Abstract
Inappropriate platelet aggregation can cause blood coagulation and thrombosis. In this study, the effect of an ethanol extract of Ramulus mori (ERM) on blood circulation was investigated. The antithrombotic activity of ERM on rat carotid arterial thrombosis was evaluated in vivo, and the effect of ERM on platelet aggregation and blood coagulation time was evaluated ex vivo. To evaluate the safety of ERM, its cytotoxicity to platelets and its effect on tail bleeding time were assessed; ERM was not toxic to rat platelets and did not prolong bleeding time. Moreover, administering ERM to rats had a significant preventive effect on carotid arterial thrombosis in vivo, and significantly inhibited adenosine diphosphate- and collagen-induced platelet aggregation ex vivo, whereas it did not prolong coagulation periods, such as prothrombin time and activated partial thromboplastin time. The results suggest that ERM is effective in improving blood circulation via antiplatelet activity rather than anticoagulation activity.
Graphical abstract
Ex vivo antiplatelet effect of the ethanol extract of Ramulus mori containing 6.79% oxyresveratrol. Platelet aggregation was induced by (A) ADP and (B) collagen.
Blood delivers essential substances, such as oxygen and nutrients, to tissues and transports metabolic wastes away from tissues. Continuous blood flow allows for proper bodily functioning; however, hemostasis is also important. When a blood vessel is damaged, rapid blood coagulation and aggregation minimize blood loss. Maintaining a balance between blood flow and hemostasis is very important because excessive bleeding can cause death, whereas excessive coagulation and aggregation can cause thrombus formation in the blood vessels, blocking blood flow and causing death. Furthermore, a thrombus can cause thrombosis, and thrombosis in brain vessels or in coronary arteries can lead to acute thromboembolic occlusion, ischemic stroke, and arteriosclerosis, among other disorders.Citation1) The incidence of these types of thrombosis is drastically increased in the elderly population.Citation2) Due to the rising aging population, it is increasingly important to treat thrombosis. To reduce or prevent thrombosis-related diseases, researchers are seeking to improve blood circulation and prevent the formation of blood clots.
Blood clotting occurs due to the aggregation of platelets and/or the activation of the coagulation cascade by coagulation-activating factors. Platelets play a very important role in coagulation.Citation3) When a blood vessel is damaged, the platelets within it employ coagulation and aggregation mechanisms to halt the bleeding. In particular, platelets are activated by multiple physiological molecules that interact with their specific receptors and trigger the activation of various signaling pathways.Citation4) Platelets interact with the subendothelial matrix through the binding of glycoprotein (GP) Ib-IX-V and αIIbβ3 on the platelet membrane to collagen and von Willebrand factor in the matrix. The binding of GP VI to collagen is required for platelet activation, which causes platelets to release adenosine diphosphate (ADP) and thromboxane A2 (TXA2), which accelerate platelet recruitment and activation, resulting in thrombus formation.Citation5) Thus, excessive platelet activation can cause the generation of a thrombus that obstructs blood flow. A thrombus can block blood flow and initiate the pathogenesis of platelet-mediated cardiovascular diseases. The formation of pathogenic thrombi can cause acute vascular atherothrombotic diseases, such as acute coronary syndrome, ischemic stroke, and symptomatic peripheral arterial disease.Citation6) Moreover, platelets are a source of inflammatory mediators, and platelet activation by inflammation may be a critical component of atherothrombosis.Citation7)
Morus alba L., which belongs to the Mulberry family (Moraceae), has long been a popular medicinal plant. All of the medicinal parts of this plant, including the leaves (Sangye), twigs (Sangzhi), root barks (Sangbaipi), and fruits (Sangshen), are described in the Chinese Pharmacopoeia 2010.Citation8) Moreover, Ramulus mori (called Sangzhi in Chinese), which was used in this study, contains quercetin, kaempferol, mulberroside A, resveratrol, trans-resveratrol, oxyresveratrol, trans-oxyresveratrol, moracin M, 1-deoxynojirimycin, and R. mori polysaccharides.Citation9) Kaempferol has many properties, such as anticancer, anti-inflammatory, and antioxidant activities. Resveratrol has many properties that are beneficial to health, such as antioxidant, blood thinning, and lifespan-extending activities. Oxyresveratrol, which is an effective tyrosine inhibitor, also has many health benefits.Citation10) In a recent study, polysaccharides derived from R. mori exhibited an anti-diabetic effect.Citation11) Moreover, R. mori components have an inhibitory effect on melanogenesisCitation12) and possess anti-inflammatory and analgesic activities.Citation13) In this study, we investigated the inhibitory effects of an ethanol extract of R. mori (ERM) containing 6.79% oxyresveratrol on arterial thrombosis in vivo, platelet aggregation ex vivo, and coagulation in vitro.
Materials and methods
Materials and animals. The Ginkgo biloba extract (Ginkgo extract, GE, Lot No. 6066J01834), which was used as a comparative material, was purchased from GNC (Pittsburgh, PA, USA). β-nicotinamide adenine dinucleotide, reduced disodium salt hydrate (β-NADH), pyruvate, and aspirin (Lot No. MKBQ8444 V; purity, 99.5 ≥) were purchased from Sigma (St. Louis, MO, USA). Five-week-old male Sprague-Dawley (SD) rats were purchased from the Dae-Han Biolink (Eum sung, South Korea). Seven-week-old male SD rats and 8-week-old male ICR mice were purchased from Koatech (Pyeongtaek, Korea). The animals were acclimatized for 1 week at 22 ± 2 °C and 50 ± 5% humidity, with 12-h cycles of light and dark. All experimental procedures were approved by the Korea University Institutional Animal Care and Use Committee (Approval No. KUIACUC-20140515-2), and were performed in accordance with the guidelines in the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1996). During treatment, body weight was measured twice a week.
Preparation of ERM. ERM was prepared as described previously.Citation14) In brief, R. mori was collected from Gwangchen (Chungnam, Korea) and was dried for 3 days at 55 °C using a hot air dryer (KDO-150, Kukje Engineering, Goyang, Korea). The dried sample (1 kg) was cut, washed with distilled water, and then extracted for 48 h in a sonicator (HO429115, Hwashin Instrument Co., Seoul, Korea) using 8 l of 80% ethanol. The ethanol extract was vacuum filtered using a Whatman™ 1822-047 GF/C glass fiber filter (pore size: 1.2 μM, Uppsala, Sweden) and then was concentrated to 500 mL using a rotary evaporator (N-1000, Eyela, Tokyo, Japan). The concentrated extract was frozen at −80 °C and then lyophilized for 48 h using a freeze-dryer (FD8512, Ilshin Lab Co., Dongducheon, Korea), which yielded 30.0 g of R. mori ethanol extract. The concentration of oxyresveratrol in the R. mori ethanol extract was determined using high-performance liquid chromatography (HPLC) (YL9100, Young Lin Instruments, Anyang-si, Korea). This analysis was performed using a Luna-Pak® C-18 (5 μm, 4.6 mm i.d. × 250 mm) column. The mobile phase was acetonitrile (CH3CN) and 10 mM phosphoric acid (H3PO4) (35:65, v/v). The flow rate was 1.0 mL/min, and the effluent was monitored at 325 nm using a photodiode array detector. The concentration of oxyresveratrol in the ethanol extract of R. mori was 6.79%. In addition to oxyresveratrol, ERM contains 2.19% of mulberroside A, a glycosylated form of OXY, and a very low amount of resveratrol, quercetin, and quercetin-3-β-glucoside. The HPLC profile of ERM is shown in Supplemental Fig. 1. The ERM (Registration Number: MAEXT01) used in this study was deposited in the laboratory of professor J.K. Kim in the Department of Chemical Engineering and Biotechnology, Korea Polytechnic University.
Determination of cytotoxicity. The cytotoxicity of ERM to platelets was determined by measuring the amount of LDH released from platelets.Citation6) Eight-week male SD rats weighing 200−250 g were lightly anesthetized using ethyl ether, and 8−10 mL of blood was collected from the abdominal aorta in BD vacuum tubes containing sodium citrate (3.8%). After centrifugation at 200 g for 10 min at room temperature, the supernatant (platelet-rich plasma, PRP) was removed. Then, the remaining material was centrifuged at 1,200 g for 10 min at room temperature, and the supernatant obtained was the PPP (platelet-poor plasma). The number of platelets in the PRP was counted using a hemocytometer (MELET SCHLOESING Laboratoires, Osny, France), and this solution was diluted to 2 × 108 platelets/mL using PPP. The collected plasma was used within 2 h. After dilution, the PRP was incubated with the vehicle (distilled water) or with 0.5 mg/mL, 1.25 mg/mL, or 2.5 mg/mL of ERM for 5 min, after which, the treated plasma was centrifuged at room temperature for 1 min at 10,000 g. An aliquot of the supernatant (50 μL) was mixed with 1.8 mL of β-NADH solution (0.17 mM reduced form of β-NAD disodium salt in Tris-EDTA buffer, pH 7.4) and incubated for 5 min at 37 °C.Citation15) Then, 100 μL of a pyruvate solution (14 mM pyruvate in Tris-EDTA buffer, pH 7.4) that had been preincubated at 37 °C was added, and the absorbance at 340 nm was immediately measured. The decrease in the absorbance at 340 nm of the supernatant due to the conversion of NADH to NAD, which is as an indicator of LDH activity, was determined. The amount of LDH released from platelets was calculated as a percentage (%) of the total LDH activity of platelets that had been lysed using 0.5% Triton X-100. The positive control group was treated with 0.5% Triton X-100, and the amount of LDH released by this group was considered to be 100%.
In vivo antithrombotic activity assay. Six-week male SD rats (180−200 g) were divided into five groups (n = 6). The rats were orally administered the vehicle, GE (100 mg/kg), or ERM at doses of 20, 50, and 100 mg/kg daily for 3 weeks. At 45 min after the last treatment, the rats were anaesthetized using isoflurane (Piramal, Bethlehem, PA, USA), and a segment of the right carotid artery was exposed. At 60 min after the last treatment, thrombosis was induced by placing 2 mm2 of Whatman No. 1 filter paper soaked in 70% ferric chloride (FeCl3) on the carotid artery, and the blood flow rate was measured using a laser Doppler flowmeter (FLO-C1, Omegawave, Tokyo, Japan) for 10 min.Citation16) Then, the Whatman filter paper was removed and the blood flow rate was measured for 50 min. The occlusion time was set as the time for the blood flow rate to slow to 10% of the initial blood flow rate.Citation17)
Ex vivo platelet aggregation assay. Six-week male SD rats (180−200 g) were orally administered the vehicle, GE (100 mg/kg), or ERM at doses of 20, 50, and 100 mg/kg daily for 3 weeks. The antiplatelet aggregation activity was determined using a slightly modified method that was previously described.Citation16) Briefly, blood was collected from the abdominal aorta and treated with 3.8% sodium citrate. PRP and PPP were obtained as described above. Then, the number of platelets in the PRP was counted using a hemocytometer and adjusted to 3 × 108 platelets/mL using PPP. Platelet aggregation was measured using a Chrono-Log aggregometer (Chrono-Log, Havertown, PA, USA), using ADP (10 μM) and collagen (10 μg/mL) as stimulators.
Coagulation assay. Six-week male SD rats (180−200 g) were orally administered the vehicle, GE (100 mg/kg), or ERM at doses of 20, 50, and 100 mg/kg daily for 3 weeks. To evaluate the anticoagulation effect of ERM, PT and aPTT were determined using an automated ACL 100 coagulometer (Instrumentation Laboratory, Milan, Italy). After the PPP was obtained as described above, it was incubated at 37 °C for 7 min. Then, 50 μL of plasma was mixed with 100 μL of the PT reagent (RecombiPlasTin 2G, Instrumentation Laboratory, Bedford, MA, USA) in the processing plate, and the PT was measured. Additionally, in the aPTT assay, 50 μL of incubated plasma was mixed with 53 μL of the aPTT reagent (SynthASil, Instrumentation Laboratory, Bedford, MA, USA) and coagulation was initiated by adding CaCl2.
Tail bleeding time of mice. The bleeding times were determined as previously described.Citation18) Male ICR mice weighing 30−35 g were orally administered the vehicle, ERM at doses of 20, 50, 100 or 50 mg/kg of aspirin. Two hours after the treatment, the mice were lightly anaesthetized using ether and were maintained at body temperature, 37 °C. The tail was severed 3 mm from the tip using a surgical blade and then immersed in a transparent 15-mL conical tube containing normal saline that had been pre-warmed to 37 °C. The time until bleeding ceased was measured. Bleeding times exceeding 200 s were recorded as 200 s for statistical analysis.
Statistical analyses. Statistical analysis was performed using a one-way ANOVA followed by Dunnett’s test,Citation6) and significance was set at p values of < 0.05.
Results and discussion
Before investigating the anticoagulation effect of ERM, its possible cytotoxicity to platelets was investigated by determining the level of released lactate dehydrogenase (LDH) activity. The levels of LDH activity released by platelets treated with ERM at doses of 0.5, 1.25, and 2.5 mg/mL and that of the untreated control group were 6.5 ± 4.8%, 12.9 ± 3.5%, 9.9 ± 3.5%, and 9.9 ± 2.4%, respectively, of the total LDH activity of platelets that were lysed using 0.5% Triton X-100. There were no significant increases in the leakage of LDH in the ERM-treated groups compared with that of the untreated control group (Table ), which demonstrated that ERM was not cytotoxic to platelets.
Table 1. Effects of ERM on LDH release by platelets.
The effect of ERM on blood flow was evaluated by determining its antithrombotic activity, antiplatelet activity, and anticoagulation activity. To evaluate the effects of ERM, Ginkgo extract (GE), which is a well-known functional food that improves blood circulation, was used as a comparative material. During the period of oral administration of ERM to the animals used to prepare the FeCl3-induced arterial thrombosis model or to conduct the antithrombotic and antiplatelet assays, no significant differences between the body weights of each test group and those of the negative control group were found during the experimental period (Supplemental Table 1 and Table 2). There was no difference between the body weights of the control group and those of the ERM-treated group during the experimental period. In addition, there were no significant signs and symptoms in the experiment animals, which suggested that ERM did not exert any toxicity.
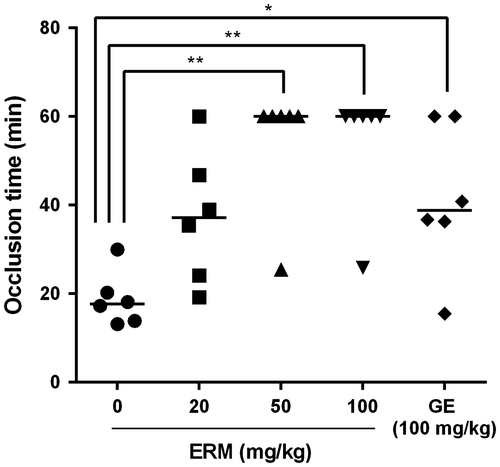
The in vivo antithrombotic effect of ERM was evaluated using the rat FeCl3-induced arterial thrombosis model. After 3 weeks of oral administration of ERM, carotid arterial injury was induced in rats using FeCl3 to evaluate the effect of ERM on occlusive thrombus formation in vivo. The injured vessels in the control group were occluded within 18.7 ± 6.1 min, whereas those of the group treated with ERM at a dose of 20 mg/kg were occluded 37.3 ± 14.9 min, those of the group treated at a dose of 50 mg/kg were occluded 54.2 ± 14.1 min, and those of the group treated at a dose of 100 mg/kg were occluded 54.3 ± 14.0 min. The occlusion time was significantly (p < 0.05) prolonged in the groups treated with 50 mg/kg and 100 mg/kg of ERM, which exhibited a more prolonged time than did the group treated with the GE (100 mg/kg; 41.5 ± 16.8 min; Fig. ).
Fig. 1. In vivo antithrombotic effect of ERM.
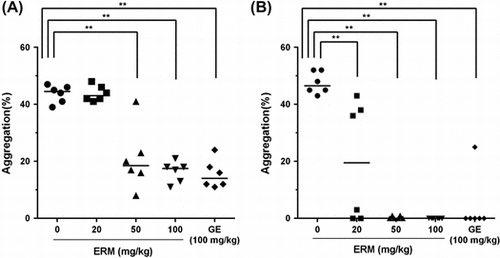
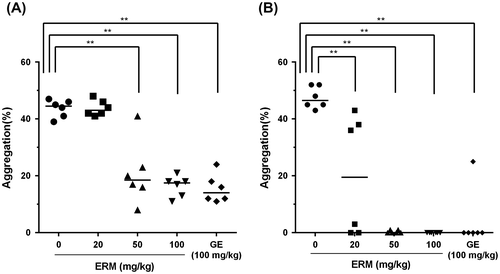
The results of the antiplatelet assay showed that the percentages of ADP-induced platelet aggregation were 43.7 ± 3.1%, 43.8 ± 2.7%, 20.8 ± 11.1%, 16.3 ± 3.7%, and 15.5 ± 5.0% in the control group, the groups treated with ERM at doses of 20, 50, and 100 mg/kg, and the group treated with 100 mg/kg of GE, respectively (Fig. (A). ERM significantly (p < 0.01) inhibited ADP-induced platelet aggregation in a dose-dependent manner compared with the control value. The percentages of collagen-induced platelet aggregation were 43.8 ± 2.8%, 20.0 ± 21.0%, 0.3 ± 0.5%, 0.0 ± 0.0%, and 4.2 ± 10.2% in the control group, the groups treated with ERM at doses of 20, 50, and 100 mg/kg, and the group treated with 100 mg/kg of GE, respectively (Fig. (B). ERM significantly (p < 0.01) inhibited collagen-induced platelet aggregation compared with the control value. ERM showed strong antiplatelet activity, particularly regarding collagen-induced platelet aggregation, which was completely inhibited by treatment with 50 mg/kg and 100 mg/kg of ERM. The IC50 values of ERM against ADP- and collagen-induced platelet aggregation are 72.7 ± 6.72 mg/kg and 23.8 ± 17.46 mg/kg, respectively. At 10 μM ADP concentration, Ginkgo biloba extract (Indena SpA, Italy) at 4 mg/mL inhibited platelet aggregation by almost 42% compared with the control.Citation19) IC50 value of Ginkgo extract against platelet activating factor (PAF, 1-O-Octadecyl-2-Oacetyl-sn-glycero-3 phosphorylcholine)-induced aggregation of human PRP was 36.3 μg/mL.Citation20) Unfortunately, the IC50 values of ERM obtained in this study could not be directly compared with those of Ginkgo extracts previously reported because we measured platelet aggregation using ex vivo assay, while the previous reports had measured it using in vitro assay.
Fig. 2. Ex vivo antiplatelet effect of ERM.
Platelets play a very important role in the hemostatic process. Collagen is exposed from endothelial cells when blood vessel is damaged. As platelets adhere to collagen, platelets become stickier and release chemical messengers such as ADP, serotonin, and thromboxane A2, causing more aggregation of nearby free platelets. Each of these processes is thought to involve specific platelet receptors.Citation21) ERM has more antiplatelet activity against collagen-induced platelet aggregation than that against ADP-induced platelet aggregation, which suggests that ERM might be more effective in early stage of platelet aggregation via different platelet receptors.
To investigate the anticoagulation effect of ERM, plasma was collected from rat blood after a 3-week oral administration of ERM. The anticoagulation effect of ERM was evaluated by measuring the prothrombin time (PT) and the activated partial thromboplastin time (aPTT). The results showed no significant increase in either the PT (Fig. (A) or the aPTT (Fig. (B) in the ERM-treated groups compared with those of the control. The results showed that ERM had a significant antithrombotic effect on rat carotid arterial thrombosis in vivo and inhibited platelet aggregation ex vivo but did not extend PT or aPTT. These results indicate that ERM intake may prevent thrombotic and cardiovascular disease by inhibiting platelet aggregation and not by inhibiting blood coagulation.
Fig. 3. Ex vivo anticoagulation effect of ERM.
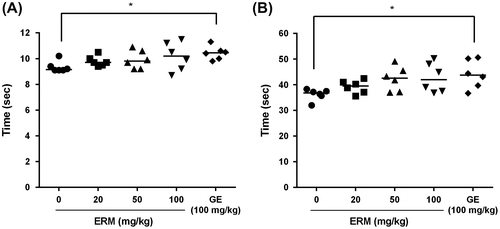
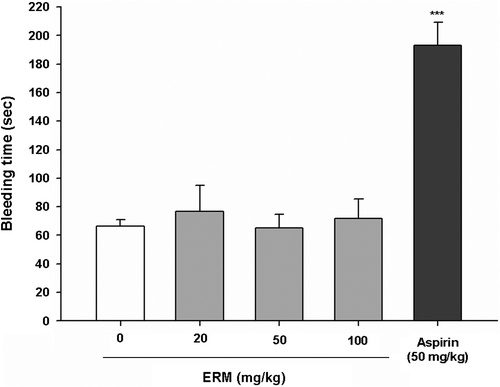
To evaluate the effects of ERM on hemostasis, a bleeding time assay was performed. The mean bleeding time of the control group was 66.8 ± 5.0 s, that of the group treated with ERM at a dose of 20 mg/kg was 76.7 ± 18.2 s, that of the group treated with ERM at a dose of 50 mg/kg was 65.2 ± 9.4 s, and that of the group treated with ERM at a dose of 100 mg/kg was 71.8 ± 13.4 s. These results showed that the bleeding time of the control group and the ERM-treated groups were not significantly different, whereas the bleeding time was significantly prolonged to 193.2 ± 16.2 s by aspirin treatment at a dose of 50 mg/kg (Fig. ). In addition to not being cytotoxic to platelets, ERM did not prolong the bleeding time, which is a side effect of aspirin treatment.
Fig. 4. Effects of ERM on mouse tail bleeding times.
Glucose derivatives that occur naturally in fruits and vegetables, such as D-glucono-1,4-lactone, sodium D-gluconate, and calcium D-glucarate, prevent excessive platelet activation through antioxidant-based mechanisms.Citation22) A phenolic fraction of Alhagi maurorum roots showed strong antioxidative and antiplatelet activities in plasma and blood platelet samples that were treated with a strong biological oxidant, hydrogen peroxide.Citation23) A polyphenolic fraction isolated from the aerial parts of Tribulus pterocarpus showed antiplatelet activity by decreasing the level of the free radical O2-, which indicated that the antiplatelet activity of this fraction might be associated with its antioxidative activity.Citation24) In the in vivo FeCl3-mediated rat arterial thrombosis model used for the antithrombotic assay, FeCl3 induces oxidative damage of the exposed subendothelial matrix. The ERM used in this study contained oxyresveratrol (6.79%), which has shown a strong antioxidant activityCitation25) and might contribute to the antithrombotic effect of ERM.
ERM showed remarkable antiplatelet effects. ERM significantly inhibited ADP-induced platelet aggregation as well as collagen-induced platelet aggregation in a dose-dependent manner. ADP and collagen play an important role in the platelet activation and aggregation process by inducing conformational change in platelet glycoprotein (GP)IIb/IIIa receptor.Citation26) The results obtained in this study suggest that the antiplatelet mechanism of ERM may block the activation of GP IIb/IIIa receptor on the platelet. Further study is required to identify the precise pathway underlying the antiplatelet aggregation activity of ERM. However, ERM treatment did not extend the plasma coagulation time, indicating that ERM did not have anticoagulation activity. Overall, ERM had antiplatelet effects similar to those of GE, which has been used as a functional food for improving blood flow, although, unlike ERM, GE showed anticoagulation activity.
The results obtained in the present study suggest that ERM has potential use in preventing thrombosis and improving blood circulation. ERM showed no cytotoxicity and did not have the side effect of prolonging the bleeding time. In addition, R. mori has long been an approved food source in Korea. ERM may be a substantially safer therapeutic for individuals at risk for atherothrombosis than other products,Citation27) considering the narrow therapeutic ranges and side effects of aspirin and heparin, which are the drugs most commonly used to prevent blood clot formation.Citation28) Furthermore, before thrombosis progresses to cardiovascular disease, it is important to prevent thrombus formation and eliminate thrombi. In this respect, ERM might be beneficial for individuals with a high risk of developing thrombotic and cardiovascular diseases.
In conclusion, ERM had a significant protective effect against arterial thrombosis in vivo, which may be due to antiplatelet activity and not due to anticoagulation activity. ERM was not only nontoxic to platelets but also did not prolong the bleeding time. Therefore, ERM has certain advantages for its development as a functional food that improves blood circulation.
Authors contribution
Y.H.L. designed the research. J.L., G.K., J.P., S.Y.C., and Y.S. performed the experiments. J.K.K. prepared the extract; Y.H.L. and J.L. wrote the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This research was supported by the High Value-added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea [grant number: 313028-3].
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2016.1156479.
TBBB_1156479_Supplemental.docx
Download MS Word (94.6 KB)Notes
Abbreviations: ERM, ethanol extract of Ramulus mori; GE, Ginkgo extract; ADP, adenosine diphosphate; PT, prothrombin time; aPTT, activated partial thromboplastin time; LDH, lactate dehydrogenase; PRP, platelet-rich plasma; PPP, platelet-poor plasma.
References
- Jin YR, Ryu CK, Moon CK, et al. Inhibitory effects of J78, a newly synthesized 1,4-naphthoquinone derivative, on experimental thrombosis and platelet aggregation. Pharmacology. 2004;70:195–200.10.1159/000075548
- Bauersachs RM. Use of anticoagulants in elderly patients. Thromb. Res. 2012;129:107–115.10.1016/j.thromres.2011.09.013
- Walsh PN. Platelet coagulation-protein interactions. Semin. Thromb. Hemost. 2004;30:461–471.10.1055/s-2004-833481
- Nieswandt B, Aktas B, Moers A, et al. Platelets in atherothrombosis: lessons from mouse models. J. Thromb. Haemost. 2005;3:1725–1736.10.1111/jth.2005.3.issue-8
- Furie B, Furie BC. Thrombus formation in vivo. J. Clin. Invest. 2005;115:3355–3362.10.1172/JCI26987
- Yu HY, Park SW, Chung IM, et al. Anti-platelet effects of yuzu extract and its component. Food Chem. Toxicol. 2011;49:3018–3024.10.1016/j.fct.2011.09.038
- Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb. Haemost. 2009;102:248–257.
- Pharmacopoeia Committee of P. R. China. Pharmacopoeia of the People’s Republic of China. Beijing: Chemical Industry Publichers; 2010.
- Jiang N, Bo M, Wu Z, et al. A review on the chemical constituents and pharmacological activity studies of Ramulus Mori. Jiangsu Sericult. 2006;28:4–7.
- Wei H. Morus alba L. Sang, white mulberry. In: Anonymous, editor. Dietary Chinese herbs. Springer, Vienna; 2015. p. 721–730.
- Xu L, Yang F, Wang J, et al. Anti-diabetic effect mediated by Ramulus mori polysaccharides. Carbohydr. Polym. 2015;117:63–69.10.1016/j.carbpol.2014.09.052
- Lee KT, Lee KS, Jeong JH, et al. Inhibitory effects of Ramulus mori extracts on melanogenesis. J. Cosmet. Sci. 2003;54:133–142.
- Zhang Z, Shi L. Anti-inflammatory and analgesic properties of cis-mulberroside A from Ramulus mori. Fitoterapia. 2010;81:214–218.10.1016/j.fitote.2009.09.005
- Hwang D, Jo SP, Lee J, et al. Antihyperlipidaemic effects of oxyresveratrol containing Ramulus mori ethanol extract in rats fed a high-cholesterol diet. J. Funct. Foods. 2015;19:353–362.10.1016/j.jff.2015.09.039
- Kim Y, Bae O, Chung S, et al. Improvement of haemostasis mediated by anti-platelet activities by plant vinegar. Toxicol. Res. 2004;20:137–142.
- Jin Y, Yu JY, Lee J, et al. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin. Pharmacol. Toxicol. 2007;100:170–175.10.1111/pto.2007.100.issue-3
- Jang JY, Kim TS, Cai J, et al. Perilla oil improves blood flow through inhibition of platelet aggregation and thrombus formation. Lab. Anim. Res. 2014;30:21–27.10.5625/lar.2014.30.1.21
- Lee BK, Lee D, Sy Ha, et al. Anti-platelet effects of mixtures of onion and aloe extract. Taehan Yakhakhoe. 2014;58:322–327.
- Dutta-Roy AK, Gordon MJ, Kelly C, et al. Inhibitory effect of Ginkgo biloba extract on human platelet aggregation. Platelets. 1999;5:298–305.
- Koch E. Inhibition of platelet activating factor (PAF)-induced aggregation of human thrombocytes by ginkgolides: considerations on possible bleeding complications after oral intake of Ginkgo biloba extracts. Phytomedicine. 2005;12:10–16.10.1016/j.phymed.2004.02.002
- Clemetson KJ. Platelets and primary haemostasis. Thromb. Res. 2012;129:220–224.10.1016/j.thromres.2011.11.036
- Saluk-Juszczak J. A comparative study of antioxidative activity of calcium-D-glucarate, sodium-D-gluconate and D-glucono-1,4-lactone in a human blood platelet model. Platelets. 2010;21:632–640.10.3109/09537104.2010.512210
- Olas B, Hamed AI, Oleszek W, et al. Comparison of biological activity of phenolic fraction from roots of Alhagi maurorum with properties of commercial phenolic extracts and resveratrol. Platelets. 2015;26:788–794.10.3109/09537104.2015.1031650
- Olas B, Morel A, Hamed AI, et al. Evaluation of polyphenolic fraction isolated from aerial parts of Tribulus pterocarpus on biological properties of blood platelets in vitro. Platelets. 2013;24:156–161.10.3109/09537104.2012.671979
- Aftab N, Likhitwitayawuid K, Vieira A. Comparative antioxidant activities and synergism of resveratrol and oxyresveratrol. Nat. Prod. Res. 2010;24:1726–1733.10.1080/14786410902990797
- Calvete JJ. On the structure and function of platelet integrin alpha IIb beta 3, the fibrinogen receptor. Proc. Soc. Exp. Biol. Med. 1995;208:346–360.10.3181/00379727-208-43863A
- Buettner C, Yeh GY, Phillips RS, et al. Systematic review of the effects of ginseng on cardiovascular risk factors. Ann. Pharmacother. 2006;40:83–95.
- Lowe G. Measurement of thrombosis and its prevention. Br. J. Clin. Pharmacol. 2002;54:96–100.10.1046/j.1365-2125.2002.01626.x
