Abstract
ERECTA controls both developmental processes and disease resistance in Arabidopsis. We investigated the function of ERECTA in non-host resistance to Magnaporthe oryzae in Arabidopsis. In the pen2 er mutant, penetration resistance and post-penetration resistance to M. oryzae were compromised. These results suggest that ERECTA is involved in both penetration and post-penetration resistance to M. oryzae in Arabidopsis.
Plants have evolved effective basal defense systems to detect and limit the growth of pathogens. Pathogens can be recognized by the host through the perception of conserved pathogen-associated molecular patterns (PAMPs). PAMPs are recognized by transmembrane pattern recognition receptors (PRRs) that bind with specific PAMPs and initiate intracellular immune responses. Plant PRRs are either surface-localized receptor-like kinases or receptor-like proteins that contain various ligand-binding ectodomains that perceive PAMPs or damage-associated molecular patterns (DAMPs).Citation1)
Disease resistance shown by an entire plant species to all genetic variants of a non-adapted pathogen species is the most common form of plant immunity and termed non-host resistance (NHR).Citation2) Arabidopsis mutants with altered non-host interactions following non-adapted powdery mildew Blumeria graminis hordei infection have been described, and three genes have been identified: PENETRATION 1 (PEN1), PEN2, and PEN3.Citation3–6) PEN1 encodes a plasma membrane-anchored syntaxin with a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) domain.Citation3) PEN2 encodes an atypical myrosinase involved in glucosinolate metabolism in defense responses.Citation4,7,8) PEN3 encodes a pleiotropic drug resistance-type (PDR) ATP-binding cassette transporter.Citation5,6) Although the identified pen mutants (pen1, pen2, and pen3) compromise NHR at the cell periphery, each mutant line retains the ability to mount effective post-invasion immunity, which is linked to a cell death response. Collectively, these studies demonstrated that Arabidopsis NHR to non-adapted biotrophic powdery mildews has two successive multicomponent defense layers: penetration resistance and post-penetration resistance.
Rice blast caused by Magnaporthe oryzae is a devastating disease of rice. The mechanisms of resistance to M. oryzae have been extensively studied, and the rice-M. oryzae pathosystem has become a model system in plant–microbe interaction studies.Citation9,10) However, the mechanisms of NHR to M. oryzae in other plants remain poorly understood. We have found that PEN2, powdery mildew resistance 5 (PMR5), Arabidopsis G-protein beta subunit 1 (AGB1), and mildew locus O 2 (MLO2) are involved in both penetration resistance and post-penetration resistance to M. oryzae in Arabidopsis.Citation11–16) We have also found that penetration resistance to M. oryzae in Col-0 was stronger than that of Ler in Arabidopsis. To examine the genetic basis underlying natural variation in the responses, we used a set of recombinant inbred lines derived from a Col × Ler cross and identified three quantitative trait loci (QTL) that govern the expression of NHR in Arabidopsis against M. oryzae. Among the three QTL, QTL2 on chromosome 2 contained an ERECTA coding sequence.Citation11) The leucine-rich repeat receptor-like kinase ERECTA controls both developmental processes and disease resistance in Arabidopsis.Citation17) ERECTA has been identified as a positive mediator of resistance against bacteria (Ralstonia solanacearum),Citation18) fungi (Plectosphaerella cucumerina and Verticillium longisporum),Citation19,20) and oomycetes (Pythium irregulare).Citation21) The deposition of β-1,3-glucan callose, a defense response of plants to pathogen attack or PAMP treatment, was impaired in the er mutant upon Pl. cucumerina inoculation, but not after inoculation with Pl. parasitica (Peronospora parasitica) or treatment with the flg22 elicitor.Citation19)
In this study, we examined the function of ERECTA in NHR to M. oryzae in Arabidopsis.
Arabidopsis plants were grown under short-day conditions (9:15 L:D) at 22 °C in a growth room. The Arabidopsis accession code was Col-0. We used the following mutants: pen2–1Citation4) and er (SALK_044110)Citation22,23) (all with the Col-0 background). The pen2 er double mutant was generated by crossing er with pen2–1. Plants homozygous for both er and pen2–1 loci were identified in the F2 progeny by PCR genotyping, as previously described.Citation11,13) Fungal inoculation and quantification of cell entry and fungal growth were conducted as previously described.Citation11–16) Data were compared using Tukey’s highly significant difference (HSD) tests. Calculations were performed on three data-sets (n = 3), and p < 0.05 indicated statistically significant effects. For visualization of callose, samples were stained with aniline blue as previously described.Citation11)
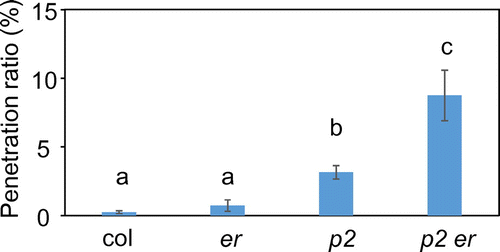
To determine whether ERECTA would affect NHR to M. oryzae in Arabidopsis, the er mutant was inoculated with M. oryzae and monitored by microscopy. Analysis of the M. oryzae challenge for the er mutant at 72-h post-inoculation (hpi) revealed that the rate of entry was slightly higher than that of the wild-type plants, although there were no significant differences between them (Fig. ). Double mutants were generated between the pen2 and er mutants to evaluate the role of ERECTA in NHR to M. oryzae in plants with a pen2 background. We harvested leaves of infected plants at 72 hpi and examined them microscopically. The rate of entry into pen2 er plants was significantly higher than that into pen2 plants (Fig. ).
Fig. 1. Quantitative analysis of penetration resistance to M. oryzae in Arabidopsis mutants.
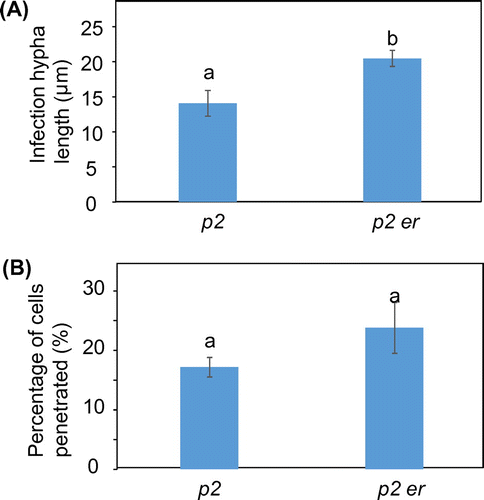
We investigated the role of ERECTA in post-penetration resistance by measuring the lengths of the longest infection hyphae in pen2 er plants at 72 hpi. The infection hyphae in pen2 er plants were significantly longer than those in pen2 plants (Fig. (A)). We also examined the proportion of branched hyphae development in pen2 er plants. The proportion of branched hyphae development in pen2 er plants was slightly higher than that in pen2 plants, although there were no significant differences between them (Fig. (B)).
Fig. 2. Quantitative analysis of post-penetration resistance to M. oryzae in Arabidopsis mutants.
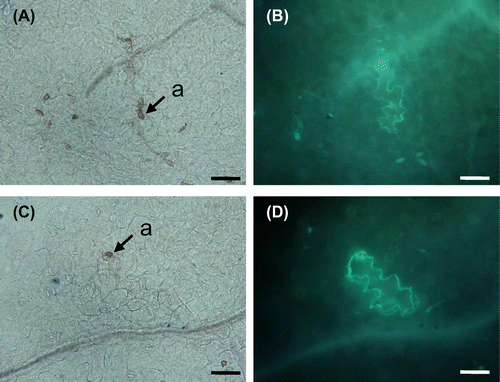
To determine whether ERECTA would affect callose accumulation in NHR to M. oryzae in Arabidopsis, infection sites were stained with aniline blue. We harvested leaves of the infected plants at 72 hpi and examined them microscopically. UV-induced fluorescence with aniline blue was detected in invaded epidermal cells. We found that callose accumulated in pen2 er plants after M. oryzae infection (Fig. ).
Fig. 3. Accumulation of callose at infection sites in Arabidopsis mutants.
In conclusion, penetration resistance and post-penetration resistance in the pen2 er mutant were compromised. This suggests that ERECTA is a positive regulator of NHR to M. oryzae in Arabidopsis.
Author contributions
A.I. designed the research; T.T., H.S., and A.I. performed the research; A. I. wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by JSPS KAKENHI [grant number 26450058].
Acknowledgments
We would like to acknowledge ABRC for providing seeds of Col-0 and er (SALK_044110). We thank Dr H. Koga (Ishikawa Prefectural University) for providing the M. oryzae isolate and Dr P. Shulze-Lefert (Max Planck Institute for Plant Breeding Research) for seeds of pen2-1.
References
- Zipfel C. Plant pattern-recognition receptors. Trends Immunol. 2014;35:345–351.10.1016/j.it.2014.05.004
- Lipka U, Fuchs R, Lipka V. Arabidopsis non-host resistance to powdery mildews. Curr. Opin. Plant Biol. 2008;11:404–411.10.1016/j.pbi.2008.04.004
- Collins NC, Thordal-Christensen H, Lipka V, et al. SNARE-protein-mediated disease resistance at the plant cell wall. Nature. 2003;425:973–977.10.1038/nature02076
- Lipka V, Dittgen J, Bednarek P, et al. Pre- and Postinvasion Defenses Both Contribute to Nonhost Resistance in Arabidopsis. Science. 2005;310:1180–1183.10.1126/science.1119409
- Stein M, Dittgen J, Sanchez-Rodriguez C, et al. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell. 2006;18:731–746.10.1105/tpc.105.038372
- Kobae Y, Sekino T, Yoshioka H, et al. Loss of AtPDR8, a plasma membrane ABC transporter of Arabidopsis thaliana, causes hypersensitive cell death upon pathogen infection. Plant Cell Physiol. 2006;47:309–318.10.1093/pcp/pcj001
- Bednarek P, Pislewska-Bednarek M, Svatos A, et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science. 2009;323:101–106.10.1126/science.1163732
- Clay NK, Adio AM, Denoux C, et al. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science. 2009;323:95–101.10.1126/science.1164627
- Koga H. Cytological aspects of infection by the rice blast fungus Pyricularia oryzae. In: Sreenivasaprasad S, Johnson R, editors. Major fungal diseases of rice: recent advances. Netherlands: Springer; 2001. p. 87–110.10.1007/978-94-017-2157-8
- Ebbole DJ. Magnaporthe as a model for understanding host-pathogen interactions. Annu. Rev. Phytopathol. 2007;45:437–456.10.1146/annurev.phyto.45.062806.094346
- Maeda K, Houjyou Y, Komatsu T, et al. AGB1 and PMR5 contribute to PEN2-mediated preinvasion resistance to Magnaporthe oryzae in Arabidopsis thaliana. Mol. Plant Microbe. Interact. 2009;22:1331–1340.10.1094/MPMI-22-11-1331
- Maeda K, Houjyou Y, Komatsu T, et al. Nonhost resistance to Magnaporthe oryzae in Arabidopsis thaliana. Plant Signal. Behav. 2010;5:755–756.10.4161/psb.5.6.11770
- Nakao M, Nakamura R, Kita K, et al. Non-host resistance to penetration and hyphal growth of Magnaporthe oryzae in Arabidopsis. Sci. Rep. 2011;1:171.
- Nozaki M, Kita K, Kodaira T, et al. AtRbohF contributes to non-host resistance to Magnaporthe oryzae in Arabidopsis. Biosci. Biotechnol. Biochem. 2013;77:1323–1325.10.1271/bbb.130092
- Okawa C, Ishikawa A. MPK6 contributes to non-host resistance to Magnaporthe oryzae in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2013;77:1320–1322.10.1271/bbb.130082
- Okawa C, Nakao M, Ishikawa A. Penetration and post-penetration resistance to Magnaporthe oryzae in Arabidopsis. Biosci. Biotechnol. Biochem. 2013;77:1776–1778.10.1271/bbb.130213
- van Zanten M, Snoek LB, Proveniers MC, et al. The many functions of ERECTA. Trends Plant Sci. 2009;14:214–218.10.1016/j.tplants.2009.01.010
- Godiard L, Sauviac L, Torii KU, et al. ERECTA, an LRR receptor-like kinase protein controlling development pleiotropically affects resistance to bacterial wilt. Plant J. 2003;36:353–365.10.1046/j.1365-313X.2003.01877.x
- Llorente F, Alonso-Blanco C, Sánchez-Rodriguez C, et al. ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant J. 2005;43:165–180.10.1111/tpj.2005.43.issue-2
- Häffner E, Karlovsky P, Splivallo R, et al. ERECTA, salicylic acid, abscisic acid, and jasmonic acid modulate quantitative disease resistance of Arabidopsis thaliana to Verticillium longisporum. BMC Plant Biol. 2014;14:85.10.1186/1471-2229-14-85
- Adie BA, Perez-Perez J, Perez-Perez MM, et al. ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell. 2007;19:1665–1681.10.1105/tpc.106.048041
- Hunt L, Gray JE. The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development. Curr. Biol. 2009;19:864–869.10.1016/j.cub.2009.03.069
- Durbak AR, Tax FE. CLAVATA signaling pathway receptors of Arabidopsis regulate cell proliferation in fruit organ formation as well as in meristems. Genetics. 2011;189:177–194.10.1534/genetics.111.130930
