Abstract
Effective anti-Botrytis strategies leading to reduce pesticides on strawberries are examined to provide the protection that is harmless to humans, higher animals and plants. Calcium treatments significantly inhibited the spore germination and mycelial growth of B. cinerea. The intracellular polygalacturonase and CMCase showed low activities in B. cinerea cultivated by medium containing calcium. On the other hand, calcium-stimulated β-glucosidases production occurred. Our findings suggest that the calcium treatments keep CMCase activity low and cause low activities of cell-wall degrading enzymes of B. cinerea in the late stage of growth.
Graphical abstract
Effect of calcium on cellulolytic enzymes and pathogenicity of B. cinerea.
Botrytis cinerea (Which will referred to as BC) is a fungus that has been used in making the so-called “noble rot” wine. On the other hand, BC is a widespread fungus able to infect the aerial parts of many plant species. BC causes gray mold disease, which results in great damage to agricultural crops. Strawberry (Fragaria × ananassa Duch.) is one of the most commercially important fruit crops. Strawberry diseases are a major limiting factor that severely impact the plant agronomic performance and lead to economic losses. Moreover, most strawberry cultivars are highly susceptible to BC.Citation1)
Using fungicides is the most effective way of preventing the occurrence of Botrytis disease.Citation2) However, following an increase public health concern and fast development of resistance to novel fungicides by fungi, new methods which eliminate or reduce the amount of fungicides have been needed.
Calcium, as a constituent of the cell wall, plays an important role in forming cross-bridges which influence cell-wall strength.Citation3) Exogenously applied calcium stabilizes the plant cell wall and protects it from cell-wall degrading enzymes.Citation4–6) Preharvest calcium sprays provided an increase of calcium content in peach fruits which corresponded to an increase of tissue firmness after removal from cold storage.Citation7) Moreover, calcium has direct effects on pectinasesCitation8) and cell walls of BC.Citation9)
In previous papers,Citation10–12) we tested the relation between the cellulase activity and the pathogenicity of BC. The pathogenicity of BC against apple fruits was found to correlate positively with the β-glucosidase activity and negatively with CMCase activity. Thus, the β-glucosidase was purified and characterized. This enzyme has a novel ability to degrade substrate with considerable polymerization. Further, the β-glucosidase underwent intensive induction by host plant cell walls and showed a high activity against fibers made from apple fruit tissues.
The aim of this present study is to investigate whether calcium affects cell-wall degrading enzyme productions and the pathogenicity of BC isolated from strawberry.
Materials and methods
Plant pathogen
Botrytis cinerea was isolated from commercially available strawberry fruits (Tochiotome) by single spore isolation and was grown on potato dextrose agar (PDA). Morphological characteristics such as conidiophores length, conidial dimension were measured. Cultural characteristics such as colony appearance, conidial shape, color and shape were also examined. The identification was confirmed by PCR molecular method.Citation13) Conidial suspensions were prepared from the PDA plates after 2 weeks of growth and the suspensions were kept at −80 °C.
First culture
BC was inoculated on potato dextrose agar (PDA) in petri dishes and was maintained at 25 °C for a week.
Experimental culture
The culture media used in this study were inorganic medium (made of 0.2% NH4NO3, 0.6% KH2PO4, and 0.05% MgSO4 (pH 5.0)), PY-medium (made of 0.6% peptone and 0.3% yeast extract in inorganic medium), and PYA-medium (made of 1% avicel in PY- medium).
Preparation of crude enzyme solution
(a) Preparation from PYA-medium cultivation: after cultivation, cells were removed by gauze filtration and the filtrate was centrifuged at 8000 rpm and 0 °C for 30 min. The supernatant was dialyzed overnight against 0.02 M acetate buffer (pH 5.0). The dialyzed solution was used as the crude extracellular enzyme preparation. The cells harvested by gauze filtration were washed sufficiently with deionized water and ground in a mortar with a suitable amount (equal to wet cell wt.) of sea sand in 0. 1 M acetate buffer (pH 5.0), then centrifuged at 12,000 rpm and 0 °C for 30 min. The precipitate was washed 3 times with 0.1 M acetate buffer (pH 5.0). The supernatant was dialyzed overnight against 0.02 M acetate buffer (pH 5.0). The dialyzed solution was designated the crude intracellular enzyme.
(b) Preparation from lesions of strawberry fruit: after cultivation, the whole lesion was ground in a mortar with a suitable amount (equal to wet wt.) of sea sand in 0.1 M acetate buffer (pH 5.0), and centrifuged at 12,000 rpm and 0 °C for 30 min. The supernatant was dialyzed overnight against 0.02 M acetate buffer (pH 5.0).
Enzyme activity
Enzyme activities were measured by the activity against 1% avicel (avicelase), 1% carboxymethyl cellulose (carboxymethyl cellulase: CMCase), 0.25% salicin (β-glucosidase) and 1% pectic acid (polygalacturonase). A 0.05-mL sample of an appropriately diluted enzyme solution was added to 0.05 mL of substrate in 0.1 M acetate buffer (pH 4.0), and the mixture was incubated at 30 °C. The reaction was stopped by the addition of a copper reagent, and the released reducing sugar was measured by the Somogyi- Nelson method.Citation14) One unit of the activity was defined as the amount of enzyme needed to release 1 μ mol of reducing sugar (glucose equivalent) per min under the assay conditions. The assays were repeated six times.
Infection studies
The host plants used in this study were commercially available strawberry fruit (Tochiotome). They were washed with water and wiped with dry paper towels, and their surfaces were sterilized with 5% sodium hypochlorite solution. Each lot of the plant was inoculated with BC spores (i.e. 104 spores), and was kept at 25 °C in polyethylene bags. The size of the lesion appearing on the surface of host plants was measured every 24 h and expressed as an index of the pathogenicity. Infection studies were carried out in triplicate.
Mineral treatment
Mineral treatment for strawberries: Calcium was applied directly onto strawberry fruits by dipping in 0.5–1 M calcium solution (pH 4.0) for 1 h. Mineral treatment for spores: spores were suspended in 1 M solution (pH 4.0) for 1 h. BC spores (i.e. 104 spores) were inoculated on ager plate and were kept at 25 °C and then germinating spores were counted under microscope. Mineral treatment for mycelium: mycelium of BC grown on a filter paper (5 × 5 mm square) which was taken from the surface of the first cultures was inoculated at the center of PDA plate containing 1-M mineral solution (pH 4.0). The length of mycelium from the center was measured.
Results and discussion
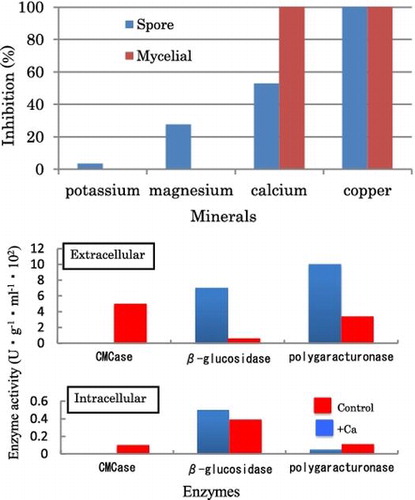
Effect of minerals on the spore germination of BC
The Spore germination of BC was inhibited by 1 M of K+(3%), Mg2+(30%), Ca2+(52%), and Cu2+(100%). Calcium and copper showed a strong inhibitory effect on the spore germination of BC (Fig. (a)). Copper has long been known for its antimicrobial properties, which have been linked to the production of toxic hydroxyl radicals and the inhibition of Fe–S-dependent enzymes.Citation15) However, copper is known to be poisonous to human, we decided to focus on other minerals. Calcium is found in nearly all cell organelles, including mitochondria, vacuoles, golgi-like bodies, nuclei, and endoplasmic reticulum. Normally, an electrochemical gradient allows passive diffusion of calcium ions into the cell through protein channels, but the low cytoplasmic calcium concentration is mainly maintained through the activity of Ca2+/ATPase pumps.Citation16,17) Incubating spores of BC in high concentrations of CaCl2 resulted in decreased spore-germination (Fig. (a)). The result suggests that the excess amount of calcium allows BC to accumulate calcium in the cells and the accumulated calcium could inhibit enzymes involved in the germination processes of BC.
Fig. 1. Effect of minerals on the spore germination and the mycelial growth of B. cinerea.
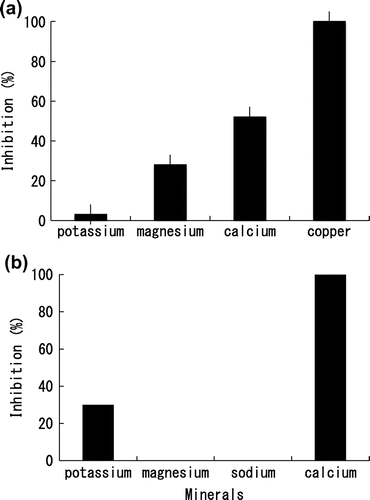
Effect of minerals on the mycelial growth of BC
The mycelial growth of BC was inhibited by 1 M of K+ (30%) and Ca2+ (100%) on PDA plate (Fig. (b)). Magnesium and Na+ showed no inhibitory effect on the mycelial growth of BC on PDA plate (Fig. (b)). It has been reported that calcium in a high concentration increases the thickness of the fungal cell wall.Citation9) Thickening the fungal cell wall could retard the elongation phase of the hyphae and results in a loss of cell wall elasticity.Citation9) In our study, the spore germination of BC was also inhibited by calcium as described above. Sexual spores need a post-maturation phase, the thickness of wall becomes progressively thinner by digestion of its inner layers, before they can germinate. By contrast, nonsexual spores show exogenously imposed dormancy.Citation18) Botrytis cinerea produces nonsexual spores whose germination depends on the environment not on the thickness of the spore wall. Our results suggest that increasing calcium in the cytoplasm might affect the fungal growth and spore germination.
The mycelial growth of BC was inhibited by 1 M of K+, Na+, and Ca2+ in PYA medium (Fig. ). On the other hand, 1 M Na+ showed no inhibitory effect on the mycelial growth of BC on PDA plate (Fig. (b)). High ionic levels are known to be potentially damaging to cells. A common method of balancing a high external osmotic environment is to accumulate sugars or sugar derivartives that do not interfere with the central metabolic pathways.Citation19) Glucose contained in PDA medium could reduce the inhibitory effect of Na+ ion. In the case of 1 M NaCl-PYA medium, avicel in the medium was degraded (ca. 50%) at day 9, and once decreased growth grew back later (data not shown). It indicates that glucose might be produced by the result of avicel degradation and might reduce the inhibitory effect of Na+ ion.
Fig. 2. Effect of minerals on the mycelial growth of B. cinerea cultivated in PYA-medium.
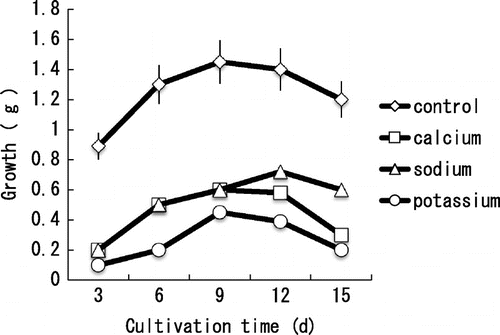
The mycelial growth in 1 M CaCl2-PYA medium was inhibited (>50%) compared with control culture (Fig. ), and was inhibited completely on 1 M CaCl2- PDA plate (Fig. (b)). Although glucose contained in PDA could account for its tolerance to minerals, the mycelial growth was strongly inhibited on 1-M CaCl2-PDA plate. There must be some other reason except osmotic stress for the inhibition by 1-M CaCl2. Extracellular Mg2+ is known to regulate Ca2+ channels in the plasma membrane.Citation20) Magnesium inhibits Ca2+ uptake, but does not inhibit growth and conidiation in Trichoderma viride mycelium.Citation21) Moreover, upon Mg2+ depletion external Ca2+ is rapidly internalized, leading to high cytoplasmic Ca2+ similar to that observed in the presence of high Ca2+ concentrations in the medium.Citation22) Magnesium contained in PYA medium might act at Ca2+ channels to regulate Ca2+ influx, and the reduced Ca2+ concentration in BC might allow the mycelia to grow. On the other hand, Mg2+ depletion and high Ca2+ concentration in the 1-M CaCl2-PDA medium might cause Ca2+channels to open and influx of Ca2+.
Morphology changes were shown on hyphae in high concentrations of minerals, especially the septation of hypha was different and the tips swelled on the shoulders of the apex in the culture from 1-M CaCl2-PDA plates (Fig. ). Jackson and Heath showed that treatment of hyphae with cytochalasin E/dimethyl sulphoxide, which disrupt cell dynamics by binding to actin microfilaments, induced rapid changes in actin caps. Cap disruption is accompanied by transient growth rate increases, subsequent rounding and swelling of apices and a shift of osmotically induced burst points closer to the apex.Citation23) In B. cinerea, several components of the Ca2+ signaling pathway have been identified and characterized. It has been shown that the Ca2+/calcineurin (CN) pathway is involved mainly in processes such as hyphal growth and septation and, to a lesser extent, in differentiation processes such as spore production, germination, and sexual development.Citation24)
Fig. 3. Effect of minerals on the morphological characters of B. cinerea.
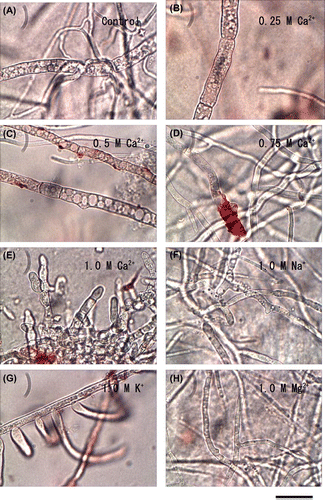
Our results described above suggest that high intracellular Ca2+ might affect signaling pathways involving Ca2+ and the function of cytoskeleton.
Effect of calcium on the susceptibility of the strawberry fruits inoculated by BC
Calcium significantly influenced the mycelial growth and the spore germination of BC (Fig. ). Then, effect of calcium on the susceptibility of the strawberry fruits against BC was tested. After calcium treatments (1 M), the symptom on the strawberry fruit inoculated with BC had not been found until day 7 (delays of up to 3 days) (Table ). External calcium can play an indirect role in fungal growth by altering internal calcium which controls the cytoplasmic calcium gradient, vesicle migration to the tip and the activity of fungal enzymes involved in cell-wall expansion.Citation25) Calcium is also known to reduce decay by stabilizing the host cell wall and thus reducing maceration by pectinase produced by fungal pathogens.Citation26,27) Both the symptom appearing and the lesion expansion on the strawberry fruits were delayed by calcium treatments (Table ). These results suggest that calcium could affect not only the lesion formation but also the initial infection by BC. In a previous paper, we reported that cell wall-degrading enzymes are involved in the initial infection and the lesion formation of BC.Citation10,11) Our findings suggest that calcium affects cell-wall degrading enzymes involved in infection by BC.
Table 1. Effect of the calcium treatments on susceptibility of strawberry inoculated by BC.
Cell-wall degrading enzyme activities in the strawberry fruit lesion by BC
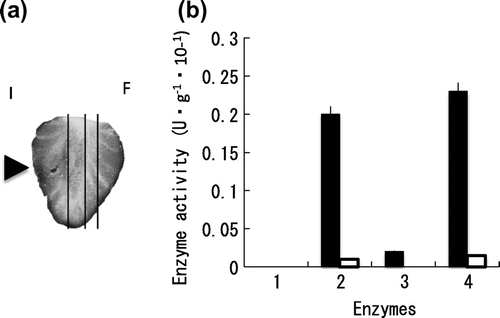
The activity of cell-wall degrading enzymes in the strawberry fruit lesion due to BC was measured following the progress of the lesion. At day 5 after inoculation of BC, the strawberry fruit lesion was sliced as shown in Fig. (a). Each slice was assayed for the enzyme activity. In describing cell-wall degrading enzyme activities, the activities caused by strawberry fruit itself were not differentiated from the activities caused by the fungus. As shown in Fig. (b), β-glucosidase was present only in the infected tissue, while the polygalacturonase and CMCase activities were found both in healthy and infected tissues. On the other hand, no activity was shown on avicelase. The results indicate that polygalacturonase and CMCase of strawberry fruit itself was caused by BC infection. In cases on apple fruit infection, the highest activity of β-glucosidase was found in the advancing margin of the lesion, as shown in a previous paper.Citation11) In the strawberry lesion, β-glucosidase activity was rather low, while polygalacturonase and CMCase had high activities. These results suggest that polygalacturonase and CMCase could play an important role to develop the lesion by BC in strawberry fruits.
Fig. 4. Cell-wall degrading enzyme activities in the strawberry fruit lesion by B. cinerea.
Effect of minerals on the production of cell wall-degrading enzymes
The BC strain isolated from strawberry was cultured in 1-M mineral-PYA medium and PYA medium. The growth of BC was inhibited by PYA medium containing 1-M CaCl2, NaCl or KCl (Fig. ). The CMCase showed certain activities in 1-M NaCl-PYA, 1-M KCl-PYA and PYA, whereas no activity in 1-M CaCl2 -PYA. The β-glucosidase had high activities in 1-M CaCl2-PYA (Fig. ). Therefore, the effect of calcium on the production of cell-wall degrading enzymes was tested in detail. The BC was cultured in 1-M CaCl2-PYA medium and PYA medium, and the course of changes in enzyme activities was measured. In control medium (PYA medium), the intracellular and extracellular polygalacturonase, CMCase and β-glucosidase activities showed peaks at day 6. The intracellular β-glucosidase was produced at day 6, decreased at day 9, and after that increased again (Fig. (b)). In 1-M CaCl2-PYA media (Fig. (d)), low activities were shown on intracellular polygalacturonase and CMCase during cultivation. It has been reported that the activity of the pectinolytic enzymes obtained from BC is drastically inhibited by calcium.Citation8) Calcium cross-linkages between polygalacturonic acid chains favor gel formation and make them less accessible to polygalacturonases. Calcium, by its ability to interact with the substrate of the enzyme, could reduce polygalacturonases activity.Citation8) In this study, we found that the production of polygalacturonase is also affected by calcium. The polygalacturonase was released earlier than usual in culture medium containing calcium (Fig. (c) and (d)). The enzyme usually has high activity in the late stages of fungus growth (Fig. (a) and (b)). Maintaining high activity of the polygalacturonase in the late stages could be important for BC to develop the lesion. The polygalacturonase with low activity in the late stage of growth could affect the lesion formation by BC.
Fig. 5. Effect of minerals on cell-wall degrading enzyme productions of B. cinerea isolated from strawberry.
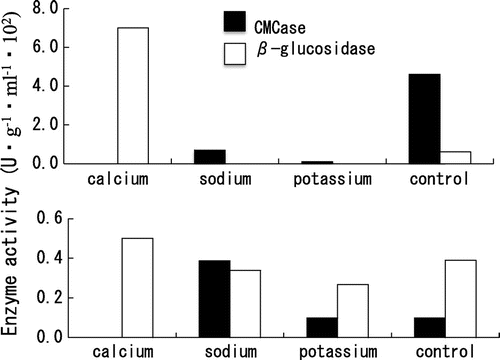
Fig. 6. Effect of calcium on the production of cell-wall degrading enzymes of B. cinerea.
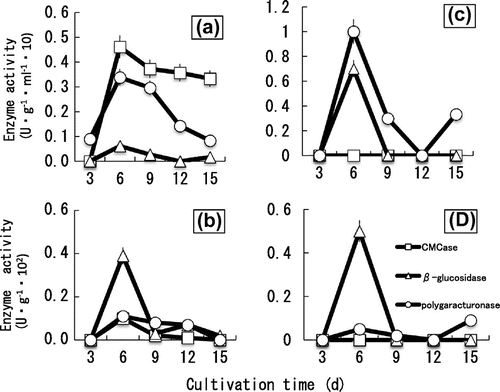
The β-glucosidase had higher activity than the other intracellular enzymes (Fig. (b) and (d)). The β-glucosidase was localized in the cell interior of hyphae cultivated in the control medium (Fig. (a) and (b)). The result supports an observation on cellobiohydrolases and beta-1,4 glucanase localized within cisternae of endoplasmic reticulum and within membrane complexes of cellulose-grown hyphae of Trichoderma reesei.Citation28) In this study, we found that the β-glucosidase shows high activities both inside and outside of the cells grown in CaCl2-PYA medium (Fig. (c) and (d)). The result suggests that the calcium treatments could stimulate transportation of the enzyme or change the permeability of the cell wall. Calcium has been thought to be involved in tip growth of several fungi.Citation18) So any localized ingress of calcium through the plasma membrane would cause a perturbation, including an interaction with the cytoskeleton because calcium is known to cause the contraction of F-actin.Citation18) Moreover, calcium seems to alter the distribution of intracellular membrane organelles favoring the forward movement of cargo-loaded vesicles from ER to Golgi and then from Golgi to the cell membrane.Citation29) Our findings suggest that calcium could stimulate production of the active β-glucosidase. The excess extracellular enzyme might trigger the host plants to activate phytoalexins increasing the resistance against BC.
The CMCase activity remained low during the incubation in CaCl2-PYA medium (Fig. (c) and (d)). The extracellular CMCase had high activities in the control medium (Fig. (a)). The cereal pathogens produce the necessary cell-wall degrading enzymes constitutively, in the absence of host wall material, and the production of these enzymes is not repressed by sugars.Citation18) In our previous work,Citation10–12) pectinases, avicelase, and CMCase of BC are produced constitutively, while the β-glucosidase is strongly induced by avicel, but slightly by cellobiose, and not at all by glucose. The β-glucosidase is only inducible enzyme among the glucanases of BC. Our results indicate that calcium inhibit the productions of active CMCase and pectinases, which are produced constitutively. It has been reported that calcium enhance cellulase production and them structural stability. CMCase production by Aspergillus terreus is enhanced with the addition of calcium chloride (3 mM) in the medium.Citation30) Cellulolytic enzymes from Clostridium thermocellum are stabilized by calcium ions, and Ca2+ is a constituent of many thermostable proteins.Citation31) Little attention has been given to the inhibitory effect of calcium on the production of cellulases. Our findings suggest that CMCase could play an important role in pathogenicity of BC on strawberry fruits. Thus, calcium could affect the pathogenicity depending on CMCase.
In most cases research indicates that the improved biocontrol efficacy is due to an indirect effect of the calcium inhibiting the fungal pathogen or increasing the host resistance. We have now pointed out that calcium affects directly the production of active cellulolytic enzymes involved in the pathogenicity of BC. Controlling the enzymes could offer a possible target for plant protection against the gray mold fungus. It is hope that the findings that have been presented in this paper will contribute to a better understanding of the inhibition mechanism of calcium on BC.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hahn M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014;7:133–141.10.1007/s12154-014-0113-1
- Leroux P. Recent developments in the mode of action of fungicides. Pesticide Sci. 1996;47:191–197.10.1002/(SICI)1096-9063(199606)47:2<>1.0.CO;2-7
- Fry SC. Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytol. 2004;161:641–675.10.1111/j.1469-8137.2004.00980.x
- White PJ, Broadley MR. Calcium in Plants. Ann. Bot. 2003;92:487–511.10.1093/aob/mcg164
- El-Mougy NS, Abdel-Kader MM, Aly DEH. Application of plant resistance inducers for controlling early and late blights of tomato under plastic houses conditions. J. Appl. Sci. Res. 2012;8:3406–3414.
- Volpin H, Elad Y. Influence of calcium nutrition on susceptibility of rose flowers to Botrytis cinerea. Phyopathology. 1991;81:1390–1394.10.1094/Phyto-81-1390
- Manganaris GA, Vasilakakis M, Mignani I, et al. The effect of preharvest calcium sprays on quality attributes, physicochemical aspects of cell wall components and susceptibility to brown rot of peach fruits (Prunus persica L. cv. Andross). Sci. Hortic.. 2005;107:43–50.10.1016/j.scienta.2005.06.005
- Cabanne C, Donèche B. Purification and characterization of two isozymes of polygalacturonase from Botrytis cinerea. Microbiol. Res. 2002;157:183–189.10.1078/0944-5013-00147
- Chardonne CO, Sams CE, Conway WS. Calcium effect on the mycelial cell walls of Botrytis cinerea. Phytochemistry. 1999;52:967–973.10.1016/S0031-9422(99)00315-5
- Sasaki I, Nagayama H. Induction of β-glucosidase in Botrytis cinerea by cell wall fractions of the host plant. Biosci. Biotechnol. Biochem. 1997;61:1073–1076.10.1271/bbb.61.1073
- Sasaki I, Nagayama H. β-glucosidase of Botrytis cinerea: its involvement in the pathogenicity of this fungus. Biosci. Biotechnol. Biochem. 1996;60:54–56.10.1271/bbb.60.54
- Sasaki I, Nagayama H. Purification and characterization of β-glucosidase from Botrytis cinerea. Biosci. Biotechnol. Biochem. 1995;59:100–101.10.1271/bbb.59.100
- Khazaeli P, Zamanizadeh H, Morid B, et al. Morphological and molecular identification of Botrytis cinerea causal agent of gray mold in rose greenhouses in centeral regions of Iran. IJASR. 2010;1:20–24.
- Somogyi M. Notes on sugar determination. J. Biol. Chem. 1952;195:19–23.
- Hofer U. Good copper, bad copper. Nat. Rev. Microbiol. 2013;11:299.10.1038/nrmicro3013
- Carafoli E. Calcium pump of the plasma membrane. Physiol. Rev. 1991;71:129–153.
- Boumaaza B, Benkhelifa M, Belkhoudja M. Effects of two salts compounds on mycelial growth, sporulation, and spore germination of six isolates of Botrytis cinerea in the western north of Algeria. Int. J. Microbiol. 2015;2015:1–8.
- Deacon JW. Fungal Biology. 4th ed. Oxford: Wiley-VCH, Blackwell; 2005.10.1002/9781118685068
- Wisniewski M, Droby S, Chalutz E, et al. Effects of Ca²+ and Mg²+ on Botrytis cinerea and Penicillium expansum in vitro and on the biocontrol activity of Candida oleophila. Plant Pathol. 1995;44:1016–1024.
- Brunet S, Scheuer T, Klevit R, et al. Modulation of CaV1.2 channels by Mg2+ acting at an EF-hand motif in the COOH-terminal domain. J. Gen. Physiol. 2005;126:311–323.10.1085/jgp.200509333
- Krystofová S, Varecka L, Betina V. The 45Ca2+ uptake by Trichoderma viride mycelium. Correlation with growth and conidiation. Gen. Physiol. Biophys. 1995 Aug;14(4):323–337.
- Wiesenberger G, Steinleitner K, Malli R, et al. Mg2+ deprivation elicits rapid Ca2+ uptake and activates Ca2+/calcineurin signaling in Saccharomyces cerevisiae. Eukaryot. Cell. 2007;6:592–599.10.1128/EC.00382-06
- Jackson SL, Heath IB. Evidence that actin reinforces the extensible hyphal apex of the oomycete Saprolegnia ferax. Protoplasma. 1990;157:144–153.10.1007/BF01322647
- Harren K, Schumacher J, Tudzynski B. The Ca2+/calcineurin-dependent signaling pathway in the gray mold Botrytis cinerea: the role of calcipressin in modulating calcineurin activity. PLoS ONE. 2012;7:e41761.10.1371/journal.pone.0041761
- Jackson SL, Heath IB. Roles of calcium ions in hyphal tip growth. Microbiol. Rev. 1993;57:367–382.
- Bateman DF, Lumsden RD. Relation between calcium content and nature of the pectic substances in bean hypocotyls of different ages to susceptibility to an isolate of Rhizoctonia solani. Phytopathology. 1965;55:734–738.
- Elad Y, Evensen K. Physiological aspects of resistance to Botrytis cinerea. Phytopathology. 1995;85:637–643.
- Chapman CM, Loewenberg JR, Schaller MJ, et al. Ultrastructural localization of cellulase in Trichoderma reesei using immunocytochemistry and enzyme cytochemistry. J. Histochem. Cytochem. 1983;31:1363–1366.10.1177/31.12.6195212
- Martín JF. Calcium-containing phosphopeptides pave the secretory pathway for efficient protein traffic and secretion in fungi. Microb. Cell Fact. 2014;13:117–127.10.1186/s12934-014-0117-0
- Shahriarinour M, Wahab MNA, Mohamad R, et al. Effect of medium composition and cultural condition on cellulase production by Aspergillus terreus. Afric. J. Biotech. 2011;10:7459–7467.
- Kateva IA, Uversky VN, Ljungdahl LG. Calcium and domain interactions contribute to the thermostability of domains of the multimodular cellobiohydrolase, CbhA, a subunit of the Clostridium thermocellum cellulosome. Biochem. J. 2003;372:151–161.10.1042/bj20021621
