six-week-old male ICR mice were purchased from Orient Bio (Gangneung, Korea) and housed in wire cages at 20−22 °C with a relative humidity of 40−50%. All animals were given ad libitum access to standard laboratory chow and water. The Institutional Animal Care and Use Committee (IACUC) at Yonsei University (Wonju, Korea) approved the protocol for this study.
Cell viability assay
RBL-2H3 cells (2 × 104 cells/well) were incubated overnight in 96-well plates and then treated with PIW at various concentrations (10, 25, 50, or 100 μg/mL) for 24 h. Cell viability was determined with the Ez-Cytox kit (Daeil Lab, Seoul, Korea) by measuring the absorbance at 450 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
RBL-2H3 cell stimulation & β-hexosaminidase release assay
RBL-2H3 cells (4 × 105 cells/mL) were primed overnight and sensitized with DNP-specific IgE (400 ng/mL). IgE-sensitized RBL-2H3 cells were washed twice with PIPES buffer (25 mM PIPES, pH 7.2, 110 mM NaCl, 4 mM KCl, 0.4 mM MgCl, 40 mM HCl, 5.6 mM glucose, 1 mM CaCl2, and 0.1% BSA) and treated for 30 min at 37 °C with PIW in PIPES buffer at the indicated concentrations. The cells were subsequently stimulated with DNP-BSA (dinitrophenyl-conjugated bovine serum albumin, 50 ng/mL) at 37 °C for 15 min and chilled on ice to stop the stimulation. Supernatant from IgE-Ag-stimulated cells in PIPES buffer was transferred to 96-well plates and mixed with β-hexosaminidase substrate (P-nitrophenyl-N-acetyl-β-D-glucosaminide) in 0.1 M citrate buffer (pH 4.5) and incubated at 37 °C for 1 h. The reaction was terminated via the addition of 0.1 M carbonate buffer (pH 10.5), and the absorbance was measured at 405 nm using a microplate reader.
Passive cutaneous anaphylaxis
DNP-specific IgE antibody (0.5 μg) was injected intradermally into one ear each of 7-week-old ICR mice. After 24 h, the mice were orally challenged with PIW. One hour later, 200 μg of DNP-BSA containing 3% Evans blue was intravenously injected. Two hours after the Ag challenge, the mice were euthanized, and the stimulated ear was excised to measure the amount of dye that had extravasated in response to the Ag. The dye was extracted from the ear in 500 μL of formamide at 63 °C overnight and quantified by measuring dye absorbance at 620 nm using a microplate reader.
DPPH assay & measurement of intracellular ROS
Free radical scavenging activity was measured by DPPH (2,2-diphenyl-1-picrylhydrazyl) assay. PIW was added to an ethanolic solution (100 mL) of DPPH radicals (final concentration of DPPH was 0.2 mM), and the mixture was shaken and left to stand at room temperature for 30 min. Finally, the absorbance of the resulting solution was measured at 517 nm using a microplate reader. The level of intracellular ROS was assessed by fluorescence with DCF-DA (2,7-dichlorofluorescein diacetate) according to the method of Lee et al. with some modifications.Citation19) RBL-2H3 cells (4 × 105 cells/mL) were primed overnight and sensitized with DNP-specific IgE (400 ng/mL). After 12 h, PIW was added at various concentrations (10, 25, 50, or 100 μg/mL). Then, RBL-2H3 cells were incubated with 10 μM DCF-DA (2,7-dichlorofluorescein diacetate, dissolved in DMSO) for 30 min at 37 °C. Next, the RBL-2H3 cells were stimulated with DNP-BSA (50 ng/mL) and fixed in 4% formaldehyde for 10 min. Finally, images of intracellular ROS production were acquired with a fluorescence microscope.
Protein extraction and Western blot analysis
RBL-2H3 cells were harvested and lysed with RIPA buffer containing 2 mM EDTA, 137 mM NaCl, 20 mM Tris-HCl (pH 8.0), 1 mM sodium vanadate, 10 mM NaF, 1 mM PMSF, 1% Triton X-100, 10% glycerol, and a protease inhibitor cocktail. The protein concentration of each sample was determined using the Bradford assay kit (Bio-Rad, CA, USA) according to the manufacturer’s instructions. The whole lysates were analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10–15% polyacrylamide gels. The proteins were transferred to PVDF membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked overnight at 4 °C in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) and 5% skimmed milk powder and then incubated with each primary antibody. Antibodies against Syk, phospho-Syk, phospho-p44/42 MAP kinase (Thr 202/Tyr 204), p44/42 MAP kinase, Akt, and phospho-Akt were obtained from Cell Signaling (Beverly, MA, USA). Blots were washed with TBS-T and incubated with secondary antibody conjugated with horseradish peroxidase. Proteins were detected using an enhanced chemiluminescence detection reagent for immunoblot analysis (GE Healthcare, Buckinghamshire, UK).
RNA preparation and real-time PCR
Total RNA was isolated using Tri reagent (Sigma–Aldrich, St, Louis, MO, USA) according to the manufacturer’s instructions. The concentration of total RNA was determined using a spectrophotometer (GE Healthcare, Buckinghamshire, UK). Total RNA was used as a template for cDNA synthesis, which was performed using a cDNA synthesis kit (Takara Bio, Shiga, Japan). Real-time PCR analysis was carried out using SYBR Green I and a Lightcycler ® 96 instrument (Roche, Basel, Switzerland). The following primers were used: mouse IL-4 (interleukin 4) sense 5′-GGTCTCAACCCCCAGCTAGT-3′; IL-4 antisense 5′-GCCGATGATCTCTCTCAAGTGA-3′; mouse IL-13 (interleukin 13) sense 5′-CCTGGCTCTTGCTTGCCTT-3′; IL-13 antisense 5′-GGTCTTGTGTGATGTTGCTCA-3′; mouse TNF-α sense 5′-CTGTAGCCCACGTCGTAGC-3′; TNF-α antisense 5′-TTGAGATCCATGCCGTTG-3′; mouse β-actin sense 5′-ATGCCATCCTGCGTCTGGACCTGGC-3′; and β-actin antisense 5′-AGCATTTGCGGTGCACGATGGAGGG-3′. Reactions were carried out for 35 cycles of denaturation, annealing, and extension at 94 °C for 60 s, 49 °C for 45 s, and 72 °C for 45 s, respectively.
AD-like mouse model
ICR mice were divided into 3 groups (normal, untreated DNCB-induced, and PIW-treated DNCB-induced; n = 6 per group). After complete removal of dorsal hairs, 250 μL of 1% 2,4-dinitrochlorobenzene (DNCB) solution (dissolved in a 3:1 mixture of acetone and olive oil) was applied to a 7.5 cm2 area of dorsal skin on the first day and sensitization occurred for 4 days. After sensitization, the 7.5 cm2 area of dorsal skin was challenged with 200 μL of 0.5% DNCB solution every 2 days for 2 weeks. As indicated, 200 μL of 10 mg/mL PIW was applied to the DNCB-induced dorsal skin of the mice a total of 6 times during the 2-week treatment period. After 18 days from the first application of DNCB, animals were sacrificed.
Evaluation of skin dermatitis severity & scratching behavior
After treatment with PIW, the severity of dermatitis in the dorsal skin lesions was evaluated. To ensure reliability of the skin severity test, 2 researchers who were unaware of the treatment group performed a blinded examination, and the findings were double-checked. The evaluated symptoms of (1) erythema, (2) pruritus and dry skin, (3) edema and excoriation, (4) erosion, and (5) lichenification were scored as follows: none = 0; mild = 1; moderate = 2; and severe = 3. The sum of the scores for each evaluated symptom (maximum score: 15) was taken as the skin severity score. For evaluation of scratching behavior, we videotaped the behavior patterns of the mice after the initial sensitization for 60 min every 2 days over 2 weeks. The recorded behavior was then reviewed, and the number of scratching episodes was counted and multiplied by 6. Two researchers who were blinded to the group assignments evaluated the video, and the data were double-checked. Scratching of the rostral back and biting of the caudal back were recorded. Licking of the belly and dorsal skin during grooming was disregarded.
Histopathological assay & measurement of IgE content in serum
The dorsal skin was isolated from each mouse, fixed in a 4% paraformaldehyde solution, and stabilized in a 30% sucrose solution. The fixed skin tissue was subsequently embedded in Tissue-Tek O.C.T Compound (Sakura Finetek USA, Inc., Torrance, CA, USA), sectioned, and stained with hematoxylin and eosin. Histological analysis was performed by light microscopy. Dorsal skin thickness was measured with a micrometer (NIKON ECLIPSE TE2000-U, ACT-1 for DXM 1200, Japan). For measurement of IgE content in serum, IgE level in serum was measured using a mouse IgE kit (BioLegend, San Diego, CA, USA) according to the manufacturer’s instructions. IgE concentrations were calculated using a linear regression equation obtained from standard absorbance values, and the absorbance of the resulting solution was measured at 450 nm using a microplate reader.
Statistical analysis
Experimental results are expressed as the mean ± standard deviation. One-way or two-way analysis of variance was used for multiple comparisons, followed by Tukey–Kramer post hoc analysis. SPSS statistical software Version 21.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. We considered P values <0.05, <0.01, and <0.001 to be statistically significant.
Results
PIW decreases IgE/Ag-induced RBL-2H3 cell degranulation
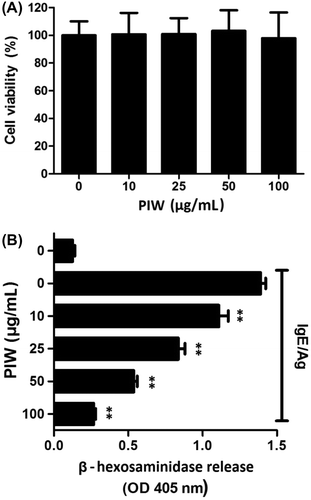
β-Hexosaminidase, a granule-associated exoglycosidase, has been used as a hallmark of mast cell degranulation as an alternative to histamine. We measured β-hexosaminidase released by IgE/Ag-induced RBL-2H3 cell degranulation. IgE/Ag treatment increased β-hexosaminidase release eightfold compared to non-treatment. However, PIW at 100 μg/mL inhibited IgE/Ag-induced β-hexosaminidase release in a dose-dependent manner, with 80% effective inhibition compared to no PIW. To determine whether PIW has a cytotoxic effect against mast cells, RBL-2H3 cells were incubated with PIW for 24 h, and cell viability was determined with an Ez-Cytox kit. No significant cytotoxicity was detected in cells treated with 10–100 μg/mL PIW (Fig. (A)).
Fig. 1. Effects of PIW on IgE/Ag-induced RBL-2H3 cell viability and degranulation.
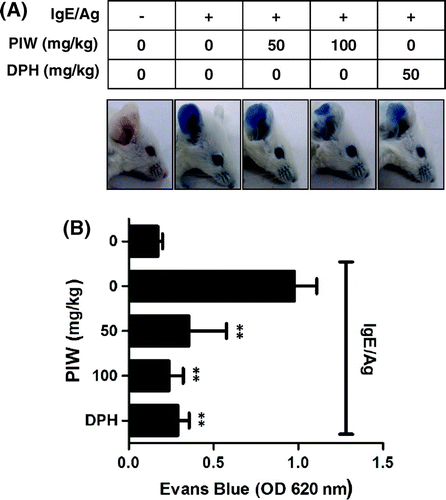
PIW suppresses IgE/Ag-induced passive cutaneous anaphylaxis
Mast cells play prominent roles in allergic response and anaphylaxis.Citation1) The passive cutaneous anaphylaxis (PCA) animal model is widely used to evaluate the local allergic reaction in vivo. We induced a PCA reaction by local sensitization with IgE in the mouse ear and then injected Ag intravenously along with Evans blue dye. The extravasated intensity of blue dye decreased 80% in the PIW treatment group (100 mg/kg) compared with the PIW-untreated group (Fig. (A) and (B)). In addition, PIW (100 mg/kg) suppressed the anaphylaxis as effectively as antihistamines such as diphenylhydramine (DPH, 50 mg/kg).
Fig. 2. Effect of PIW on IgE/Ag-induced passive cutaneous anaphylaxis.
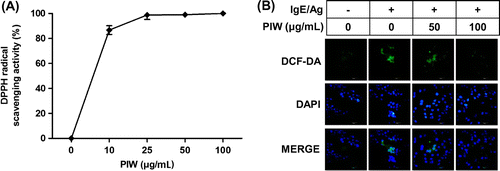
PIW decreases IgE/Ag-induced production of intracellular ROS
Intracellular ROS production is induced by IgE/Ag-mediated activation of FcεRI.Citation20,21) Thus, we measured the free radical scavenging activity of PIW at various concentrations (10, 25, 50, and 100 μg/mL). PIW dramatically scavenged the free radical of DPPH (Fig. (A)) and decreased the expression of DCF fluorescence compared with IgE/Ag-induced activation of mast cells (Fig. (B)).
Fig. 3. Effect of PIW on IgE/Ag-induced production of intracellular ROS.
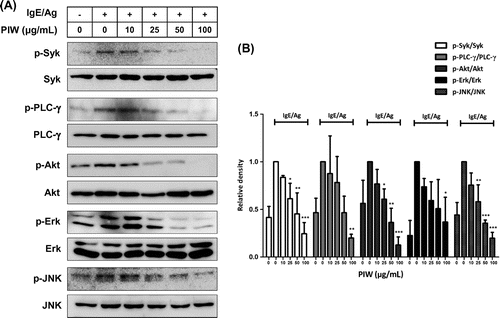
PIW inhibits IgE/Ag-induced phosphorylation of Syk, PLC-γ, Akt, Erk, and JNK
To identify the signaling pathways involved in PIW-induced inhibition of RBL-2H3 cell degranulation, we measured the phosphorylation status of various signaling molecules. PIW downregulated the phosphorylation of Erk, Akt, JNK, and PLC-γ. Phosphorylation of Syk, an early signaling molecule, was suppressed 75% in PIW-treated IgE/Ag-induced cells compared with untreated IgE/Ag-induced RBL-2H3 cells, and phosphorylation of PLC-γ, which cleaves phospholipids, decreased 78% compared to untreated IgE/Ag-induced cells. The phosphorylation of Akt, another molecule downstream of Syk, was strongly suppressed by 80% in PIW-treated IgE/Ag-induced cells compared with untreated IgE/Ag-induced cells. In addition, phosphorylation of Erk and JNK, major subfamilies of MAPKs, were suppressed 60 and 77%, respectively, by PIW treatment (Fig. ).
Fig. 4. Effect of PIW on IgE/Ag-induced phosphorylation of Syk, PLC-γ, Akt, Erk, and JNK.
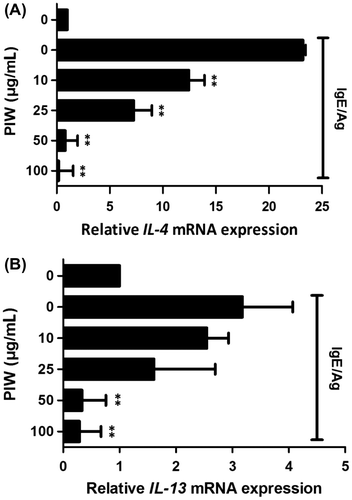
PIW reduces IgE/Ag-induced IL-4 and IL-13 mRNA expression in RBL-2H3 cells
Proinflammatory cytokines such as IL-4 and IL-13 are instrumental effectors in the allergic response by inducing IgE production in B cells and T-cell differentiation.Citation22) Accordingly, we investigated the effects of PIW on the expression of IL-4 and IL-13 mRNA in IgE/Ag-induced RBL-2H3 cells. PIW significantly decreased expression of IL-4 and IL-13 mRNA in a dose-dependent manner (Fig. (A) and (B)).
Fig. 5. Effect of PIW on IgE/Ag-induced expression of IL-4 and IL-13 mRNA.
PIW improves the condition of DNCB-induced skin & attenuates AD symptoms in AD-like ICR mice
Repeated application of DNCB in mice induced dermatitis symptoms of dryness, erythema, pruritus, edema, excoriation, erosion, and lichenification. Addition of PIW caused the skin to resemble that not treated with DNCB (Fig. (A)). To quantify the response, we calculated a dermatitis severity score every other day for 18 days. Repeated topical application of DNCB increased clinical severity scores in ICR mice, whereas the application of PIW suppressed AD symptoms (Fig. (B)). Itching is a significant problem in AD patients because scratching aggravates dermatitis.Citation17) DNCB application substantially increased the frequency of scratching behavior, whereas PIW treatment significantly suppressed DNCB-induced scratching behavior after 4 applications (Fig. (C)). Dermal infiltration with eosinophils and mononuclear cells was noted as a clinical AD symptom. Histological examination of AD-like ICR mice also showed that PIW reduced the thickening of the dorsal skin and attenuated the severity of AD (Fig. (D)).
Fig. 6. Effect of PIW on DNCB-induced AD-like ICR mice.
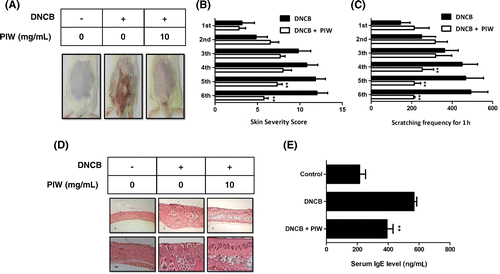
PIW downregulates serum IgE level in AD-like ICR mice
Mast cells are known as key effector cells in IgE-mediated allergic disorders. Increased IgE level in serum is an important component of AD. When ICR mice were treated with DNCB, IgE level in serum increased about 2.4-fold compared to mice not treated with DNCB. However, administration of PIW reduced serum IgE level by 33% compared with increases mediated by DNCB (Fig. (E)).
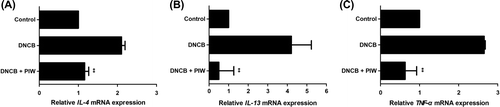
PIW decreases IgE/Ag-induced IL-4, IL-13, and TNF-α mRNA expression in vivo
Cytokines such as IL-4 and IL-13 are known to induce overproduction of IgE. Further, tumor necrosis factor alpha (TNF-α), a pro-inflammatory cytokine, affects epidermal morphogenesis and barrier function.Citation23) mRNA expression of inflammatory mediator cytokines such as IL-4, IL-13, and TNF-α increased in DNCB-induced AD-like mice, and PIW significantly suppressed 50, 86, and 77%, respectively, of the expression of each compared with DNCB treatment alone (Fig. (A)–(C)).
Discussion
Mast cells play a critical role in allergic and inflammatory disorders, including anaphylaxis shock, asthma, and AD, by degranulating and releasing inflammatory cytokines. In particular, activated mast cells release various vasoactive amines such as histamine, neukotriene, and serotonin, among others.Citation8) We evaluated the inhibitory effect of PIW on IgE/Ag-induced allergic activation in a DNCB-induced AD-like mouse model. IgE/Ag-induced allergic activation was also investigated in stimulated mast cells and an anaphylactic mouse model. To determine the effect of PIW on IgE/Ag-stimulated RBL-2H3 mast cells, we first confirmed that PIW had no cytotoxic effect on RBL-2H3 cells (Fig. (A)). Mast cells release mediators such as histamine, leukotrienes, and β-hexosaminidase through Ag-stimulated degranulation.Citation8) PIW effectively decreased the release of β-hexosaminidase (Fig. (B)). In addition, the binding of Ag and IgE leads to FcεRI cross-linking, which activates Lyn and Syk.Citation6) Syk is a critical signaling molecule that activates many downstream signaling molecules such as Erk and Akt.Citation8,9) In previous report, Suzuki et al. reported that FcεRI signaling of mast cells activated intracellular production of hydrogen peroxide and the production of ROS was decreased by Syk inhibitor.Citation20) In our study, the PIW was effective to be scavenging of free radical and inhibition of intracellular ROS (Fig. ). Also, phosphorylation of Syk, an early signaling molecule, was suppressed in PIW-treated IgE/Ag-induced cells compared with untreated IgE/Ag-induced RBL-2H3 cells, and the PIW suppressed the phosphorylation of downstream signal molecules of Syk such as PLC-γ, Erk, JNK, and Akt in IgE/Ag-induced activation of RBL-2H3 cells (Fig. ). These results including ours suggest that PIW may have a decrease in the ROS through inhibition of Syk signal molecule as well as a decrease in ROS level through antioxidant activity, partially.
IgE/Ag-induced activation of mast cell signaling molecules induces the synthesis and release of pro-inflammatory cytokines such as IL-4, IL-13, and TNF-α. Real-time RT-PCR analysis revealed that PIW inhibited the expression of IL-4 and IL-13 mRNA (Fig. ). Clinically, it is well known that mast cells are principle actors in the acute phase of IgE-associated allergic disorders.Citation24,25) The PCA animal model is widely used to evaluate antiallergic effects in mice, and we found that PIW significantly reduced anaphylactic shock in this mouse model (Fig. (A) and (B)).Citation26)
Activated mast cells release a variety of active substances that play critical roles in allergic reactions such as those of AD.Citation4) We first confirmed that PIW effectively inhibited the allergic activation of mast cells and investigated the effect of PIW on a DNCB-induced AD-like mouse model. We separated the animals into control, AD-like model, and PIW treatment groups. Repeated application of DNCB increased clinical severity score and thickness of the dorsal skin in ICR mice, but the application of PIW attenuated the infiltration of inflammatory cells and clinical AD severity score (Fig. (A), (B), and (D)). In addition, application of DNCB increased the frequency of scratching behavior, whereas treatment with PIW reduced scratching behavior (Fig. (C)). IgE expression is known to cause acute and chronic phase skin inflammation, and an increase in total serum IgE is a hallmark of AD.Citation27) Of note, serum IgE concentrations were significantly lower in PIW-treated mice compared with untreated AD-like mice (Fig. (E)). PIW also considerably suppressed the expression of IL-4, IL-13, and TNF-α mRNA in a dose-dependent manner (Fig. ). Recently, Hon et al. reported that the skin infection of bacteria induced the AD by breaking down the skin barrier.Citation28) Also, PIW has been known as effective in various antibacterial activities.Citation18) These results including ours suggest that PIW may have prevention of AD such as breaking down of the skin barrier through inhibition of the skin infection, partially.
In summary, this study is the first to demonstrate PIW suppression of IgE/Ag-stimulated degranulation and cytokine production in mast cells by blocking the phosphorylation of Syk and associated downstream signaling (Fig. ) in a PCA mouse model. Also, PIW attenuated DNCB-induced AD symptoms in mice. Accordingly, PIW shows promise as a new candidate therapeutic agent for mast cell-mediated allergic diseases, including AD.
Author contributions
HS Cha and WJ Kim designed the experiments, and MH LEE, SH Kim, and SY Kim carried out the DNCB-induced AD-like mouse model analysis. KH Lee and TJ Kim formulated the hypothesis and wrote the article. All authors reviewed and approved the final draft.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
We are grateful to Dr. Yusu Shin (National Institute of Horticultural & Herbal Science, RDF, KOREA) for providing us with PIW.
Notes
Abbreviations: PIW, Pterocarpus indicus Willd water extract; Syk, spleen tyrosine kinase; PLC-γ, phospholipase C-gamma; ROS, reactive oxygen species; IL-4, interleukin 4; IL-13, interleukin 13; PCA, passive cutaneous anaphylaxis; DPH, diphenylhydramine; AD, atopic dermatitis; TNF-α, tumor necrosis factor-alpha; IgE, immunoglobulin E; Ag, antigen; FcεRI, Fc epsilon receptor 1; PKC, protein kinase C; DAG, diacylglycerol; PI3K, phosphatidylinositol-3-kinases; ITAMs, immunoreceptor tyrosine-based activation motifs; MAPKs, mitogen-activated protein kinases; Erks, extracellular-signal-regulated kinases; JNKs, c-Jun NH2-terminal kinases; Th, T helper; MEM, minimal essential medium; DNP-BSA, dinitrophenyl-conjugated bovine serum albumin; DPPH, 2,2-diphenyl-1-picrylhydrazyl; DCF-DA, 2,7-dichlorofluorescein diacetate; TBS/T, Tris-buffered saline containing 0.1% Tween-20; DNCB, 2,4-dinitrochlorobenzene.
References
- Theoharides TC, Alysandratos KD, Angelidou A, et al. Mast cells and inflammation. Biochim. Biophys. Acta. 2012;1822:21–33.10.1016/j.bbadis.2010.12.014
- Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J. Neuroimmunol. 2004;146:1–12.10.1016/j.jneuroim.2003.10.041
- Zuidscherwoude M, van Spriel AB. The origin of IgE memory and plasma cells. Cell. Mol. Immunol. 2012;9:373–374.10.1038/cmi.2012.21
- Kawakami T, Ando T, Kimura M, et al. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009;21:666–678.10.1016/j.coi.2009.09.006
- Furumoto Y, Gomez G, Gonzalez-Espinosa C, et al. The role of Src family kinases in mast cell effector function. Novartis. Found. Symp. 2005;271:39–53.10.1002/SERIES1767
- Xiao W, Nishimoto H, Hong H, et al. Positive and negative regulation of mast cell activation by Lyn via the Fc RI. J. Immunol. 2005;175:6885–6892.10.4049/jimmunol.175.10.6885
- Kim DK, Kim HS, Kim AR, et al. DJ-1 regulates mast cell activation and IgE-mediated allergic responses. J. Allergy. Clin. Immunol. 2013;131:1653–1662.10.1016/j.jaci.2012.10.012
- Siraganian RP, Zhang J, Suzuki K, et al. Protein tyrosine kinase Syk in mast cell signaling. MolImmuno. 2002;l38:1229–1233.
- Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat. Immunol. 2008;9:1215–1223.10.1038/ni.f.216
- Mocsai A, Zhang H, Jakus Z, et al. G-protein-coupled receptor signaling in Syk-deficient neutrophils and mast cells. Blood. 2003;101:4155–4163.10.1182/blood-2002-07-2346
- Woolley DE, Tetlow LC. Mast cell activation and its relation to proinflammatory cytokine production in the rheumatoid lesion. Arthritis. Res. 2000;2:65–74.10.1186/ar70
- Jin H, Oyoshi MK, Le Y, et al. IL-21R is essential for epicutaneous sensitization and allergic skin inflammation in humans and mice. J. Clin. Invest. 2009;119:47–60.
- Grewe M, Bruijnzeel-Koomen CA, Schöpf E, et al. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol. Today. 1998;19:359–361.10.1016/S0167-5699(98)01285-7
- Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J. Clin. Cell. Immunol. 2011;2:110–122.
- Leung DY, Boguniewicz M, Howell MD, et al. New insights into atopic dermatitis. J. Clin. Invest. 2004;113:651–657.10.1172/JCI21060
- Hong J, Buddenkotte J, Berger TG, et al. Management of itch in atopic dermatitis. SeminCutan. Med. Surg. 2001;30:71–86.
- Ständer S, Luger TA. Itch in atopic dermatitis – pathophysiology and treatment. Acta. Dermatovenerol. Croat. 2010;18:289–296.
- Khan MR, Omoloso AD. Antibacterial activity of Pterocarpus indicus. Fitoterapia. 2003;74:603–605.10.1016/S0367-326X(03)00149-7
- Lee YH, Kim WJ, Lee MH, et al. Anti-skeletal muscle atrophy effect of Oenothera odorata root extract via reactive oxygen species dependent signaling pathways in cellular and mouse model. Biosci. Biotechnol. Biochem. 2015;2015:1075861.
- Suzuki Y, Yoshimaru T, Matsui T, et al. Fc RI signaling of mast cells activates intracellular production of hydrogen peroxide: role in the regulation of calcium signals. J. Immunol. 2003;171:6119–6127.10.4049/jimmunol.171.11.6119
- Yoshimaru T, Suzuki Y, Matsui T, et al. Blockade of superoxide generation prevents high-affinity immunoglobulin E receptor-mediated release of allergic mediators by rat mast cell line and human basophils. Clin. Exp. Allergy. 2002;32:612–618.10.1046/j.0954-7894.2002.01263.x
- Gauchat JF, Henchoz S, Mazzei G, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–343.10.1038/365340a0
- Danso MO, van Drongelen V, Mulder A, et al. TNF-α and Th2 cytokines induce atopic dermatitis–like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J. Invest. Dermatol. 2014;134:1941–1950.10.1038/jid.2014.83
- Wershil BK, Mekori YA, Murakami T, et al. 125I-fibrin deposition in IgE-dependent immediate hypersensitivity reactions in mouse skin. Demonstration of the role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. J. Immunol. 1987;139:2605–2614.
- Mukai K, Matsuoka K, Taya C, et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23:191–202.10.1016/j.immuni.2005.06.011
- Ishizuka T, Oshiba A, Sakata N, et al. Aggregation of the FcepsilonRI on mast cells stimulates c-Jun amino-terminal kinase activity. A response inhibited by wortmannin. J. Biol. Chem. 1996;271:12762–12766.
- Arshad SH, Holgate S. The role of IgE in allergen-induced inflammation and the potential for intervention with a humanized monoclonal anti-IgE antibody. Clin. Exp. Allergy. 2001;31:1344–1351.10.1046/j.1365-2222.2001.01162.x
- Hon KL, Tsang YC, Lee VW, et al. Efficacy of sodium hypochlorite (bleach) baths to reduce Staphylococcus aureus colonization in childhood onset moderate-to-severe eczema: A randomized, placebo-controlled cross-over trial. J. Dermatolog. Treat. 2015;13:1–7.10.3109/09546634.2015.1093586