Abstract
The peptide linker between variable domains of heavy (VH) and light (VL) chains is one of important factors that influence the characteristics of scFv, including binding activity and specificity against target antigen. The scFvs against daidzin (DZ-scFvs) with different linker lengths were constructed in the format of VH-(GGGGS)n-VL (n = 1, 3, 5, and 7). They were expressed in the hemolymph of silkworm larvae using the Bombyx mori nucleopolyhedrovirus (BmNPV) bacmid DNA system, and their reactivity against daidzin and related compounds were evaluated using an indirect competitive enzyme-linked immunosorbent assay (icELISA), which is applicable for quantitative analysis of daidzin. The results showed that the reactivity of scFvs against daidzin was increased, whereas specificity slightly decreased when their peptide linker was lengthened. These results suggested that the linker length of DZ-scFvs contributes to its reactivity. In addition, the results emphasize that the linker length could control the reactivity of DZ-scFvs.
Graphical abstract
Construction, expression, and characterization of multiform scFvs against daidzin (DZ-scFvs) with different linker lengths.
Because monoclonal antibody (MAb) technology has been developed,Citation1) applications of antibody have attracted much attention in the fields of diagnosis, targeted therapy, and analytical chemistry due to its high affinity and specificity against target molecules. In the case of analytical chemistry, MAbs against low molecular weight compounds have been shown to be important reagent in various disciplines, including drug monitoring during clinical trials, food and environmental analysis of toxin contamination, screening for drug abuse, and pharmaceutical research and development.Citation2–5) With DNA recombinant technology, now recombinant antibodies (rAb) have been developed to eliminate some limitations of conventional MAb and polyclonal antibody (PAb).
Various types of rAbs have been developed because they can be engineered to obtain specific properties for various applications.Citation6) Moreover, rAbs can be produced more economically, which broadens their usage. Nowadays, single-chain variable fragment (scFv) antibody, the smallest rAb retaining the binding activity, is a popular format for therapeutic and analytical applications because of its small size and possibility of genetic engineering. The scFv is constructed from the variable domains of the heavy (VH) and light (VL) chain, where the C-terminus of the VH is linked to the N-terminus of the VL using a flexible peptide linker.
The success of scFv construction largely depends on the peptide linker between the VH and VL domains, in which the linker neither interferes the folding and association of the VH and VL domains, nor reduces stability and binding activity.Citation7) In the previous investigations of linker length (0–15 amino acids), the results showed that the affinity constant of scFv increased when the linker between the VH and VL was shortened, which resulted in dimer and trimer formation.Citation8) Using the two types of peptide linker including (GGGGS)2 and (GGGGS)3, the scFv against the CD20 antigen exhibited a higher affinity with the shorter linker.Citation7) These results related to the fact that monomeric scFv cannot form due to flexibility limited by shorten linker, and scFv was forged into dimer, trimer, or multimer;Citation8,9) however, the effect of linker length on binding specificity of scFv has not been evaluated yet.
It is well known that characteristics of scFv seem to be similar with its parent MAb, especially when the target antigen is a high molecular weight molecule. However, in the case of low molecular weight compounds, some scFvs have been reported to exhibit different characteristics from its parental MAb, such as plumbagin,Citation10) 2,4-dichlorophenoxyacetic acid,Citation11) mycotoxin deoxynivalenol,Citation12) zearalenone,Citation13) and digoxinCitation14). As such, the characteristics of scFv are supposed to be easily affected by some factors, which include the complementarity-determining region (CDR) of variable domains, sequences and length of peptide linker between two variable domains.
Until now the effects of the peptide linker on binding characteristics of scFv against low molecular weight compounds have not been evaluated yet. The specificity is one of important factors that significantly affect reliability when scFv was applied in the analytical field. In the present study, therefore, scFvs against daidzin (DZ-scFvs) with different linker lengths in the format of VH-(GGGGS)n-VL (n = 1, 3, 5, and 7), have been constructed as a model to investigate the effects of peptide-linker length on their binding characteristics. These scFvs were expressed as active form in the hemolymph of silkworm larvae using the Bombyx mori nucleopolyhedrovirus (BmNPV) bacmid DNA system, which was developed previously.Citation15) Their characteristics, including activity and specificity against daidzin (DZ) and its related compounds were evaluated using indirect competitive enzyme-linked immunosorbent assay (icELISA). The construction, expression, purification, and characterization of these scFvs are described in this paper.
Materials and methods
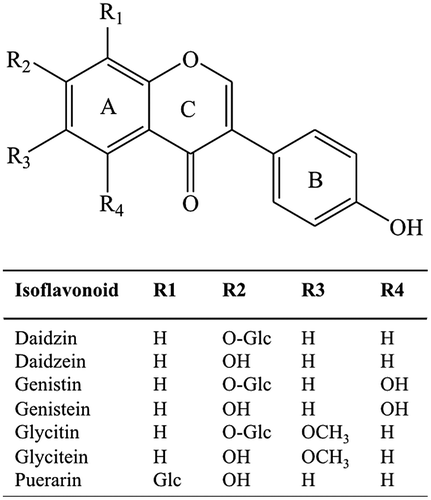
Chemical and immunochemicals. Daidzin (DZ,≥99%), daidzein (≥97%), and glycitein (≥95%) were purchased from Fujicco Co. (Kobe, Japan). Genistin (≥98%) and genistein (≥98%) were obtained from Wako Pure Chemicals Industries (Osaka, Japan), while puerarin (≥98%) and glycitin (≥99%) were obtained from Tokyo Chemical Industry Co. (Tokyo, Japan) and LC Laboratories (MA, USA), respectively. Ovalbumin (OVA,≥98%) was purchased from Sigma-Aldrich (Steinheim, Germany). Horseradish peroxidase (HRP)-conjugated goat IgG to mouse IgG Fc and HRP-conjugated mouse IgG against T7-Tag were individually purchased from Organon Teknika Cappel Products (PA, USA) and Novagen (MA, USA), respectively. Toyopearl CM-650 M cation exchange resin was obtained from Tosoh Co. (Tokyo, Japan). DNA polymerases and DNA restriction enzymes were purchased from Takara (Kyoto, Japan). All other chemical and immunological reagents that have not been specified were standard commercial products of analytical grade.
Preparation of daidzin-ovalbumin (DZ-OVA) conjugate. DZ-OVA conjugate, which was used as immobilized DZ for ELISA, was synthesized by periodate oxidation, and followed by reductive amination as described previously.Citation16) Briefly, 3 mg of DZ was dissolved in dimethyl sulfoxide (0.4 mL) and then was added dropwise to an aqueous solution (0.4 mL) of sodium periodate (3.1 mg). The resultant mixture was stirred at room temperature for 1 hour. Subsequently, the mixture was added into 50 mM carbonate buffer solution (pH 9.6, 2.0 mL) containing OVA (5.0 mg), and then the mixture was stirred for another 5 h. The resultant mixture was dialyzed against distilled water (4 °C) to remove salt and unconjugated DZ, after that it was lyophilized to obtain powders of the DZ-OVA conjugates (5.0 mg).
Construction of multiform DZ-scFv genes and their expression vectors. Each gene encoding the VH and VL was constructed from hybridoma cells secreting MAb against DZ (DZ-MAb). The development and characterization of the DZ-MAb have been described previously.Citation16) Total RNA from the hybridoma was extracted using the Sepasol RNA I super reagent (Nacalai Tesque, Kyoto, Japan), and first strand cDNA was synthesized using random hexamer primers from the First-Strand cDNA Synthesis Kit (Amersham Biosciences, Buckinghamshire, UK). The VH and VL genes were amplified by polymerase chain reaction (PCR) with the VH and VL specific primers.Citation17) Amplified PCR products were ligated into the pGEM-T vector (Promega, Madison, WI), and then the ligated vector was transformed into E. coli JM109 cells. Finally, the vector containing the expected genes was purified and sequenced (BigDye® Terminator v1.1 Cycle sequencing Kit, Applied Biosystems, CA, USA).
Specific primers for the construction of multiform DZ-scFv genes were designed from the exact sequence of the VH and VL genes. Genes encoding DZ-scFvs were constructed in the VH-linker-VL orientation, where several linkers in the format of (GGGGS)n (n = 1, 3, 5, and 7) were utilized. The primers for construction of the multiform DZ-scFv genes are summarized in Table . As for the format of the VH-(GGGGS)1-VL (DZ-scFv1), the VH gene was amplified by primers 1 and 3, whereas the VL was amplified by primers 2 and 4. The genes for the VH and VL were linked to be in the VH-(GGGGS)1-VL format by splicing by overlapping extension-PCR (SOE-PCR), where primers 1 and 2 were used. The VH-(GGGGS)n-VL (n = 3, 5, and 7) was individually named DZ-scFv3, DZ-scFv5, and DZ-scFv7. The DZ-scFv3, DZ-scFv5, and DZ-scFv7 were constructed by amplified VH and VL genes, where 1, 5 and 2, 6; 1, 7 and 2, 8; 1, 9 and 2, 10 primer pairs with DZ-scFv1, DZ-scFv3, and DZ-scFv5 genes as templates were used, respectively. Then, the same SOE-PCR was performed (Fig. (A)). All formats of DZ-scFvs were sub-cloned into the pET28a(+) expression vector (Novagen, Darmstadt, Germany) to utilize the hexahistidine (His6)-tag system and T7 sequences present in this vector.
Table 1. List of primers for amplification of DZ-scFvs.
Fig. 1. The multiform construction of DZ-scFvs donor vectors, pFastBac1Mel/DZ-scFvs.
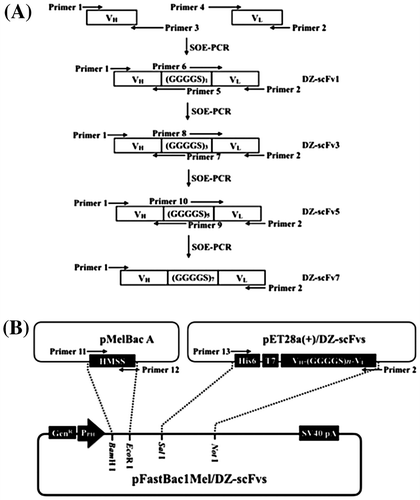
Subsequently, a baculovirus donor vector for the BmNPV bacmid DNA system was constructed. In Fig. (B), the DZ-scFv genes were amplified from the pET28a(+)/DZ-scFv vectors with N-terminal His6 and T7 sequences, using primers 2 and 13. The resultant PCR products were digested and ligated into downstream of honeybee melittin secretion signal (HMSS) sequences in pFastBac1Mel baculovirus donor vector. HMSS sequence was amplified from pMelBac A vector (Invitrogen) using primers 11 and 12, and then was sub-cloned into pFastBac1 baculovirus donor vector (Invitrogen). This HMSS mediates secretion of expressed DZ-scFvs into the hemolymph of silkworm larvae.
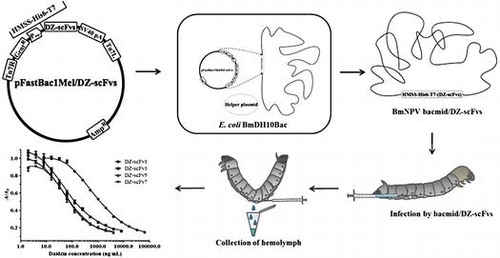
Recombinant BmNPV bacmids containing DZ-scFv1, 3, 5, and 7 genes (BmNPV bacmid/DZ-scFvs) were prepared using E.coli BmDH10Bac (Fig. ). The pFastBac1Mel/DZ-scFvs donor vector (10 ng) was transformed into E.coli BmDH10Bac competent cells (100 μL), and then the E. coli was selected using Luria–Bertani agar (1% (w/v) polypeptone, 0.5% (w/v) yeast extract, 0.5% (w/v) NaCl, and 1.5% (w/v) agar) plates containing kanamycin (50 μg/mL), gentamicin (7 μg/mL), tetracycline (10 μg/mL), isopropyl-β-D-thiogalactopyranoside (40 μg/mL), and 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (300 μg/mL). Colony PCR using M13 universal primer was performed to screen the recombinant bacmid in white colonies. Finally, recombinant BmNPV bacmid/DZ-scFvs were extracted using a QIAGEN Plasmid Maxi Kit (Valencia, CA, USA) for their expression.
Fig. 2. Construction of recombinant BmNPV bacmid DNA.
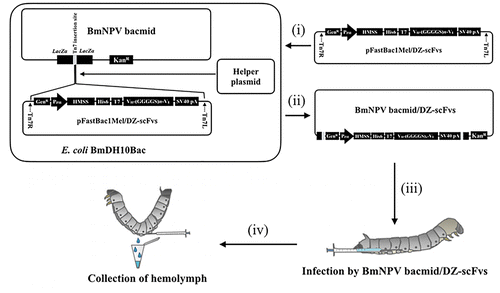
Expression of DZ-scFvs in the hemolymph of silkworm larvae. The expression of DZ-scFvs in the hemolymph of silkworm larvae was performed by the transfection of BmNPV bacmid/DZ-scFvs to the fifth instar silkworm larvae (Fig. ). The recombinant BmNPV bacmid/DZ-scFvs (1 μg) were suspended in the DMRIE-C transfection reagent (Invitrogen; 3 μL/silkworm larvae) and then kept at room temperature for 45 min. The resultant mixture was diluted with sterilized and deionized water (50 μL/silkworm larvae; Nacalai Tesque, Kyoto, Japan), and directly injected into the dorsal node of the larvae. The injected silkworm larvae were cultured at 25 °C for 6 days, where silkworm larvae could access food freely. The hemolymph was collected in a microtube containing 5% (w/v) sodium thiosulfate (50 μL) to avoid the oxidation of silkworm-derived protein. The hemolymph was centrifuged at 12,000 rpm for 20 min (4 °C). The clear hemolymph was kept at 4 °C until next experiment was performed.
Indirect ELISA (iELISA). The reactivity of DZ-scFvs was evaluated by iELISA, in which DZ-OVA conjugate was utilized as immobilized DZ in the following procedure. A transparent 96-well plate (Maxisorb Nunc, Roskilde, Denmark) was coated with DZ-OVA conjugates (1 μg/mL, 100 μL/well) in coating buffer consisting of 50 mM sodium carbonate buffer (pH 9.6) for 1 h. The plate was washed three times with 0.05% (v/v) tween 20 in 10 mM phosphate-buffered saline (T-PBS). Thereafter, the plate was treated with 5.0% (w/v) skimmed milk in phosphate-buffered saline (S-PBS, 300 μL/well) for 1 h to reduce non-specific adsorption. The plate was washed again, and then hemolymph or purified DZ-scFvs was reacted with immobilized DZ-OVA conjugates (100 μL/well). Subsequently, the plate was washed with T-PBS thrice and incubated for 1 h with a diluted solution (1:5000) of HRP-conjugated mouse IgG against T7-Tag (100 μL/well). As the last step, the substrate solution of 100 mM sodium citrate buffer (pH 4.0) containing 0.003% (v/v) H2O2 and 0.3 mg/mL of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) was added (100 μL) to each well after washing the plates three times with T-PBS. Fifteen minutes later, the absorbance at 405 nm was measured using a microplate reader (Multiskan FC, Thermo Scientific). All reactions were carried out at 37 °C.
Purification of DZ-scFvs expressed in the hemolymph of silkworm larvae. The hemolymph of each silkworm was directly analyzed for DZ-scFvs presence using iELISA. The hemolymph containing DZ-scFvs was pooled and subjected to cation exchange chromatography. Cation exchanger TOYOPEARL CM-650 M (20 mL; Tosoh Corp.) was packed and equilibrated with starting buffer (10% (v/v) glycerol in 50 mM Tris–HCl, pH 6.8). The hemolymph treated with starting buffer and proteinase inhibitors was centrifuged to remove insoluble aggregates before the clear solution was subjected to cation exchanger. Unbound proteins were washed out using starting buffer, and then the bound proteins were eluted with starting buffer containing a gradient concentration of NaCl from 0 to 300 mM. Each fraction (20 mL) was collected and analyzed using iELISA and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to evaluate the presence of DZ-scFvs.
Characterization of DZ-scFvs by iELISA and icELISA. To evaluate the effects of linker lengths on the binding characteristics of DZ-scFvs, icELISA was performed using free DZ and related compounds as competitor. The same procedures that were used in the iELISA were used until the blocking step. In the competitive step, serial concentrations of DZ in 20% (v/v) methanol in water (50 μL) were added to each well, and then purified DZ-scFvs (diluted in T-PBS) were added (50 μL). An hour later, the plate was washed three times with T-PBS, and then bound DZ-scFvs were allowed to react with a diluted solution (1:5000) of HRP-conjugated mouse IgG against T7-Tag (100 μL/well) for 1 h. In the last step, the substrate solution (100 μL/well) was added and incubated for 15 min. The developed color was then measured using a microplate reader with an absorbance at 405 nm.
Using the above procedure of icELISA with the optimal concentration of DZ-scFvs, the serial concentrations of DZ were tested to evaluate whether DZ-scFvs reacted with free DZ in a concentration-dependent manner. The sensitivity of icELISA for DZ determination could be estimated.
A cross-reactivity (CR) test was performed to assess the specificity of DZ-scFvs. In one assay, the serial concentrations of DZ or related compounds (Fig. ) were reacted with DZ-scFvs, in which their comparative binding activity against investigated compound and DZ was expressed as a percentage of CR calculated using the following equation.Citation18) In the equation, IC50 represents the concentration of the DZ or investigated compound that gives 50% inhibition of the control, which was the absorbance in the absence of DZ or investigated compounds.
Results and discussion
Construction, expression, and purification of DZ-scFvs
The sequencing of the VH and VL domains, which were cloned from the cDNA of hybridoma cells secreting DZ-MAb, revealed that they were composed of 118 and 114 amino acids, respectively. The CDR for both the VH and VL domains was assigned according to the Kabat and Chothia numbering scheme (http://www.bioinf.org.uk/abs/) as shown in Fig. . They were then linked together with the peptide linker consisting of (GGGGS)n (n = 1, 3, 5, and 7) by SOE-PCR to construct four formats of DZ-scFvs (DZ-scFv1, 3, 5, and 7). These nucleotide sequences were assigned in DDBJ as accession number LC075518. These were subsequently sub-cloned into the pET28a(+) expression vector, followed by a pFastBac1Mel donor vector to construct pFastBac1Mel/DZ-scFvs (Fig. (B)). The pFastBac1Mel/DZ-scFvs donor vector was designed to contain HMSS to enhance the secretion of DZ-scFvs into the hemolymph of silkworm larvae. When the conventional bacterial expression system (BL21 (DE3) E. coli) was used, scFvs were expressed as inclusion bodies, and this requires time- and cost-consuming refolding steps that can affect the 3-D characteristics of scFv, ultimately influencing function.Citation14) Consequently, the specificity of scFv expressed in E. coli was changed when compared with its parent MAb, whereas the employment of an insect cell expression system resulted in the retention of the MAb binding specificity of scFv.Citation14) Therefore, to diminish the effects of refolding steps on scFv reactivity and to ensure that its reactivity and specificity are only reflected from linker length, all DZ-scFvs were expressed as native form in the hemolymph of silkworm larvae. Six days after silkworm was infected by recombinant BmNPV bacmid/DZ-scFvs, DZ-scFvs were expressed and secreted into the hemolymph of all infected silkworm larvae. The hemolymph containing DZ-scFvs was pooled and subjected to cation exchanger, where the high purity of DZ-scFvs was eluted with 250−300 mM NaCl.
Fig. 4. The sequencing of the VH and VL domains revealed that they were composed of 118 and 114 amino acids, respectively.

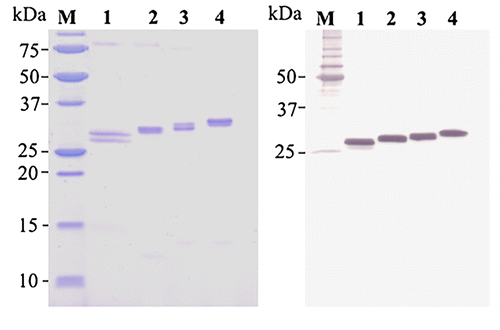
SDS-PAGE analysis (Fig. ) revealed that high purity of DZ-scFvs was obtained after single cation exchange chromatography. The bands of DZ-scFvs in SDS-PAGE analysis also correlated to their theoretical molecular masses of 30.18, 30.81, 31.44, and 32.07 kDa for DZ-scFv1, 3, 5, and 7, respectively, which corresponds to the chimera proteins of N-terminal His6 and T7 sequences. As SDS-PAGE analysis (non-reducing condition) of DZ-scFv1, two bands were separated clearly, which it might be resulted variable folding because properly folded DZ-scFv1 (oxidized form) migrated faster than improperly folded DZ-scFv1 (reduced or partially reduced form).Citation19) This phenomenon may be also accounted for the fact that the reactivity of DZ-scFv1 exhibited the lowest reactivity to immobilized and free DZ, which were evaluated by iELISA and icELISA, respectively (Fig. ). In case of DZ-scFv3, 5, and 7, there are two closely spaced bands, this phenomenon was expected to appear by variable glycosylation.Citation19) However, both bands were detected in western blots using antibody to 6 × His epitope tag.
Fig. 5. SDS-PAGE (left) and western blotting (right) analysis of purified scFvs.
Reactivity of DZ-scFvs
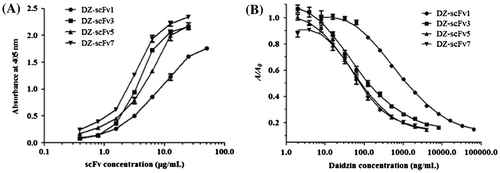
Using iELISA, all DZ-scFvs reacted with DZ-OVA conjugates in a scFv concentration-dependent manner (Fig. (A)). The DZ-scFv3, 5, and 7 showed similar activities to each other against DZ-OVA conjugates, whereas DZ-scFv1 displayed the weakest reactivity of the four DZ-scFvs. When DZ-scFvs concentrations for icELISA were optimized based on the absorbance at 405 nm given ~1.0 using iELISA, the concentration of 12.50, 3.13, 6.25, and 3.13 μg/mL was found to be the optimal concentrations of scFv1, 3, 5, and 7, respectively. The inhibitory curves of DZ using DZ-scFvs-based icELISA were showed (Fig. (B)) where the sensitivity of DZ determination by the icELISA can be evaluated. As a result, all DZ-scFvs reacted in a concentration-dependent manner with free DZ. Moreover, the IC50 of individual DZ-scFv1, 3, 5, and 7 against free DZ was calculated to be 1271.96, 125.09, 84.95, and 79.34 ng/mL, respectively. This suggests that the inhibitory activity was increased as the n of (GGGGS)n increased, where the degree of increase was large from n = 1 to 3 and small from n = 3 to 7. The results of the icELISA revealed that increased linker length improved icELISA sensitivity for DZ determination. Therefore, the length of linker peptide should be optimized when constructing each scFv. In addition, DZ-scFv1 exhibited the lowest reactivity against immobilized and free DZ when compared with other formats, although it has been reported that shorter linker peptides result in higher affinity for target molecules.Citation7,8) When the linker peptide is less than 15 amino acids, scFv has a tendency to form dimers or trimers, which results in a stronger activity against antigens compared with monomers. Our results showed that DZ-scFv1 has the lowest activity against immobilized and free DZ, suggesting that DZ-scFv1 might not form multimers, but it remained as a monomer.
Subsequently, the CR test of DZ-scFvs was carried out using six structure-related compounds; these were daidzein, genistin, genistein, glycitin, glycitein, and puerarin (Fig. ), in which parental DZ-MAb exhibited different degrees of CR against these compounds (Table ). The CR of DZ-scFvs exhibited a tendency to be increased as the n of (GGGGS)n increased, with the exception of some values for which CR against genistin was decreased (Table ). Long peptide linker was estimated to prevent associations between the VH and VL domains and affected the function of scFv, which caused the broad specificity of DZ-scFvs as n increased.
Table 2. Cross-reactivity (CR) of DZ-scFvs and its parental DZ-MAb against isoflavones.
The sensitivity and specificity of scFv are important factors when used as a tool for quantitative analysis. In this study, a multiform DZ-scFv was successfully constructed and expressed using a BmNPV bacmid DNA system to investigate the effects of peptide linker length on the reactivity of DZ-scFvs. Their characterizations revealed that sensitivity through icELISA was increased, whereas their specificity against DZ was decreased by increasing the n of (GGGGS)n. These data suggest that the linker length of scFv donates its reactivity when the target antigen is a low molecular weight compound.
Therefore, optimization of linker length is a useful approach for construction of scFv against a low molecular weight compound since the highest sensitivity of immunoassay is needed when only small amount of target compound was found in sample. Moreover, CR also has an effect on immunoassay performance, in which total related compound can be analyzed in the same immunoassay using group-specific antibody.
Authors’ contribution
Gorawit Yusakul, Seiichi Sakamoto, Benyakan Pongkitwitoon, Hiroyuki Tanaka, and Satoshi Morimoto designed the experiments. Gorawit Yusakul and Benyakan Pongkitwitoon performed experiments and analyzed results. Seiichi Sakamoto, Hiroyuki Tanaka, and Satoshi Morimoto provided the reagents and equipment. Finally, Gorawit Yusakul and Seiichi Sakamoto wrote the paper.
Funding
This work was supported by the Fuji Foundation for Protein Research.
Acknowledgments
We also appreciate the technical supports from the Research Support Center, Graduate School of Medical Sciences, Kyushu University, Japan.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497.10.1038/256495a0
- Alak AM. Measurement of tacrolimus (FK506) and its metabolites: a review of assay development and application in therapeutic drug monitoring and pharmacokinetic studies. Ther. Drug Monit. 1997;19:338–351.10.1097/00007691-199706000-00016
- Emon JMV, Lopez-Avila V. Immunochemical methods for environmental analysis. Anal. Chem. 1992;64:78A–88A.10.1021/ac00026a715
- Franek M, Hruska K. Antibody based methods for environmental and food analysis: a review. Vet. Med-Czech. 2005;50:1–10.
- Huestis MA, Gustafson RA, Moolchan ET, et al. Cannabinoid concentrations in hair from documented cannabis users. Forensic Sci. Int. 2007;169:129–136.10.1016/j.forsciint.2006.08.005
- Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat. Biotech. 2005;23:1126–1136.10.1038/nbt1142
- Gu X, Jia X, Feng J, et al. Molecular modeling and affinity determination of scfv antibody: proper linker peptide enhances its activity. Ann. Biomed. Eng. 2010;38:537–549.10.1007/s10439-009-9810-2
- Wang S, Zheng C, Liu Y, et al. Construction of multiform scFv antibodies using linker peptide. J. Genet. Genomics. 2008;35:313–316.10.1016/S1673-8527(08)60045-4
- Dolezal O, Pearce LA, Lawrence LJ, et al. ScFv multimers of the anti-neuraminidase antibody NC10: shortening of the linker in single-chain Fv fragment assembled in VL to VH orientation drives the formation of dimers, trimers, tetramers and higher molecular mass multimers. Protein Eng. 2000;13:565–574.10.1093/protein/13.8.565
- Sakamoto S, Taura F, Putalun W, et al. Construction and expression of specificity-improved single-chain variable fragments against the bioactive naphthoquinone, plumbagin. Biol. Pharm. Bull. 2009;32:434–439.10.1248/bpb.32.434
- Sakamoto S, Pongkitwitoon B, Nakamura S, et al. Construction, expression, and characterization of a single-chain variable fragment antibody against 2,4-dichlorophenoxyacetic acid in the hemolymph of silkworm larvae. Appl. Biochem. Biotechnol. 2011;164:715–728.10.1007/s12010-011-9168-4
- Maragos CM, Li L, Chen D. Production and characterization of a single chain variable fragment (scFv) against the mycotoxin deoxynivalenol. Food Agric. Immunol. 2011;23:51–67.
- Yuan Q, Clarke JR, Zhou HR, et al. Molecular cloning, expression, and characterization of a functional single-chain Fv antibody to the mycotoxin zearalenone. Appl. Environ. Microbiol. 1997;63:263–269.
- Lemeulle C, Chardès T, Montavon C, et al. Anti-digoxin scFv fragments expressed in bacteria and in insect cells have different antigen binding properties. FEBS Lett. 1998;423:159–166.10.1016/S0014-5793(98)00029-5
- Motohashi T, Shimojima T, Fukagawa T, et al. Efficient large-scale protein production of larvae and pupae of silkworm by Bombyx mori nuclear polyhedrosis virus bacmid system. Biochem. Biophys. Res. Commun. 2005;326:564–569.10.1016/j.bbrc.2004.11.060
- Sakamoto S, Yusakul G, Pongkitwitoon B, et al. Simultaneous determination of soy isoflavone glycosides, daidzin and genistin by monoclonal antibody-based highly sensitive indirect competitive enzyme-linked immunosorbent assay. Food Chem. 2015;169:127–133.10.1016/j.foodchem.2014.08.004
- Krebber A, Bornhauser S, Burmester J, et al. Reliable cloning of functional antibody variable domains from hybridomas and spleen cell repertoires employing a reengineered phage display system. J. Immunol. Methods. 1997;201:35–55.10.1016/S0022-1759(96)00208-6
- Weiler EW, Zenk MH. Radioimmunoassay for the determination of digoxin and related compounds in Digitalis lanata. Phytochemistry. 1976;15:1537–1545.10.1016/S0031-9422(00)88933-5
- Hirohata S, Wang LW, Miyagi M, et al. Punctin, a novel ADAMTS-like Molecule, ADAMTSL-1, in extracellular matrix. J. Biol. Chem. 2002;277:12182–12189.10.1074/jbc.M109665200
- Sung LY, Chen CL, Lin SY, et al. Efficient gene delivery into cell lines and stem cells using baculovirus. Nat. Protoc. 2014;9:1882–1899.10.1038/nprot.2014.130