Abstract
Recently, it has been reported that increased expression of WNT1 accelerates the differentiation of melanocyte stem cells (McSCs) in solar lentigines (SLs), hyperpigmented maculae commonly seen on sun-exposed areas of the skin. In this study, to establish an in vitro SL model, human epidermal squamous carcinoma cell line HSC-1, which expresses higher levels of WNT1 than normal human epidermal keratinocytes, was co-cultured with early passage normal human epidermal melanocytes (NHEMs) as an in vitro McSC model. As a result, mRNA expression levels of melanocyte differentiation-related genes MITF and TYR in NHEMs were significantly increased by co-culturing with HSC-1 cells. Furthermore, Phalaenopsis orchid extract (Phex) inhibited McSCs differentiation by suppressing WNT1 expression via down-regulation of DLX2, a transcriptional activator of WNT1, in HSC-1 cells. Therefore, our finding suggested that extracts such as Phex, which suppresses WNT1 expression, may be useful as a novel treatment of SLs.
Graphical abstract
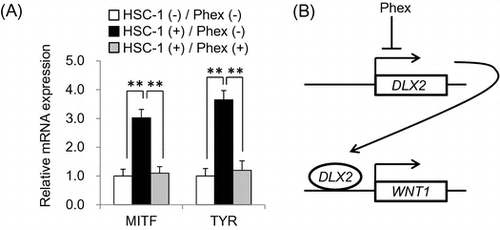
Phalaenopsis orchid extract (Phex) suppressed McSC differentiation (A) by inhibiting WNT1 expression via down-regulation of DLX2 (B).
Melanocytes are located in the epidermis and hair bulbs, contributing to the color of the skin and hair, respectively.Citation1) They play an important role in protecting the skin from UV radiation by producing melanin. Tyrosinase (TYR) is a rate-limiting enzyme in the biosynthesis of melanin, and its expression is regulated by micophthalmia-associated transcription factor (MITF); therefore, these factors are essential for melanogenesis. Melanocytes produce melanin in melanosomes, lysosome-related organelles, and transfer several melanosomes packaged in a vesicle to surrounding keratinocytes.Citation2) Melanosomes are normally located above the superior pole of the nucleus in keratinocytes, forming a so-called “melanin cap”.
Melanocytes can also be involved in aesthetic appearance, because melanocyte dysfunction such as abnormalities in melanogenesis, melanosome transfer, and survival can cause abnormal coloration of the skin and hair. Solar lentigines (SLs) are hyperpigmented maculae that arise owing to excess melanin synthesis and an increased number of melanocytes in the lesions.Citation3,4) Because SLs are commonly seen on sun-exposed areas such as face and arms, they are considered to be mainly caused by UV radiation.Citation5) Such abnormal coloration of the skin may have a negative impact on an individual’s appearance. This has led to the development of skin-lightening agents, which appropriately control melanin synthesis, to improve the quality of life of people who are negatively impacted by SLs.Citation6) Previous studies demonstrated that the melanogenesis-stimulating factors, stem cell factor (SCF), and endothelin 1 (EDN1) were up-regulated, and TYR expression levels were increased, in SL lesions.Citation7,8) Therefore, many studies have been conducted to develop skin-lightening agents that suppress melanogenesis by means of TYR inhibition and down-regulation of melanogenesis-stimulating factors using the B16F10 mouse melanoma cell line, normal human epidermal melanocytes (NHEMs), and so on. For example, arbutin, one of the most popular skin-lightening compounds, suppresses melanogenesis by inhibiting TYR activityCitation9) and down-regulating TYR expressionCitation10). Although conventional treatments are based on such a strategy, and the inhibition of melanogenesis in epidermal melanocytes is effective, it is not capable of completely restoring the normal skin color of SL lesions.
It has been demonstrated the origin of melanocytes in adult mammals, termed a melanocyte stem cell (McSC), is located in the bulge area of hair follicles.Citation11) McSCs differentiate into mature melanocytes and migrate to hair bulbs to supply melanin to hair shafts. According to a recent study, McSCs also supply mature melanocytes to the epidermis in response to UV radiation.Citation12) In these processes, Wnt/β-catenin signaling triggers McSC differentiation.Citation12,13) Furthermore, increased expression of WNT1 in keratinocytes and accelerated differentiation of McSCs were found in SL lesions,Citation14) which were considered to be the cause of the increased number of melanocytes. These findings led to the assumption that appropriate regulation of McSC differentiation is necessary to develop a novel treatment for SLs, because conventional skin-lightening agents are only a symptomatic treatment. To address this issue, we established a novel in vitro SL model focusing on McSC differentiation to screen skin-lightening agents to determine the effectiveness of novel treatments, and we found that the Phalaenopsis orchid extract (Phex) suppressed the differentiation of NHEMs at early passage, an in vitro McSC model, by down-regulating WNT1 expression by HSC-1 cells, a WNT1-expressing epidermal keratinocyte cell line. These results suggested that Phex will be useful for the treatment of SLs.
Materials and methods
Preparation of Phalaenopsis orchid extract
Dried mericlone of Phalaenopsis orchid [Phalaenopsis (Shirane × lueddemanniana) “Harugasumi”] (1 g), which was grown in house, was extracted with 20 ml of hot water and dried using a freeze dryer. The yield of the water extract was about 0.2 g. The extract was dissolved in PBS and added to cell culture media.
Cell culture
NHEMs were purchased from TOYOBO (Osaka, Japan) and maintained between passages 1 and 3 in Medium254 containing Human Melanocyte Growth Supplement (Life Technologies, Carlsbad, CA, USA). A human skin squamous carcinoma cell line, HSC-1, and a human oral squamous carcinoma cell line, HSC-3, were obtained from JCRB Cell Bank (Japanese Collection of Bioresources Cell Bank) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS).
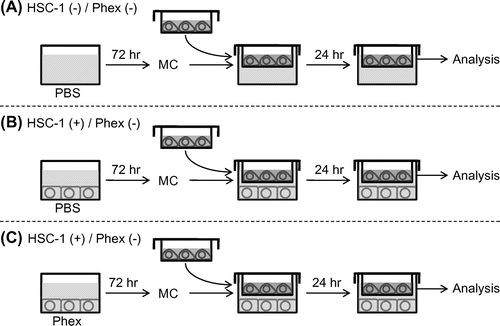
As shown in Fig. , HSC-1 and NHEMs were co-cultured using membrane inserts with a pore size of 0.4 μm in 24-well culture plates (BD Biosciences, San Jose, CA, USA). Briefly, HSC-1 cells were seeded onto 24-well plates at a density of 4 × 104 cells per well and cultured for 24 h. The culture medium was changed to DMEM containing 2% FBS with or without Phex. After 72 h, culture medium was replaced with DMEM without FBS for co-culturing with NHEMs. As a negative control, DMEM was added into an empty well. NHEMs (4 × 104 cells) were seeded in the upper chamber coated with 0.1% gelatin. After 24 h, the culture medium was replaced with Medium254 without growth supplement, and these chambers were moved onto a plate in which HSC-1 cells were cultured. After 24 h of co-culture, NHEMs were harvested for gene expression analysis.
Fig. 1. Experimental design of the in vitro solar lentigines (SL) model.
Cell viability assay
To analyze the cell toxicity of Phex, HSC-1 cells were seeded onto 96-well plates at a density of 1 × 104 cells per well and cultured for 24 h. The culture medium was changed to DMEM containing 2% FBS with or without Phex. After 24 h, the relative cell number was measured by a modified MTT assay using Cell Counting Kit-8 (DOJINDO Laboratories, Kumamoto, Japan) in accordance with the manufacturer’s protocol. In brief, after removing the medium, 100 μL of CCK-8 solution was added to the cells, followed by 2 h of incubation. Optical density values were determined at an absorbance of 450 nm using a microplate reader (Molecular Devices, Inc., Menlo Park, CA, USA).
Small interfering RNA transfection
HSC-1 cells were seeded onto 24-well plates at a density of 4 × 104 cells per well in DMEM containing 10% FBS and cultured for 24 h. The culture medium was replaced with OPTI-MEM® (Life Technologies) containing 50 nM negative control small interfering RNA (siRNA), or a mixture of three types of siRNAs (Bioneer, Daejeon, Korea, siRNA No. 1042398, siRNA No. 1042397, siRNA No. 1042395) targeting DLX2 to avoid off-target effects. After 4 h of incubation, OPTI-MEM was replaced with fresh culture medium, and the cells were cultured for another 24 h.
Real-time RT-PCR
Total RNA was extracted from cells using RNAIso Plus (Takara Bio, Shiga, Japan). cDNA was synthesized by reverse transcription using a High Capacity RNA-to-cDNA kit (Applied Biosystems, Tokyo, Japan). Real-time semiquantitative RT-PCR was performed with SYBR® Select Master Mix (Applied Biosystems) using a StepOnePlus Real-time RT-PCR system (Applied Biosystems) in accordance with the manufacturer’s protocol. The primer sequences used were as follows: ACTB forward, 5′-CACTCTTCCAGCCTTCCTTCC-3′ and reverse, 5′-GTGTTGGCGTACAGGTCTTTG-3′; MITF forward, 5′-GAGGCAGTGGTTTGGGCTT-3′ and reverse, 5′-AATTCTGCACCCGGGAATC-3′; TYR forward, 5′-TGCGGTGGGAACAAGAAATC-3′ and reverse, 5′-GAAGAATGATGCTGGGCTGAGT-3′; DLX2 forward, 5′-AGCCAACAACGAGCCTGAGA-3′ and reverse, 5′-TTTCTTTGGCTTCCCGTTCA-3′; and SIX3 forward, 5′-GTATTCCGCTCCCCCCTAGA-3′ and reverse, 5′-TGGTGAGAATCGGCGAAGTT-3′; SCF forward, 5′-CCGGGATGGATGTTTTGC-3′ and reverse, 5′-CAAGCTGTCTGACAATTGTACTACCA-3′; EDN1 forward, 5′-TACTTCTGCCACCTGGACATCA-3′ and reverse, 5′-CTCACGGTCTGTTGCCTTTGT-3′; WNT4 forward, 5′-GAGGAGGAGACGTGCGAGAA-3′ and reverse, 5′-CAGGTTCCGCTTGCACATCT-3′; WNT7A forward, 5′-TCAGTTTCAGTTCCGCAATGG-3′ and reverse, 5′-CTTTGAGCTCCTTCCCGAAGA-3′; WNT10A forward, 5′-AAGCCTGGAGACTCGCAACA-3′ and reverse, 5′-TCTCGGAAACCTCTGCTGAAG-3′; amplification was normalized to the housekeeping gene β-actin (ACTB), and differences between samples were quantified based on the ΔΔCt method. All PCR products were checked by melting curve analysis to exclude the possibility of multiple products or an incorrect product size.
Immunocytochemistry
To analyze the inhibitory effect of Phex on WNT1 expression level, HSC-1 cells were seeded onto 24-well plates at a density of 2.5 × 104 cells per well and cultured for 24 h. The culture medium was changed to DMEM containing 2% FBS with or without Phex. After 72 h, HSC-1 cells were fixed with 4% paraformaldehyde for 5 min. The cells were blocked with 2% BSA for 1 h and were then incubated with rabbit anti-WNT1 antibody (1:200; Gentex, San Antonio, TX, USA) and mouse anti-β-actin antibody (1:1000; Sigma, St Louis, Mo, USA) overnight at 4 °C. After washing with PBS, the cells were incubated with secondary antibodies conjugated to Alexa488 and Alexa594 (Life Technologies, Carlsbad, CA, USA). 4′,6-Diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA) was used for nuclear staining. Stained cells were observed by fluorescent microscopy, and microscopic images were collected randomly. Relative fluorescent intensity of the images were analyzed using NIH Image, and the relative expression levels of WNT1 were normalized with fluorescent intensity of β-actin.
Western blotting
Cells were lysed with 2% sodium dodecyl sulfate (SDS) containing protease inhibitor cocktail (Sigma). After centrifugation at 5000 ×g, the supernatant was collected, and protein concentrations were determined using a BCA Protein Assay kit (Thermo Fisher Scientific, Yokohama, Japan). The protein samples were separated by SDS-polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 5% skimmed milk (BD Biosciences) in 0.1% Tween-20 Tris-buffered saline (TTBS), the membranes were incubated in TTBS containing primary antibodies against WNT1 (1:200; Gentex), DLX2 (1:100; Abgent, San Diego, CA, USA), or β-actin (1:5000; abcam, Cambridge, MA, USA). The membranes were washed in TTBS, incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson Immuno Research Laboratories, West Grove, PA, USA), and visualized using Amersham ESL Prime (GE Healthcare, Buckinghamshire, UK) in accordance with the manufacturer’s protocol.
Statistical analysis
All experiments were carried out in triplicate. Data are presented as the mean ± standard deviation (SD). p < 0.05 was considered significant. Statistical analysis was performed using analysis of variance with Tukey–Kramer multiple comparison or Student’s t-test.
Results
Establishment of in vitro SL model
WNT1 expression is increased in epidermal keratinocytes in SL lesions, and it accelerates McSC differentiation.Citation14) Therefore, it is necessary that McSC differentiation is appropriately controlled by down-regulation of WNT1 expression in order to develop a novel treatment for SLs. However, there are some important points in establishing a screening system for ingredients that suppress differentiation of McSCs induced by WNT1. Although WNT1 activates Wnt/β-catenin in McSCs, it is not appropriate to evaluate the effect on the downstream signaling. This is because it may lead to choosing ingredients that inhibit Wnt/β-catenin signaling activated by such as WNT4, WNT7A, and WNT10A,Citation12,15) which are not associated with the formation of SLs but important for differentiation of McSCs in normal skin, as candidate medication for SL. To eliminate such possibilities, it is appropriate to screen ingredients that suppress expression of WNT1. Thus, keratinocytes that highly express WNT1 should be used.
As shown in Fig. (A), normal human epidermal keratinocytes (NHEKs) expressed WNT1 at a low level. As reported previously, human oral squamous carcinoma cell line HSC-3 expressed much higher levels of WNT1 than NHEKs.Citation16) To reproduce the microenvironment of SLs in vitro, we selected human epidermal squamous carcinoma cell line HSC-1 as WNT1-expressing epidermal keratinocytes (Fig. (A)). These cells also expressed higher levels of WNT1 than NHEKs. It has been reported that NHEMs at early passage can be used as an in vitro McSC model.Citation17) While McSCs in vivo and early passage melanocytes in vitro exhibit similar response to TGF-β1 which keeps McSCs in a dormant state, melanoma cells in vitro do not exhibit such response. Considering that early passage NHEMs had similar characteristics as McSCs, we determined that NHEMs were suitable for use as McSC model cells.
Fig. 2. Establishment of in vitro SL model.
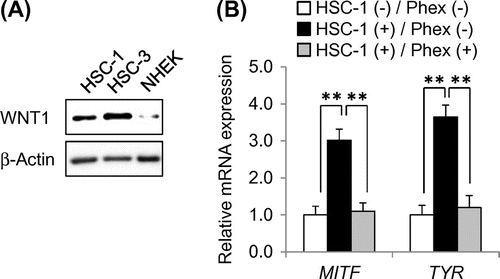
Therefore, NHEMs were co-cultured with HSC-1 to confirm whether HSC-1 cells can induce McSC differentiation. Real-time PCR analysis showed that mRNA expression levels of melanocyte differentiation-related genes MITF and TYR in NHEMs were significantly increased when co-cultured with HSC-1 cells as compared with those in NHEMs cultured alone (Fig. (B)). MITF is a transcription factor acting as a master regulator of melanocyte differentiation and necessary for expression of melanogenesis-related genes. Since TYR is a rate-limiting enzyme in melanin synthesis, TYR is critical for mature melanocytes having melanogenic ability.Citation1) These results indicated that co-culturing with HSC-1 induced the differentiation of NHEMs into mature melanocytes, and this culture system was considered to be suitable as an in vitro SL model.
Effect of Phalaenopsis orchid extract (Phex) on WNT1 expression and McSC differentiation
Because McSC differentiation was induced by a high expression level of WNT1 by HSC-1, we examined whether Phex suppressed WNT1 expression levels in HSC-1 cells. As Phex showed cell toxicity at 200 μg/mL with HSC-1 cells (Fig. (A)), Phex was added at a maximum concentration of 100 μg/mL to analyze its inhibitory effect on the expression of WNT1 by immunostaining. As a result, shown in Fig. (B) and (C), Phex suppressed WNT1 expression in a concentration-dependent manner. The same result was obtained by western blotting method (Fig. (D)). In addition, there was no remarkable change in the expressions of SCF and EDN1, major melanocyte-stimulating factors secreted by keratinocytes; and WNT4, WNT7A, and WNT10A,Citation12,15) predominant Wnt ligands in the epidermis. Furthermore, the mRNA expression levels of MITF and TYR in NHEMs co-cultured with Phex-pretreated HSC-1 cells were significantly decreased compared with those in NHEMs co-cultured with non-treated HSC-1 cells (Fig. (B)). Taken together, these results strongly suggested that Phex suppressed McSC differentiation via down-regulation of WNT1 expression in epidermal keratinocytes in SL lesions.
Fig. 3. Phex-suppressed Wnt1 expression in HSC-1 cells.
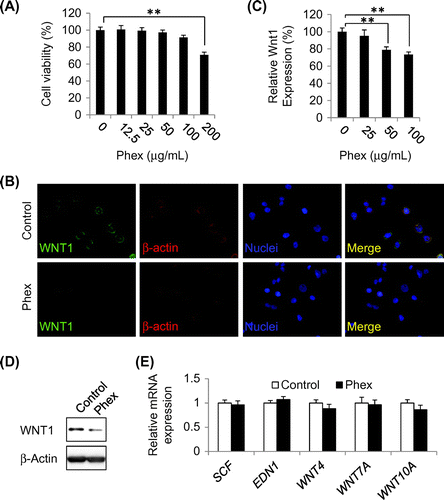
Fig. 4. Phex suppressed WNT1 expression via down-regulation of DLX2 expression in HSC-1 cells.
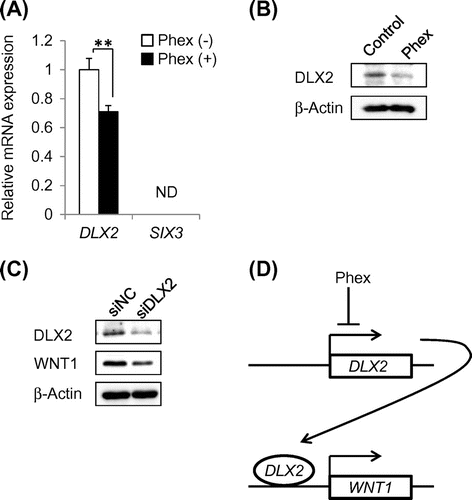
Phex-suppressed WNT1 expression via down-regulation of DLX2
To identify how Phex suppressed WNT1 expression, real-time PCR was performed to analyze the expression levels of these two transcription factors in HSC-1 cells. As a result, Phex significantly suppressed mRNA expression of DLX2 in HSC-1 cells compared with an untreated control (Fig. (A)). It has been reported that DLX2, a transcription factor, binds to the WNT1 promoter and induces mRNA expression.Citation18) On the other hand, mRNA expression of SIX3, a direct repressor of WNT1 transcription,Citation19) was not detected in HSC-1 cells (Fig. (A)). Western blotting analysis showed that Phex also suppressed DLX2 protein level in HSC-1 cells (Fig. (B)). Finally, siRNA targeting DLX2 (siDLX2) was transfected into HSC-1 cells to confirm whether DLX2 regulated WNT1 expression. DLX2 protein expression was clearly down-regulated in siDLX2-transfected cells compared with cells transfected with negative control siRNA (Fig. (C)). Because WNT1 protein expression was also suppressed in siDLX2-transfected cells, these results indicated that DLX2 up-regulates WNT1 expression in HSC-1 cells. Therefore, Phex suppressed WNT1 expression via down-regulation of DLX2, as shown in Fig. (D).
Discussion
To establish a novel in vitro SL model focusing on McSC differentiation to screen skin-lightening agents, early passage NHEMs, McSC model cells, were co-cultured with HSC-1, a WNT1-expressing epidermal keratinocyte cell line, and their differentiation was successfully induced (Fig. ). Using this novel in vitro SL model, it was demonstrated that Phex suppressed McSC differentiation (Figs. and ). siRNA-based knockdown analysis showed that Phex inhibited WNT1 expression via down-regulation of DLX2, a transcriptional activator of Wnt1 (Fig. ). Therefore, it was strongly suggested that Phex may be useful as a novel treatment of SLs. These results show that screening for active ingredients of SL medications can be carried out by examining inhibitory effect on the expression of WNT1 in HSC-1, making it possible for screening to be very simple.
Hyperpigmentation in SL lesions was thought to be caused by enhanced melanogenesis in the epidermal melanocytes. Thus, most of previous studies on developing skin-lightening agents focused on inhibiting the expression and the activity of TYR, a rate-limiting enzyme in the biosynthesis of melanin. For examples, Elephantopus mollis H. B. and K. extract reduces TYR expression,Citation20) and Salicornia herbaces extract inhibits TYR activity.Citation21) These plant extracts are expected to exert skin-lightening effect by down-regulation of melanogenesis in the epidermal melanocytes, however, they are likely to fail in complete improvement of abnormal hyperpigmentation in SLs. To provide a more effective treatment for SLs, a novel skin-lightening technology is needed to reduce the number of epidermal melanocytes caused by the accelerated differentiation of McSCs. In other words, the appropriate regulation of McSC differentiation is inevitable for the fundamental treatment of SLs. Therefore, in combination with conventional technologies, a novel skin-lightening technology based on controlling McSC differentiation may help to fully restore the skin color in SL lesions, although further investigations are needed. Phex suppressed McSC differentiation by down-regulating WNT1 expression and may be a key to a potential novel treatment of SLs. Using our novel in vitro SL model, we can screen compounds and plant extracts which regulate McSC differentiation by reduction of WNT1 expression as well as Phex.
Author contributions
Takaaki Yamada, Seiji Hasegawa, Mayumi Kunita, Kazuhisa Ohsumi, and Tsutomu Sakaida conceived and designed the experiments. Takaaki Yamada, Yu Inoue, Mayumi Kunita, and Kazuhisa Ohsumi performed the experiments. Takaaki Yamada, Seiji Hasegawa, Yu Inoue, Tsutomu Sakaida, Youichi Yashiro, and Satoru Nakata analyzed data.
Takaaki Yamada, Seiji Hasegawa, Youichi Yashiro, and Satoru Nakata wrote the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35:193–199.10.1002/biof.v35:2
- Ando H, Niki Y, Ito M, et al. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Invest. Dermatol. 2012;132:1222–1229.10.1038/jid.2011.413
- Cario-Andre M, Lepreux S, Pain C, et al. Perilesional vs. lesional skin changes in senile lentigo. J. Cutaneous Pathol. 2004;31:441–447.10.1111/cup.2004.31.issue-6
- Yamada T, Hasegawa S, Inoue Y, et al. Comprehensive analysis of melanogenesis and proliferation potential of melanocyte lineage in solar lentigines. J. Dermatol. Sci. 2014;73:251–257.10.1016/j.jdermsci.2013.11.005
- Monestier S1, Gaudy C, Gouvernet J, et al. Multiple senile lentigos of the face, a skin ageing pattern resulting from a life excess of intermittent sun exposure in dark-skinned caucasians: a case-control study. Br. J. Dermatol. 2006;154:438–444.10.1111/bjd.2006.154.issue-3
- Gillbro JM, Olsson MJ. The melanogenesis and mechanisms of skin-lightening agents–existing and new approaches. Int. J. Cosmet. Sci. 2011;33:210–221.10.1111/ics.2011.33.issue-3
- Hattori H, Kawashima M, Ichikawa Y, et al. The epidermal stem cell factor is over-expressed in lentigo senilis: implication for the mechanism of hyperpigmentation. J. Invest. Dermatol. 2004;122:1256–1265.10.1111/j.0022-202X.2004.22503.x
- Kadono S, Manaka I, Kawashima M, et al. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J. Invest. Dermatol. 2001;116:571–577.10.1046/j.1523-1747.2001.01296.x
- Maeda K, Fukuda M. Arbutin: mechanism of its depigmenting action in human melanocyte culture. J. Pharmacol. Exp. Ther. 1996;276:765–769.
- Inoue Y, Hasegawa S, Yamada T, et al. Analysis of the effects of hydroquinone and arbutin on the differentiation of melanocytes. Biol. Pharm. Bull. 2013;36:1722–1730.10.1248/bpb.b13-00206
- Nishimura EK, Jordan SA, Oshima H, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860.10.1038/416854a
- Yamada T, Hasegawa S, Inoue Y, et al. Wnt/β-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J. Invest. Dermatol. 2013;133:2753–2762.10.1038/jid.2013.235
- Rabbani P, Takeo M, Chou W, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–955.10.1016/j.cell.2011.05.004
- Yamada T, Hasegawa S, Inoue Y, et al. Accelerated differentiation of melanocyte stem cells contributes to the formation of hyperpigmented maculae. Exp. Dermatol. 2014;23:652–658.10.1111/exd.12496
- Saitoh A, Hansen LA, Vogel JC, et al. Characterization of Wnt gene expression in murine skin: possible involvement of epidermis-derived Wnt-4 in cutaneous epithelial-mesenchymal interactions. Exp. Cell Res. 1998;243:150–160.10.1006/excr.1998.4152
- Taki M, Kamata N, Yokoyama K, et al. Down-regulation of Wnt-4 and up-regulation of Wnt-5a expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Cancer Sci. 2003;94:593–597.10.1111/cas.2003.94.issue-7
- Nishimura EK, Suzuki M, Igras V, et al. Key roles for transforming growth factor β in melanocyte stem cell maintenance. Cell Stem Cell. 2010;6:130–140.10.1016/j.stem.2009.12.010
- Kuwajima T, Nishimura I, Yoshikawa K. Necdin promotes GABAergic neuron differentiation in cooperation with Dlx homeodomain proteins. J. Neurosci. 2006;26:5383–5392.10.1523/JNEUROSCI.1262-06.2006
- Kumar R, Balasenthil S, Manavathi B, et al. Metastasis-associated protein 1 and its short form variant stimulates Wnt1 transcription through promoting its derepression from Six3 corepressor. Cancer Res. 2010;70:6649–6658.10.1158/0008-5472.CAN-10-0909
- Hasegawa K, Furuya R, Mizuno H. Inhibitory effect of Elephantopus mollis H.B. and K. extract on melanogenesis in B16 murine melanoma cells by downregulating microphthalmia-associated transcription factor expression. Biosci. Biotechnol. Biochem. 2010;74:1908–1912.10.1271/bbb.100318
- Sung JH, Park SH, Seo DH. Antioxidative and skin-whitening effect of an aqueous extract of Salicornia herbacea. Biosci. Biotechnol. Biochem. 2009;73:552–556.10.1271/bbb.80601
