Abstract
To improve the catalytic activity of atrazine chlorohydrolase (AtzA), amino acid residues involved in substrate binding (Gln71) and catalytic efficiency (Val12, Ile393, and Leu395) were targeted to generate site-saturation mutagenesis libraries. Seventeen variants were obtained through Haematococcus pluvialis-based screening, and their specific activities were 1.2–5.2-fold higher than that of the wild type. For these variants, Gln71 tended to be substituted by hydrophobic amino acids, Ile393 and Leu395 by polar ones, especially arginine, and Val12 by alanine, respectively. Q71R and Q71M significantly decreased the Km by enlarging the substrate-entry channel and affecting N-ethyl binding. Mutations at sites 393 and 395 significantly increased the kcat/Km, probably by improving the stability of the dual β-sheet domain and the whole enzyme, owing to hydrogen bond formation. In addition, the contradictory relationship between the substrate affinity improvement by Gln71 mutation and the catalytic efficiency improvement by the dual β-sheet domain modification was discussed.
Graphical abstract
Structrual modification in AtzA variants.
Since 1958, atrazine (6-chloro-N2-ethyl-N4-isopropyl-1,3,5-triazine-2,4-diamine) has been widely applied as a herbicide to control broad-leaf weed species in the fields, mainly for crops resistant to atrazine, such as corn, sorghum, sugarcane, and citrus.Citation1,2) Owing to the large amount and wide range of application, as well as environmental persistence of atrazine (its half-life is estimated at between 4 and 57 weeks),Citation3) the compound has polluted the soil, ground water, and surface water, and its concentration in some areas far exceeds 2 μg/L, the maximum value ruled safe by World Health Organization (Guidelines for Drinking-water Quality). The excess atrazine is notably toxic to the environment and human health, harming sensitive crops,Citation4) interfering with the endocrine and immune systems of mammalsCitation5) and increasing the odds of ovarian cancer, breast cancer, prostate cancer, and leukemia in humans.Citation6–8) Therefore, exploring highly efficient atrazine degradation systems has considerable significance for agriculture, human health, and the environment.
Bacteria readily acclimatize to the environment and are able to evolve new enzymes to metabolize anthropogenic chemicals.Citation9,10) In response to excess atrazine in a contaminated environment, a detoxifying metabolic pathway has evolved in some bacteria within only a few decades, through which atrazine is utilized as a source of carbon and nitrogen. The atrazine-catabolic pathway was first found in Pseudomonas sp. strain ADP.Citation11) Atrazine chlorohydrolase AtzA (EC 3.8.1.8) is the first and rate-limiting enzyme of the most thoroughly characterized pathways, and catalyzes a hydrolytic dechlorination reaction, transforming atrazine into a non-toxic hydroxylated product.Citation11) The genes encoding AtzA were cloned from Nocardia, Acinetobacter, Agrobacterium, Pseudomonas, Bacillus, Xanthomonas, Arthrobacter, Rhizobium, Chelatobacter, Aminobacter, and Pseudaminobacter.Citation12–16) atzA genes from different sources are highly homologous, and the corresponding enzymes have similar characteristics and functions. The open reading frame of atzA is 1425 bp, encoding a protein of 474 amino acid residues. The native AtzA is a homotetramer or homopentamer;Citation11) the molecular mass of each subunit is 60 kDa, and the holoenzyme mass is 245 kDa. AtzA is also a metalloenzyme, and each subunit needs to bind one Fe2+ to be activated.Citation11,17)
According to the structural homology model, AtzA contains a typical (β/α)8 barrel of the amidohydrolase superfamily, with an eight-β-strand barrel core coated by eight α-helices, where the catalytic center is located. Another remarkable feature is a dual β-sheet domain, consisting of two parallel β-sheets.Citation18) The structure obtained by X-ray crystal diffraction and molecular modeling of the hexametric AtzA showed that some residues line a hydrophobic tunnel for substrate binding, from the surface to the active site of the protein. Among them, His66, His68, His243, His276, and Asp327 bind the Fe2+, while Val92, Trp87, Leu88, Tyr85, Phe84, and Asn328 make hydrophobic contacts with the N-ethyl side chain of atrazine,Citation19) respectively. However, because of the high Km of AtzA for atrazine, which exceeds the aqueous solubility of the substrate, a clear X-ray structure of the AtzA–atrazine complex has not been captured, and the detailed catalytic process is still speculative.Citation19)
Since the function of AtzA only evolved over the course of several decades, the dechlorination reaction it catalyzes is probably far from perfect. That implies that a great potential exists for the development of higher enzyme activity through artificial evolution. Rational and irrational protein designs are the two common strategies of protein engineering. For rational design, site-directed mutagenesis is utilized to modify a nucleotide sequence corresponding to a specific amino acid residue, eventually modifying the protein function. However, rational design requires a thorough knowledge of the three-dimensional structure and a clear understanding of the structure–function relationship of target proteins.Citation20) When clear structural information is not available, irrational protein design, namely directed evolution, is more suitable. Error-prone PCR, DNA shuffling, random priming, in vitro recombination, and staggered extension are used to generate random mutations, and then, through directional screening, variants with desirable properties can be obtained. This method has already been applied to improve the catalytic activity, stability, and substrate specificity of enzymes, and is effective in characterizing the structure–function relationships.Citation21,22) However, the screening efficiency is an unavoidable bottleneck in directed evolution. Site-saturation mutagenesis overcomes the disadvantages of both the rational and irrational designs.Citation23,24) It can be used to simultaneously modify multiple sites. At the same time, it is easier to reach saturated capacity for a relatively small mutagenesis library, which improves the mutation efficiency and decreases the difficulty in screening for variants to some degree.
The lack of structural information hinders rational engineering for AtzA. Previously, we have constructed a random mutagenesis library for AtzA, and established a high-throughput Haematococcus pluvialis expression screening system.Citation18,25) Characterization of the screened variants indicated that four amino acid residues (Val12, Gln71, Ile393, and Leu395) had a potential for improving the enzymatic function.Citation18) In this study, these residues were targeted to generate site-saturation mutagenesis libraries. H. pluvialis-based system was utilized to screen for variants with enhanced catalytic activity. Characterization of the evolved AtzA variants verified the importance of these residues for the enzymatic activity and will provide insights into the structure–function relationship of AtzA.
Materials and methods
Construction of site-saturation mutagenesis library of atzA
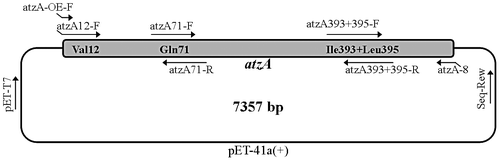
The mutation libraries of atzA were constructed according to the strategy shown in Fig . Considering that Gln71 is involved in substrate binding and Val12, Ile393, and Leu395 are all related to the structural stability of the enzyme,Citation18) four site-saturation mutagenesis libraries were constructed in the study: OEM1-1 (overlap extension PCR mutagenesis 1-1) and OEM1-2, both aimed at site 71 and combined before screening; OEM2 (aimed at sites 12, 393, and 395), and OEM3 (aimed at sites 12, 71, 393, and 395). Each library was constructed using three or four rounds of PCR, and the detailed information including primers, templates, and conditions is contained in Table S1. The sequences of primers are shown in Table S2. For each library, the upstream and downstream fragments were amplified using 2 × Pfu PCR MasterMix (TaKaRa Biotechnology Ltd. Co., Dalian, China) with a mixture of wild-type and eight mutated atzA genes,Citation18) obtained previously, as template. Full-length atzA genes were amplified by overlap extension PCR with a mixture of upstream and downstream fragments as templates. They were then inserted into pCAMBIA-1301 using SacI and SalI restriction sites. The plasmids were then transformed into Escherichia coli DH5α-FT cells and three site-saturation mutagenesis libraries were generated.
High-throughput screening and sequencing of enhanced activity variants
Plasmids were extracted from the three mutagenesis libraries and respectively used for H. pluvial transformation, as described by Wang et al.Citation18) The transformed algal cells were initially cultivated in liquid Bold’s basal medium (BBM) for 24 h for rehabilitation and then cultivated on solid medium with 0.5-mg/L atrazine for 21 d. After single algal colonies appeared on the plates, they were separately inoculated into the liquid BBM and cultivated for 15 d for activation, before being sequentially screened in 0.5, 1, and 2-mg/L atrazine, respectively. The algal lines that grew under the above selection pressure were selected and their genomic DNA was extracted, respectively, by the hexadecyltrimethylammonium bromide (CTAB) method. The mutated genes were amplified using primer pairs atzA-F-SacI/atzA-R-NotI (Table S2), and then ligated into pMD19T Simple vectors (TaKaRa Biotechnology Ltd. Co., Dalian, China) for sequencing.
Atrazine degradation abilities of H. pluvialis variants
H. pluvialis variants with potentially improved atrazine dechlorinase activities were selected and cultured in liquid BBM with 1 and 2-mg/L atrazine (chromatographic pure, purchased from Wako Pure Chemical Industries, Ltd), respectively, for 21 d. Culture aliquots (2 mL) were centrifuged to remove algal cells, and the residual atrazine was extracted from the supernatant with dichloromethane. The concentration of the residual atrazine was determined by CoM 6000 HPLC system, and used to calculate the atrazine degradation ability of H. pluvialis variants according to a method described previously.Citation18)
Specific activity and kinetic analysis of mutant enzymes
Mutant atzA was cloned into pET-41a(+) vector after digestion with SacI/NotI, and the recombinant plasmid was used to transform E. coli BL21(DE3). The protein extraction, purification, and renaturation (AtzA was expressed as inclusion bodies in E. coli) were performed according to the method described by Wang et al.Citation18) The enzymatic activity was determined based on the enzyme’s capability to convert atrazine into 2-hydroxyatrazine, and the concentration of the residual atrazine was determined by HPLC. The reactions were performed in 0.4-mL, 100 mM Tris-HCl buffer (pH 7.2) with approximately 20-μg/mL enzyme and 46-μM atrazine.Citation18) One unit was defined as the amount of enzyme that converted 1 μmol of atrazine into 2-hydroxyatrazine per minute, at room temperature. The kinetic parameters were determined in the same reaction system as described above, with atrazine concentration ranging from 4.6 to 140 μM and enzyme concentration of approximately 10 μg/mL.Citation18) The kinetic parameters were calculated using Lineweaver–Burk plots.
Homology modeling of AtzA
The amino acid sequences of the wild-type and mutant AtzA were submitted to Phyre2 server, respectively, for homology modeling in the intensive mode,Citation26) which is based on a profile–profile alignment algorithm. Templates were selected based on heuristics to maximize confidence, percentage identity, and alignment coverage. The amino acid sequences were also submitted to 3DLigandSite server for potential ligand position and binding site prediction, based on superimposition of homology models.Citation27) Based on the above analysis, PyMOL ver 1.0 was used to analyze the differences of surface charge distribution, surface structure, and hydrogen bonds between the wild-type and mutant AtzA. The plausible relationship between structural modifications and changes in enzymatic activity was analyzed.
Data analysis
All the experiments concerning data analysis were repeated four times. Data were analyzed with SPSS 11.0 software (SPSS Inc., USA). Statistical differences between the wild type and variants were assessed using independent sample t-test with 95% confidence intervals.
Results
Library construction and screening
Four site-saturation mutagenesis libraries were generated (Table ). Among the three libraries, only OEM1 reached the minimum theoretical capacity. The remaining two were far from the minimum theoretical capacity and only reached 4% of the capacity (Table ). After screening using the H. pluvialis expression system under the successive pressures of atrazine, 17 H. pluvialis variants with improved atrazine dechlorinase activity were obtained. Among them, nine were derived from OEM1, six from OEM2, and two from OEM3, respectively (Table ).
Table 1. Construction of site-saturation mutagenesis libraries and H. pluvialis variants with enhanced AtzA activity.
Sequence analysis of the improved variants
In order to investigate the relationship between the mutations and enzyme catalytic activity, sequencing of the 17 variant genes was carried out (Table ). Variant 1-79 did not have any mutations at the four target sites (12, 71, 393, and 395). Variants 1-3, 1-7, 1-73, 1-93, and 1-110 contained one mutation at site 71. Variants 1-11, 1-66, 3-2, and 3-69 contained two mutations at sites 12 and 71. Variants 2-2 and 2-11 contained two mutations at sites 393 and 395. Variants 1-23, 2-4, 2-12, and 2-16 contained three mutations at sites 12, 393, and 395. Variant 2-13 contained mutations at all the target sites. These findings indicated that all of the four target sites have an evolutionary potential for conferring improved activity to AtzA. Among these variants, Gln71 tended to be substituted by non-polar hydrophobic amino acids (with the frequency of 2/3), such as proline, glycine, methionine, and alanine. Non-polar Ile393 and Leu395 tended to be substituted by polar residues (both with the frequency of 1/2), such as arginine, serine, glutamic acid, and histidine, and of these, arginine occurred with the highest frequency (1/7 and 3/7 for Ile393 and Leu395, respectively). Val12 tended to be substituted by alanine (with the frequency of 2/3).
Table 2. Sequence analysis of the mutant and wild-type AtzA.
Besides the target sites, other mutations were also detected. Most of the mutations (except T121A and F439L), that derived from the templates used for library construction, reside in the peripheral region of the (β/α)8 domain and the dual β-sheet domain.Citation18) Among them, D30G, M315I, H399Q, N429S, and V466A occurred frequently (Table ). Such high frequency of occurrence indicated that they were beneficial for the enzymatic activity, which was further confirmed by the variant 1-79 with no mutations at target sites. A number of new mutations, which were not detected in our previous random mutagenesis work,Citation18) were also introduced due to several rounds of PCR.
The atrazine degradation capability of H. pluvialis variants
In order to investigate the atrazine degradation capability of H. pluvialis variants, they were cultured in BBM with different concentrations of atrazine. The residual atrazine concentration was determined after 21 d. These variants displayed higher degradation capability than the wild type, both in 1-mg/L and in 2-mg/L atrazine-containing medium, and the degradation rates were 1.6–5.3-fold higher than those of the wild type (Fig. ). Variant 1-11 had the highest degradation rates, which were 5.3-fold and 4.2-fold than those of the wild type in 1- and 2-mg/L atrazine medium, respectively (Fig. ). Variant 1-23 was second best, and its atrazine degradation rates were 4.7-fold and 3.0-fold greater than that of the wild type in 1- and 2-mg/L atrazine medium, respectively (Fig. ).
Fig. 2. The atrazine degradation ability of H. pluvialis variants.
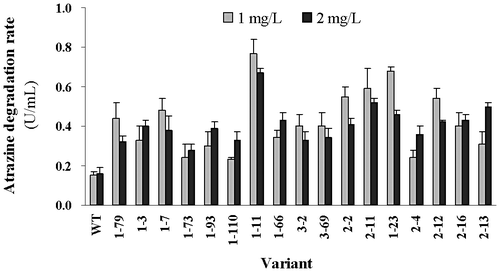
It appears that the 17 variants all displayed lower degradation abilities in 2-mg/L atrazine than in 1-mg/L atrazine, suggesting that atrazine inhibited algal growth, especially at higher concentration. Regardless of the performance of each variant under the different atrazine concentrations, the degradation trends were almost the same for all the variants, indicating that it was the mutated atzA gene that conferred higher atrazine degradation ability in H. pluvialis.
Specific activities and kinetics of the AtzA variants
The atrazine degradation capability of H. pluvialis variants reflects the characteristics of different mutant genes, to certain extent. Since no purified enzymes were used, the results did not precisely reflect the catalytic activities of the mutant enzymes. Therefore, the mutated atzA genes were expressed in E. coli. After purification and renaturation, the specific activity and kinetics of the variant enzymes were determined (Table ). Their specific activities were 1.2–5.2-fold greater than those of the wild type, and among them, variant 1-11 was the most active. The specific activities of most variants (except 2-13 and 2-2) showed similar trends with respect to atrazine degradation rates, indicating the importance of using purified enzymes to determine the specific activity and kinetic parameters, as described below.
Table 3. Specific activity and kinetics of the mutant AtzA enzymes.
Of the 17 variants, nine (1-79, 1-3, 1-73, 1-93, 1-110, 3-2, 3-69, 1-23, and 2-4) had lower Km than that of the wild type. Of them, variants 1-73 and 1-93 showed the lowest values, which were 0.6-fold and 0.7-fold with respect to that of the wild type, respectively. The average Km values of all the variants that contained mutations at each target site were compared. The average Km for enzymes with mutated residue 12 was 0.95-fold of that of the wild type, while those with mutated residues 71 and 393/395 were 0.86-fold and 0.91-fold of that of the wild type, respectively, indicating the importance of residue 71 for substrate binding of AtzA. In addition, of the seven variants with Km exceeding 153 μM, four (2-2, 2-11, 2-12, and 2-16) did not have a mutation at site 71, which further suggested its decisive role.
kcat/Km represents the catalytic efficiency of enzymes. All the mutant enzymes had higher kcat/Km values compared to the wild type, and their kcat/Km showed the same trend as specific activities. Variant 1-11 had the highest kcat/Km value, which was 3.9-fold greater than that of the wild type. Variant 1-23 came second, with kcat/Km value 3.8-fold higher than that of the wild type. Variants 2-2, 2-11, 2-12, and 2-13 had kcat/Km value 3.3-fold higher than that of the wild type, while variant 1-93 had the lowest kcat/Km, which was 1.4-fold of that of the wild type. Similarly, average kcat/Km values of all the variants containing mutations at each target site were compared. The average value for enzymes with mutated residue 12 was 3.1-fold higher than that of the wild type, whereas those of site 71 and site 393/395 were 2.77-fold and 3.18-fold higher than that of the wild type, respectively, indicating the importance of mutations at residues 12 and 393/395 for the catalytic efficiency of AtzA.
Variants 2-13 and 1-73 both had the Q71R mutation, but variant 2-13 had a much higher Km than that of variant 1-73, which was probably due to mutated residues 12, 393, and 395. These mutations improved catalytic efficiency, but simultaneously decreased the substrate affinity of variant 2-13 (increased Km). In addition, five variants (1-11, 2-13, 2-2, 2-12, and 2-11) with the highest specific activities all had Km exceeding 153 μM, implying an antagonistic relationship between the improvement of the catalytic efficiency, owing to mutated residues 12, 393, and 395, and the improvement of substrate affinity, owing to mutated residue 71 in AtzA.
As for mutations outside the target sites, variant 1-66 preserved all the mutations of the template variant 7-10 (i.e. M315I/H399Q/N429S/V466A), suggesting that some of these mutations were beneficial for catalytic activity. Compared with 1-66, variant 3-2 had an additional mutation, A173 V, but similar kinetics, indicating the relative unimportance of A173 V. Compared with 3-2, variant 3-69 had an additional mutation, T121A, a significantly improved kcat/Km but similar Km, indicating the importance of T121A for catalytic efficiency (it increased kcat/Km by about 15%). Variant 1-79 preserved two mutations, D30G and V466A, from the template but had no mutation at the target sites. It had a 3.0-fold greater kcat/Km than that of the wild type, suggesting that D30G and V466A were beneficial for catalytic efficiency.
AtzA homology modeling analysis
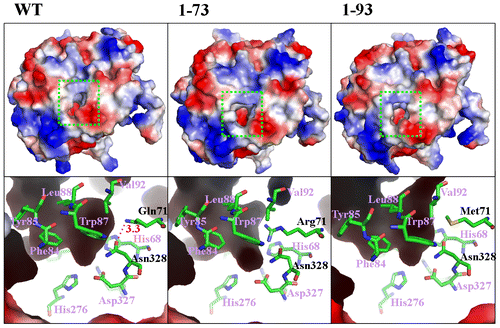
Amino acid residues that we targeted in this study are shown in the homology model of AtzA (Fig. S).Citation18) Gln71, which constituted the substrate-binding pocket,Citation19) was located in the peripheral region of the (β/α)8 domain, and in the vicinity of the Fe2+ binding site. Val12, Ile393, and Leu395, which were away from the active site, were located in the dual β-sheet domain and were involved in hydrogen bond formation between β-strands, and may help stabilize enzyme structure.
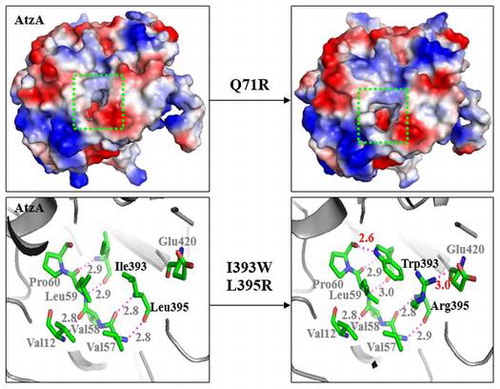
It has been reported that atrazine enters the AtzA substrate-binding pocket and the active center through a hydrophobic channel.Citation19) Therefore, the morphology of the substrate-binding channel and the pocket, which may be associated with substrate affinity, were analyzed in the variant enzymes. Two variants, 1-73 and 1-93 (with Km values 0.6-fold and 0.7-fold of that of the wild type, respectively), whose substrate affinity increased the most, had a much larger entrance to the channel than the wild type (Fig. , upper panels), which was not apparent for variants displaying no improvement in substrate affinity (data not shown). In variants 1-73 and 1-93, the morphologies of the substrate-binding pocket and the reaction center were also modified due to their mutated residue 71. Specifically, the side group of Arg71 in variant 1-73 extended into the substrate-binding site (Fig. , lower panels). In addition, it is known that Asn328 directly binds the N-ethyl side chain of atrazine through its amide group, during catalysis.Citation19) Thus, the modification of the hydrogen bond involving Asn328 is supposed to affect substrate binding. The hydrogen bond between the side chains of Gln71 and Asn328 was present in the wild type, but not in the variants 1-73 and 1-93.
Fig. 3. Substrate-binding pocket morphology of AtzA variants.
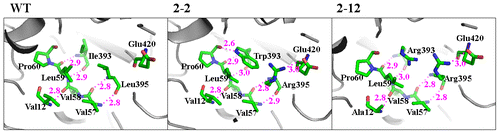
Stabilization center prediction showed that Val12 and Leu395 were located in the stabilization center of AtzA,Citation18) and were involved in the long range interaction with their respective related amino acid residues. The long range interaction is usually considered important for stabilizing the structure and prevention of the degradation of an enzyme.Citation28,29) Val12, Ile393, and Leu395 participate in the formation of hydrogen bonds between β-strands in the dual β-sheet domain, maintaining the stability of the enzyme structure. In the variants with mutations at site 393/395, new hydrogen bonds were formed (Fig. ). For instance, in variant 2-2, with mutations I393W and L395R, one new hydrogen bond between Trp393 and Pro60 was formed, and another between Arg395 and Glu420, with the bond lengths of 2.6 Å and 3.0 Å, respectively. Variant 2-12, with mutations V12A, I393R, and L395R, had a hydrogen bond between Arg395 and Glu420, with the bond length of 3 Å. Variant 2-16, with mutations V12F, I393S, and L395H, had a hydrogen bond between Ser393 and Leu59. Variant 2-4, with mutations V12C, I393E, and L395R, had one hydrogen bond between Glu393 and Phe62, and another between Glu393 and Arg395, respectively.
Discussion
In order to combine the beneficial mutations obtained from previous random mutagenesis steps and to further improve the activity of these evolved variants,Citation18) atzA genes of the wild type and these variants were used as templates to generate site-saturation libraries. As the mole ratio between the different templates was 1:1, the mutation frequencies of mutagenesis at the target residues for wild-type and the mutated genes were equal, and therefore the combination of mutations obtained was theoretically optimal. In this study, all the target sites showed evolutionary potential in improving the enzyme’s catalytic activity, suggesting their close relationship with the function of AtzA. Many mutations that were derived from the templates were retained in the 17 variants (Table ).Citation18) However, as most of these mutations reside in the peripheral region of (β/α)8 domain and dual β-sheet domain (the two targeted areas we modified in this study), the additive effect was not satisfactory and the variants did not improve their specific activities much compared to those from random mutagenesis.
Although site-saturation mutagenesis constitutes rational protein design, it is nonetheless crucial to combine it with a high-throughput screening method. Our method takes advantage of the hypersensitivity of algae to atrazine. Expression of the mutated libraries in H. pluvialis can increase its tolerance to atrazine and promote its growth into single colonies under atrazine selection pressure. However, the expression of the mutant gene in H. pluvialis was based on the insertion of the Ti vector into the genome, at a random location. Thus, the phenotype of a transformant was not only related to the transgene but also to the insertion region and copy number. This may have resulted in some improved variants unaccounted for because of inappropriate insertions, although that was compensated by repeated screening. Furthermore, this resulted in an inconsistency between the atrazine degradation capabilities of H. pluvialis transformants and the corresponding purified enzymes, which is significantly reflected in variants 2-13 and 2-2. Therefore, the screened variants should be verified by determining their enzymatic activity using purified enzymes. Nevertheless, our study confirmed again that H. pluvialis expression is simple, reliable, and efficient for screening for variants with enhanced AtzA activity.Citation18)
Of the 17 variants with enhanced atrazine degrading ability, 9 were derived from OEM1, which was possibly due to the high saturation of the library and the considerable plasticity of site 71. As Gln71 is located in the substrate-binding pocket, it possibly directly impacted the catalytic process. OEM2 targeted the sites 12, 393, and 395, which were associated with the stability of the enzyme structure. As the library’s actual capacities were much smaller than the minimum theoretical capacity, it is not surprising that less variants than OEM1 were got. OEM3 (5.0 × 105) has a capacity 50-fold greater than that of OEM2 (1.0 × 104), but the number of variants screened was less than that from OEM2 (2 vs. 6), suggesting a putative incompatibility between site 71 and the other three target sites. In other words, the catalytic center and the structure-maintaining domain may have a contradictory relationship. Namely, the improvement of the substrate affinity by mutated residue 71 is at the expense of the damage of the catalytic efficiency. In turn, the improvement of the catalytic efficiency by modified dual β-sheet domain adversely affects the substrate affinity. This contradictory relationship was also indirectly proven by the comparison of the mutations and Km values of variants 1-73 and 2-13 (described above), and by the fact that high specific activity was always associated with Km values exceeding 153 μM. If the library capacity was large enough, this contradiction could be minimized. However, the difficulty in the recombination of three fragments of atzA hindered library capacity enlargement in this study. This mutually contradictory relationship, whose mechanism needs to be further investigated, is probably a bottleneck for AtzA evolution.
Molecular docking of atrazine and AtzA showed that Gln71, which was located in the atrazine N-ethyl-binding cavity, participated in the binding of AtzA and its substrate.Citation30) In this study, variants containing mutations at site 71 had the lowest average Km value among the target sites, indicating the importance of site 71 for substrate affinity. Two possible mechanisms are proposed here. First, the mutated residue 71 interacted with atrazine directly or indirectly, facilitating substrate binding to the active center.Citation31) Alternatively, the mutated residue 71 improved the binding ability of the enzyme to its ligand Fe2+, which was beneficial for the catalytic reaction. Variant 1-73 carrying the mutation Q71R had the highest affinity for atrazine, possibly because the positively charged side chain of arginine changed the interaction status of Fe2+ and the Fe2+- binding histidines. When Gln71 was mutated into arginine or methionine, the hydrogen bond between residue 71 and Asn328, which existed in the wild type, disappeared (Fig. ). Since Asn328 directly interacted with atrazine and was largely responsible for catalytic specificity,Citation19,30) the disappearance of this hydrogen bond might greatly improve substrate-binding efficiency. The substitution of Gln71 with non-polar amino acids (methionine, proline, and alanine) increased substrate affinity, possibly because these residues were involved in hydrophobic interactions with the s-triazine ring of the substrate.Citation32) Substitution of Gln71 (apparent molar volume 86.3 mL/mole)Citation33) with the smaller alanine (53.2 mL/mole), threonine (69.7 mL/mole), proline (73.6 mL/mole), or glycine (36.1 mL/mole) also likely modified the orientation/positioning of the substrate in the active site, or the geometric relationship between the Fe2+-binding ligands, eventually increasing the enzymatic activity.
Structural stability is crucial for catalytic function of enzymes. Intramolecular interactions define the overall structure and stability of a protein.Citation34) The formation of hydrogen bonds can facilitate a catalytic reaction by stabilizing enzyme structure.Citation35,36) In AtzA, Val12, Ile393, and Leu395, which reside in the dual β-sheet domain, are involved in the formation of hydrogen bonds between the neighboring β-strands. Variants containing mutations at sites 12, 393, and 395 had higher average kcat/Km values, indicating that these mutations greatly affected the catalytic efficiency of the enzyme. As the domain is away from the active site, and β-sheet and the hydrogen bonds in it play crucial roles in structural stabilizing,Citation37) we proposed that mutations at these sites improved the enzymatic activity probably by increasing the protein structural stability and eventually affecting the catalytic reaction. Once the non-polar Ile393 and Leu395 were substituted with histidine, glutamic acid, arginine, or serine, new hydrogen bonds formed (Fig. ), which increased the stability of the dual β-sheet domain. For the variants containing mutations at site 12, V12A accounted for more than half of the mutations at this site (5/9), suggesting the substitution of valine with alanine was beneficial for enzymatic activity. The seemingly weak effects of these mutations might have a tremendous impact upon the complicated catalytic reaction on the molecular level.Citation38,39)
In this study, four residues (Val12, Gln71, Ile393, and Leu395) were targeted to generate site-saturation mutagenesis libraries, from which 17 variants with enhanced enzymatic activity were selected. These variants can serve as preferable agents for the remediation of atrazine contamination in natural and engineered environments. The structural and kinetic characterizations revealed that the substitution of Gln71 with arginine or methionine enlarged the entrance of the substrate-binding channel and modified the interaction between residues 71 and 328, thus significantly increasing the affinity for atrazine. The substitution of Ile393 and Leu395 with polar amino acids, especially charged ones such as arginine, increased the structural stability because of the formation of new hydrogen bonds, thus improving the catalytic efficiency. Our work demonstrated the effectiveness of our engineering method for enhancing enzymatic activity and provided insights into the structure–function relationship of AtzA.
Author contributions
X. Chen and D. Chen conceived and designed the study. Analysis and interpretation of data was performed by Y. Guo, W. Zhang, X. Chen, and D. Chen. Collection and assembly of data was carried out by Y. Guo, P. Zhao, and X. Li. Drafting of the article was performed by Y. Guo, P. Zhao, X. Chen, D. Chen. Critical revision of the article for important intellectual content was carried out by Y. Guo, X. Chen, and D. Chen. Final approval of the article was given by D. Chen.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Key Program of the Natural Science Foundation of Tianjin [grant number 14JCZDJC34100] and the grants of the National Natural Science Foundation of China [grant number 31070717], [grant number 31570769], [grant number 31571760].
Supplemental materials
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2016.1156481.
TBBB_1156481_Sup.doc
Download MS Word (298.5 KB)References
- Tomlin, CDS. The pesticide manual: a world compendium. 14th ed. Alton: British Crop Production Council; 2006. p. 813–814.
- Sulmon C, Gouesbet G, Binet F, et al. Sucrose amendment enhances phytoaccumulation of the herbicide atrazine in Arabidopsis thaliana. Environ. Pollut. 2007;145:507–515.10.1016/j.envpol.2006.04.018
- Belluck DA, Benjamin SL, Dawson T. Groundwater contamination by atrazine and its metabolites: risk assessment, policy and legal implications. In: Somasundaram L, Coats JR, editors. Pesticide transformation products: fate and significance in the environment. Washington, DC: ACS Press; 1991. p. 254–273.
- Gavrilescu M. Fate of pesticides in the environment and its bioremediation. Eng. Life Sci. 2005;5:497–526.10.1002/(ISSN)1618-2863
- Hayes TB, Stuart AA, Mendoza M, et al. Characterization of atrazine-induced gonadal malformations in African clawed frogs (Xenopus laevis) and comparisons with effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (17β-estradiol): support for the demasculinization/feminization hypothesis. Environ. Health Perspect. 2006;114:134–141.10.1289/ehp.8067
- Huff J, Sass J. Atrazine-a likely human carcinogen? Int. J. Occup. Environ. Health. 2007;13:356–358.
- Singh P, Suri CR, Cameotra SS. Isolation of a member of Acinetobacter species involved in atrazine degradation. Biochem. Biophys. Res. Commun. 2004;317:697–702.10.1016/j.bbrc.2004.03.112
- Sathiakumar N, MacLennan PA, Mandel J, et al. A review of epidemiologic studies of triazine herbicides and cancer. Crit. Rev. Toxicol. 2011;41:1–34.10.3109/10408444.2011.554793
- Seffernick JL, Wackett LP. Rapid evolution of bacterial catabolic enzymes: a case study with atrazine chlorohydrolase. Biochemistry. 2001;40:12747–12753.10.1021/bi011293r
- Copley SD. Evolution of efficient pathways for degradation of anthropogenic chemicals. Nat. Chem. Biol. 2009;5:559–566.10.1038/nchembio.197
- de Souza ML, Sadowsky MJ, Wackett LP. Atrazine chlorohydrolase from Pseudomonas sp. ADP: gene sequence, enzyme purification, and protein characterization. J. Bacteriol. 1996;178:4894–4900.
- Topp E, Zhu H, Nour SM, et al. Characterization of an atrazine-degrading Pseudaminobacter sp. isolated from canadian and french agricultural soils. Appl. Environ. Microbiol. 2000;66:2773–2782.10.1128/AEM.66.7.2773-2782.2000
- Rousseaux S, Soulas G, Hartmann A. Plasmid localisation of atrazine-degrading genes in newly described Chelatobacter and Arthrobacter strains. FEMS Microbiol. Ecol. 2002;41:69–75.10.1111/fem.2002.41.issue-1
- Cai B, Han Y, Liu B, et al. Isolation and characterization of an atrazine-degrading bacterium from industrial waste water in China. Lett. Appl. Microbiol. 2003;36:272–276.10.1046/j.1472-765X.2003.01307.x
- Devers M, Soulas G, Martin-Laurent F. Real-time reverse transcription PCR analysis of expression of atrazine catabolism genes in two bacterial strains isolated from soil. J. Microbiol. Methods. 2004;56:3–15.10.1016/j.mimet.2003.08.015
- Aislabie J, Bej AK, Ryburn J, et al. Characterization of Arthrobacter nicotinovorans HIM, an atrazine-degrading bacterium, from agricultural soil New Zealand. FEMS Microbiol. Ecol. 2005;52:279–286.10.1016/j.femsec.2004.11.012
- Seffernick JL, McTavish H, Osborne JP, et al. Atrazine chlorohydrolase from Pseudomonas sp. strain ADP is a metalloenzyme. Biochemistry. 2002;41:14430–14437.10.1021/bi020415s
- Wang Y, Li X, Chen X, et al. Directed evolution and characterization of atrazine chlorohydrolase variants with enhanced activity. Biochemistry (Moscow). 2013;78:1104–1111.10.1134/S0006297913100040
- Peat TS, Newman J, Balotra S, et al. The structure of the hexameric atrazine chlorohydrolase AtzA. Acta Crystallogr. D Biol. Crystallogr. 2015;71:710–720.10.1107/S1399004715000619
- Tanokura M, Miyakawa T, Guan L, Hou F. Structural analysis of enzymes used for bioindustry and bioremediation. Biosci. Biotechnol. Biochem. 2015;79:1391–1401.10.1080/09168451.2015.1052770
- You L, Arnold FH. Directed evolution of subtilisin E in Bacillus subtilis to enhance total activity in aqueous dimethylformamide. Protein Eng. 1996;9:77–83.10.1093/protein/9.1.77
- Jensen CN, Mielke T, Farrugia JE, et al. Structures of the apo- and FAD-bound forms of 2-hydroxybiphenyl 3-monooxygenase (HbpA) locate activity hotspots identified by using directed evolution. ChemBioChem. 2015;16:968–976.10.1002/cbic.201402701
- Bloom JD, Meyer MM, Meinhold P, et al. Evolving strategies for enzyme engineering. Curr. Opin. Struct. Biol. 2005;15:447–452.10.1016/j.sbi.2005.06.004
- Cai Y. Engineering a monolignol 4-O-methyltransferase with high selectivity for the condensed lignin precursor coniferyl alcohol. J. Biol. Chem. 2015;290:26715–26724.10.1074/jbc.M115.684217
- Wang H, Chen X, Hao X, et al. High throughput screening atrazine chlorohydrolase mutants with enhanced activity through Haematococcus pluvialis expression system. Chin. J. Biotechnol. 2011;27:620–628. Chinese.
- Kelley LA, Sternberg MJE. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 2009;4:363–371.10.1038/nprot.2009.2
- Wass MN, Kelley LA, Sternberg MJE. 3DLigandSite: predicting ligand-binding sites using similar structures. Nucleic Acids Res. 2010;38:W469–W473.10.1093/nar/gkq406
- Dosztányi Z, Fiser A, Simon I. Stabilization centers in proteins:Identification, characterization and predictions. J. Mol. Biol. 1997;272:597–612.10.1006/jmbi.1997.1242
- Baker D. A surprising simplicity to protein folding. Nature. 2000;405:39–42.10.1038/35011000
- Scott C, Jackson CJ, Coppin CW, et al. Catalytic improvement and evolution of atrazine chlorohydrolase. Appl. Environ. Microbiol. 2009;75:2184–2191.10.1128/AEM.02634-08
- Rothman SC, Voorhies M, Kirsch JF. Directed evolution relieves product inhibition and confers in vivo function to a rationally designed tyrosine aminotransferase. Protein Sci. 2004;13:763–772.10.1110/ps.03117204
- Noor S, Changey F, Oakeshott JG, et al. Ongoing functional evolution of the bacterial atrazine chlorohydrolase AtzA. Biodegradation. 2013;25:21–30.
- Zamyatnin AA. Protein volume in solution. Prog. Biophys. Mol. Biol. 1972;24:107–123.10.1016/0079-6107(72)90005-3
- Khan S, Vihinen M. Performance of protein stability predictors. Hum Mutat. 2010;31:675–684.10.1002/humu.v31:6
- Bandyopadhyay D, Murthy MRN, Balaram H, et al. Probing the role of highly conserved residues in triosephosphate isomerase-analysis of site specific mutants at positions 64 and 75 in the Plasmodial enzyme. FEBS J. 2015;282:3863–3882.10.1111/febs.2015.282.issue-20
- Chen X, Wang Y, Ma Z, et al. Asp141 and the hydrogen-bond chain Asp141-Asn109-Asp33 are respectively essential for GT80 sialyltransferase activity and structural stability. Biochemistry (Moscow). 2015;80:1073–1079.10.1134/S0006297915080131
- Chang S, He HQ, Shen L, et al. Understanding peptide competitive inhibition of botulinum neurotoxin a binding to SV2 protein via molecular dynamics simulations. Biopolymers. 2015;103:597–608.10.1002/bip.v103.10
- Muraki M, Goda S, Nagahora H, et al. Importance of van der Waals contact between Glu35 and Trp109 to the catalytic action of human lysozyme. Protein Sci. 1997;6:473–476.
- Zhang H, Chong H, Ching CB, et al. Random mutagenesis of global transcription factor cAMP receptor protein for improved osmotolerance. Biotechnol. Bioeng. 2011;109:1165–1172.