Abstract
We found that conditioned medium derived from Lewis Lung Carcinoma cells down-regulated Semaphorin3a (Sema3a) mRNA expression and increased the activity of mammalian target of rapamycin complex 1 (mTORC1) in osteoblast-like MC3T3-E1 cells. Furthermore, mTORC1 inhibition with rapamycin counteracted the effect of conditioned media on Sema3a mRNA expression. These results suggest that tumor cells decrease Sema3a mRNA expression in osteoblast in an mTORC1-dependent manner.
Key words:
Tumor malignancy is caused by complicated crosstalk between various factors including overexpression of oncogenic molecules and loss of function of tumor suppressor genes. Therefore, understanding the mechanisms of action of tumor suppressor molecules in tumor progression and their possible application as anticancer drugs is necessary for the development of novel therapeutic strategies. The role of the semaphorin families of proteins in pathophysiological processes such as cell migration, immune responses, angiogenesis, and cancers has been studied recently.Citation1) Among the various families, the class 3 semaphorin (Sema3) molecules are only one secreted type of semaphorin and considered to be involved in tumor suppression.Citation2) Sema3a was originally discovered as an axon guidance molecule of sensory nerves by its binding to neuropillin-1 and the plexin family receptors. In a non-small cell lung cancer study, Sema3a was downregulated in tumor tissues and its low expression level was associated with poor prognosis.Citation3) In addition, Sema3a has been shown to have suppressive roles in angiogenesis, tumor progression, and metastasis.Citation4–7) On the other hand, contradictory studies have reported that Sema3a promotes tumor cell dispersal, tumor growth, and angiogenesis,Citation8,9) indicating that the pathophysiological role of Sema3a in cancer biology is an unanswered question.
Bones are metabolically dynamic tissues characterized by bone formation and resorption regulated by the mutual interaction between osteoblasts and osteoclasts.Citation10) Aggressive tumor cells frequently disseminate from the primary site and establish metastasis in bone tissue. Tumor invasion of the bone is associated with increased osteoclastic bone resorption mediated by tumor cell-derived osteolytic factors. Increased bone resorption releases growth factors from the bone matrix, which can feed back to promote tumor growth, leading to the ‘vicious cycle’ of bone metastases.Citation11) However, the detailed mechanism by which tumor cells increase osteoclastic activity has not been elucidated completely.
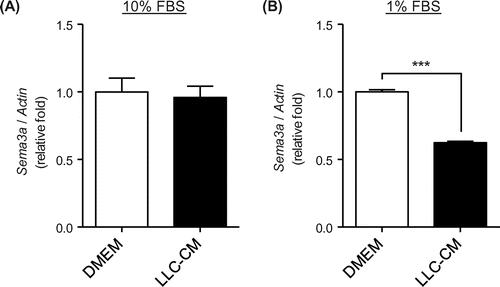
Recently, two studies reported that Sema3a expressed by osteoblasts inhibits osteoclastogenesis during normal bone development,Citation12,13) suggesting that Sema3a derived from osteoblast is a mediator to inhibit bone resorption. Thus, downregulation of Sema3a could promote bone resorption during the bone metastasis. However, both the influence of tumor progression on Sema3a expression in osteoblast and its mechanism remain unclear. We hypothesized that the putative secretory component derived from tumor down-regulate Sema3a expression in osteoblast to promote tumor-induced bone resorption. To test the hypothesis, we used both osteoblast-like cell line, MC3T3-E1, and conditioned medium derived from a tumor cell line, Lewis lung carcinoma (LLC-CM) to examine the effect of LLC-CM on Sema3a mRNA level in MC3T3-E1. LLC was incubated in DMEM containing 10% FBS for 24 h, and the medium was collected and designated as 10% FBS LLC-CM. The DMEM containing 10% FBS in which LLC was not incubated, but served as the control and was designated as 10% FBS DMEM. MC3T3-E1 cells were incubated in 10% FBS LLC-CM or 10% FBS DMEM for 24 h and then collected for qRT-PCR analysis. There was no significant difference in Sema3A mRNA levels between the 10% FBS DMEM and 10% FBS LLC-CM groups (Fig. (A)). Growth factors present in FBS, which is a supplement in conventional maintenance media at 10%, stimulate the mTOR pathway. Therefore, incubation of MC3T3-E1 cells in conventional maintenance medium may saturate the activity of this pathway, leading to possible underestimation of the effects of LLC-CM on mTOR activity. To reduce the effect of cytokines contained in FBS on mTOR pathway, LLC cells were incubated in low-serum (1% FBS) DMEM for 24 h, and then the medium was harvested and designated as 1% FBS LLC-CM. DMEM containing 1% FBS in which LLC was not incubated served as the control and was designated as 1% FBS DMEM. Compared with 1% FBS DMEM, treatment with 1% FBS LLC-CM significantly decreased Sema3a mRNA expression level in MC3T3-E1 cells (Fig. (B)). These results suggest that LLC-CM down-regulated Sema3a mRNA in MC3T3-E1 cells under low-serum conditions.
Fig. 1. Lewis lung carcinoma (LLC)-derived conditioned medium (CM) decreased Semaphorin-3a (Sema3a) expression in MC3T3-E1 cells under low-serum culture conditions.
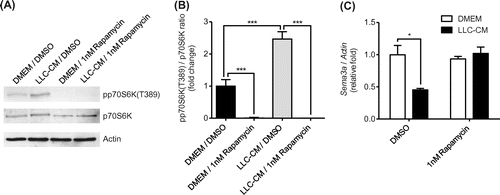
Tumor cells produce various factors that stimulate mammalian target of rapamycin complex1 (mTORC1) signaling. There are no previous reports on the relationship between mTORC1 activity and Sema3a mRNA expression and, therefore, we examined whether mTORC1 is involved in Sema3a mRNA down-regulation induced by treatment with 1% FBS LLC-CM. Rapamycin, an mTORC1 inhibitor was applied to MC3T3-E1 cells in combination with 1% FBS LLC-CM. p70S6 K phosphorylates S6 ribosomal protein to induce protein synthesis in a signaling pathway of mTORC1. The phosphorylation of p70S6 K at threonine 389 has been used as a hallmark of activation of mTORC1. Rapamycin at 1 nM blocked the phosphorylation of p70S6 K without affecting basal levels of Sema3a mRNA expression (Fig. (C)). Treatment with 1% FBS LLC-CM increased the phosphorylation of p70S6 K, which was completely reversed by rapamycin (Fig. (A) and (B)). Rapamycin at 1 nM completely abrogated the decrease in Sema3a mRNA level induced by 1% FBS LLC-CM treatment (Fig. (C)). These results indicate that 1% FBS LLC-CM treatment decreases Sema3a mRNA expression in an mTORC1-dependent manner.
Fig. 2. Rapamycin restored decrease in Semaphorin-3a (Sema3a) expression in MC3T3-E1 cells induced by Lewis lung carcinoma conditioned-medium (LLC-CM) treatment.
In the present study, we showed that mTORC1 is a negative regulator of Sema3a expression in osteoblast-like cells. Although further investigations are required, combined with the inhibitory role of Sema3a in osteoclastogenesis, mTOR inhibitors are presumed to not only suppress tumor growth, but also to correct bone metabolism disrupted by tumors.
Author contributions
Daisuke Yamada, Kohichi Kawahara, Masanobu Ozaki, and Takehiko Maeda conceived and designed the experiments. Daisuke Yamada performed the experiments. Daisuke Yamada analyzed data. Daisuke Yamada, Kohichi Kawahara, Masanobu Ozaki, and Takehiko Maeda contributed reagents/materials/analysis tools. Daisuke Yamada, Kohichi Kawahara, Masanobu Ozaki, and Takehiko Maeda wrote the paper.
Funding
This work was supported by the JSPS KAKENHI [grant number 25460108], [grant number 15K18449] and [grant number 15K08682].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat. Rev. Cancer. 2008;8:632–645.10.1038/nrc2404
- Bielenberg DR, Klagsbrun M. Targeting endothelial and tumor cells with semaphorins. Cancer Metastasis Rev. 2007;26:421–431.10.1007/s10555-007-9097-4
- Zhou H, Wu A, Fu W, et al. Significance of semaphorin-3A and MMP-14 protein expression in non-small cell lung cancer. Oncol. Lett. 2014;7:1395–1400.
- Maione F, Molla F, Meda C, et al. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J. Clin. Invest. 2009;119:3356–3372.
- Chakraborty G, Kumar S, Mishra R, et al. Semaphorin 3A suppresses tumor growth and metastasis in mice melanoma model. PLoS ONE. 2012;7:e33633.10.1371/journal.pone.0033633
- Sabag AD, Bode J, Fink D, et al. Semaphorin-3D and semaphorin-3E inhibit the development of tumors from glioblastoma cells implanted in the cortex of the brain. PLoS ONE. 2012;7:e42912.10.1371/journal.pone.0042912
- Nasarre P, Gemmill RM, Drabkin HA. The emerging role of class-3 semaphorins and their neuropilin receptors in oncology. Onco. Targets Ther. 2014;7:1663–1687.
- Bagci T, Wu JK, Pfannl R, et al. Autocrine semaphorin 3A signaling promotes glioblastoma dispersal. Oncogene. 2009;28:3537–3550.10.1038/onc.2009.204
- Casazza A, Laoui D, Wenes M, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709.10.1016/j.ccr.2013.11.007
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007;7:292–304.10.1038/nri2062
- Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat. Rev. Cancer. 2011;11:411–425.10.1038/nrc3055
- Hayashi M, Nakashima T, Taniguchi M, et al. Osteoprotection by semaphorin 3A. Nature. 2012;485:69–74.10.1038/nature11000
- Fukuda T, Takeda S, Xu R, et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497:490–493.10.1038/nature12115
