Abstract
Thiamine pyrophosphate (TPP) is a critical cofactor and its biosynthesis is under the control of TPP availability. Here we disrupted a predicted thiA gene of the fungus Aspergillus nidulans and demonstrated that it is essential for synthesizing cellular thiamine. The thiamine riboswitch is a post-transcriptional mechanism for TPP to repress gene expression and it is located on A. nidulans thiA pre-messenger RNA. The thiA riboswitch was not fully derepressed under thiamine-limited conditions, and fully derepressed under environmental stressors. Upon exposure to hypoxic stress, the fungus accumulated more ThiA and NmtA proteins, and more thiamine than under aerobic conditions. The thiA gene was required for the fungus to upregulate hypoxic branched-chain amino acids and ethanol fermentation that involve enzymes containing TPP. These findings indicate that hypoxia modulates thiA expression through the thiamine riboswitch, and alters cellular fermentation mechanisms by regulating the activity of the TPP enzymes.
Graphical abstract
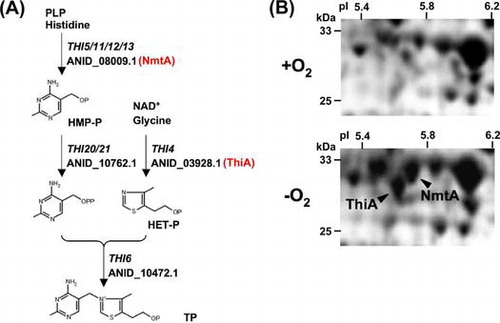
Upon exposure to hypoxic stress (B; –O2), the fungus accumulated more ThiA and NmtA proteins that involve in thiamine biosynthesis (A), and more thiamine than under aerobic conditions (B;+O2).
Hypoxia (low oxygen) imposes global metabolic responses on fungi since oxygen is critical for respiration and the synthesis of essential biological compounds. The growth of most fungi is therefore restricted by the amount of available oxygen except for the model yeast Saccharomyces cerevisiae, which thrives under hypoxic conditions by fermenting glucose to ethanol. The filamentous fungus Aspergillus nidulans, which is a genetic model for eukaryotes, alters cellular metabolic mechanisms in response to hypoxia at the levels of transcription and protein.Citation1,2) Some alterations are conserved among A. nidulans, A. oryzae, and other fungi, and others are specific to A. nidulans.Citation3,4) Biochemical studies have indicated that hypoxia induces metabolic mechanisms for nitrate reduction and branched-chain amino acid (BCAA) production as well as ethanol fermentation in A. nidulans.Citation5,6) These mechanisms oxidize NADH/NADPH and regenerate NAD+/NADP+ for oxidizing carbon sources, and thus are dissimilation mechanisms that allow the fungus to persevere under oxygen limitation.Citation6)
Thiamine (vitamin B1) is essential for all living systems, and it exists in free and phosphoester forms.Citation7–9) Thiamine pyrophosphate (TPP) is a cofactor for carbohydrate and amino acid metabolic enzymes. Micro-organisms that synthesize TPP produce thiazole and pyrimidine moieties that couple to form thiamine monophosphate.Citation10–12) Bacterial ThiG initiates thiazole (5-(2-hydroxyethyl)-4-methylthiazole (HET) phosphate) synthesis via the oxidative condensation of 1-deoxy-D-xylulose-5-phosphate, glycine (or tyrosine), and cysteine.Citation10) In contrast, an eukaryotic mechanism of thiazole synthesis is beginning to emerge. The S. cerevisiae isozyme of bacterial ThiA (Thi4p) is essential for HET phosphate synthesis and its substrate is NAD+.Citation13–16)
Fungi regulate thiamine synthesis at the transcriptional and post-transcriptional levels. Yeast THI4 is under the transcriptional control of the thiamine-responsive transcription activator complex Thi2p/Thi3p/Pdc2p and of histone acetylation at distal regions of the THI4 gene promoter.Citation17,18) The transcriptional regulation of thiamine-related genes is not as well understood in other fungi. The riboswitch is a ubiquitous post-transcriptional mechanism of gene regulation in bacteria, fungi, algae, and plants, and small ligands bind to mRNA and regulate its splicing.Citation19,20) A riboswitch that binds TPP represses the expression of Neurospora crassa thi-4, and of its ortholog, A. oryzae thiA, and functions as a feedback mechanism for thiamine biosynthesis.Citation21,22) Thiamine riboswitch also found in yeast such as Cryptococcus, Yarrowia, and Debaryomyces, but there is no report for S. cerevisiae.Citation20)
Global proteomic analyses have indicated that hypoxic A. nidulans cells upregulate enzymes containing TPP.Citation2) These include pyruvate decarboxylase (PDC), transketolase, α-ketoglutarate dehydrogenase, and acetohydroxy acid synthase, which are likely to participate in the hypoxic upregulation of ethanol fermentation, the pentose phosphate pathway (PPP), the tricarboxylate cycle, and dissimilatory BCAA fermentation, respectively.Citation6) The expression of A. nidulans thiA is also hypoxically upregulated.Citation2) These findings indicate that thiamine synthesis might become upregulated and that metabolic changes occur under hypoxic conditions. However, little is known about how oxygen regulates the expression of fungal thiA despite considerable investigation into thiamine-dependent regulation. The present study investigated the hypoxic regulation of A. nidulans thiA through the thiamine riboswitch, and found that hypoxia as well as other environmental stresses derepressed the riboswitch. This led to upregulated thiamine production, and the subsequent activation of ethanol fermentation and BCAA production, followed by NAD+ regeneration that supports survival under hypoxia.
Materials and methods
Strains, culture, and media
A. nidulans strains A26 (biA1) and A89 (biA1; argB2) were obtained from the Fungal Genetic Stock Center (University of Missouri, Kansas City, MO, USA). Conidia (1 × 108) were transferred to 500-mL Erlenmeyer flasks containing 100 mL of MMDN (10 g/L glucose, 6 g/L NaNO3, 10 mM KH2PO4, 7 mM KCl, 2 mM MgSO4, 0.2% Hutner’s trace metals) and aerobically incubated at 30 °C for 20 h at 120 rpm (preculture).Citation23) The resultant mycelia (0.1 g wet weight) were collected by filtration, washed twice with 0.85% NaCl, and then inoculated into 500-mL Erlenmeyer flasks containing 100 mL of MMEN (100 mM ethanol, 10 mM NaNO3, 10 mM KH2PO4, 7 mM KCl, 2 mM MgSO4, 0.2% Hutner’s trace metals).Citation5) Head space in the flasks was replaced with nitrogen gas by purging the air for 15 min, and then sealing the flasks with butyl rubber stoppers and rotating them at 30 °C at 120 rpm to maintain hypoxic conditions. Aerobic conditions were maintained by agitating 100 mL of MMEN medium in flasks sealed with cotton plugs but without replacing the air in the head space. Thiamine (10 μM) was added in some experiments. The growth of auxotrophic mutants was supported by adding appropriate supplements according to the instructions provided by the Fungal Genetics Stock Center.
Production of recombinant ThiA
We used PCR, A. nidulans cDNA, and the primers rthiA-f and rthiA-r to prepare thiA cDNA (Supplementary Table S1), which was then cleaved with EcoRI and HindIII before ligation into the plasmid vector pET28a (Novagen, Madison, WI, USA) that had been cut with the same restriction enzymes. Standard DNA manipulations proceeded as described.Citation24) The plasmid vector was introduced into Escherichia coli BL21 (DE3) pLysS (Merck, Darmstadt, Germany), cultured in Luria broth (1% tryptone, 0.5% yeast extract, 0.5% NaCl) containing 34 μg mL−1 kanamycin sulfate for 12 h, and then a portion (2 mL) was agitated on a rotary shaker at 120 rpm in 200 mL of Luria broth containing 34 μg mL−1 kanamycin sulfate in 500-mL flasks at 37 °C. After the optical density reached 1.0, 1 mM isopropyl 1-thio-β-D-galactoside was added to the medium and the cells were further incubated for 12 h at 120 rpm at 25 °C. The E. coli cells were then suspended in 50 mL of buffer A (50 mM potassium phosphate [pH 7.5], 1 mM dithiothreitol) and disrupted by sonication. The sonicate was centrifuged at 1,800 × g for 15 min, and the resulting cell-free extract was separated by centrifugation at 100,000 × g for 60 min. The supernatant was applied to a column (ϕ 1 × 2 cm) containing nickel-nitrilotriacetic acid agarose (Qiagen, Valencia, CA, USA) that was already equilibrated with buffer B (50 mM potassium phosphate [pH 7.5], 300 mM NaCl). The column was washed with 50 mL of buffer B containing 20 mM imidazole and protein was eluted with buffer B containing 250 mM imidazole. All manipulations proceeded at temperatures below 4 °C.
ThiA reaction with NAD+
The hydrolytic activity of NAD+ was measured as described with the following modifications.Citation15) Purified recombinant ThiA (100 μM) was incubated with 300 μM NAD+ (with or without 300 μM glycine) for 1 h at 25 °C. The reaction mixture was heat denatured (100°C, 40 s), centrifuged to remove insoluble materials, filtered through a Microcon YM-10 filter (Millipore, Billerica, MA, USA) and analyzed by high-performance liquid chromatography (HPLC) using a ϕ 15-cm × 4.6-mm Supelco LC-18-T column (Sigma-Aldrich, St. Louis, MO, USA) as described.Citation15)
Gene disruption of A. nidulans thiA
A DNA fragment encoding the 5′-region of thiA fused with appropriate restriction sites was amplified using the primers thiA5-f and thiA5-r (Supplementary Table S1), digested with restriction enzymes and ligated into pBSarg1 that had been digested with the same restriction enzymes.Citation23) The 3′-region of thiA was amplified using the primers thiA3-f and thiA3-r (Supplementary Table S1), digested with restriction enzymes, and inserted into the same restriction sites of the resulting plasmid to generate pDTA1. A. nidulans was transformed, and total fungal DNA was prepared as described.Citation5) After restriction enzyme digestion, DNA fragments were Southern blotted and hybridized using DIG DNA labeling and detection kits (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Thereafter, DNA fragments amplified by PCR using the primers thiA5-f and thiA5-r (Supplementary Table S1) served as hybridization probes.
Two-dimensional PAGE and MALDI-TOF-MS analyses
Cellular proteins were extracted and separated by two-dimensional (2D)-polyacrylamide gel electrophoresis (PAGE) as described.Citation2) Gel images were loaded into Proteomeweaver software (version 4.0, Bio-Rad) and processing, spot detection, quantitation, gel matching and warping proceeded according to the manufacturer’s instructions. The volumes of spots were normalized by dividing the volume of each by the summed volume of all spots. All proteomic differential display experiments were independently repeated three times. Proteins were identified by peptide mass fingerprinting after in-gel tryptic digestion.Citation2)
Determination of thiamine and other metabolites
Mycelia (50 mg wet weight) were washed twice with chilled water, immediately transferred to liquid nitrogen, ground into a fine powder and then suspended in 0.1 M HCl. Total amounts of thiamine and its phosphoesters were determined after taka-diastase hydrolysis of thiamine phosphates and then thiamine was quantified using a Thiamine Microbiological Assay Kit (ALPCO Diagnostics, Salem, NH, USA) according to the manufacturer’s instructions. Ethanol, lactate, valine, leucine, and isoleucine were quantified as described.Citation6)
Quantitative PCR
A. nidulans A26 and ThiAΔ were cultured in MMEN media at 30 °C for 6 and 12 h under aerobic or hypoxic conditions. Total RNA was extracted and then single-stranded cDNA was synthesized as described.Citation6) Real-time quantitative PCR proceeded using MiniOpticon™ version 3.1 (Bio-Rad) and SYBR® Premix Ex Taq™ (Bio-Rad) as described.Citation25) Supplementary Table S1 shows the gene-specific primers. The amounts of transcripts were normalized against those of the actin gene (actA), and are shown as relative values.
Detecting splice variants at the thiA 5′-region
A. nidulans A 26 was incubated in MMEN medium at 30 °C for 12 h, aeration or temperatures were changed or stressors (1 mM menadione (MD) and 1 mM hydrogen peroxide (H2O2) were added. After incubation for a further 6 h, cDNA was prepared from the resulting mycelia as described above, and introns splicing was determined using PCR and primers RTthiA-f and RTthiA-r (Supplementary Table S1).
Pyruvate decarboxylase assays
Fungal cell extracts were prepared in 50 mM histidine-HCl (pH 6.5) as described, and incubated in a reaction mixture containing 50 mM histidine-HCl (pH 6.5), 150 μM NADH, 350 μM MgCl2, 350 μM TPP, 30 μg/mL aldehyde dehydrogenase (Sigma-Aldrich, No. 82884).Citation26) The reaction then was started by adding 30 mM sodium pyruvate, and decreases in NADH were monitored at 25 °C as changes in absorbance at 340 nm using a DU-800 spectrophotometer (Beckman-Coulter, Brea, CA, USA). A molar extinction coefficient of 6.22 mM−1 cm−1 was used for NADH. Protein concentrations were determined using protein assay kits (Bio-Rad).
Results
Thiamine synthesis requires thiA in A. nidulans
Homology searches identified predicted A. nidulans proteins encoded by genes with the IDs, ANIA_03928.1, ANIA_08009.1, ANIA_10472.1, and ANIA_10762.1, and their amino acid sequences were 61, 67, 32, and 34% identical to proteins encoded by S. cerevisiae THI4, THI5, THI6, and THI20, respectively (Fig. ). The A. nidulans genome contained no other genes encoding proteins with identity as high as those to the yeast isozymes. The present study focused on the THI4 ortholog of gene ID ANIA_03928.1, designated thiA, which should encode a key enzyme for synthesizing the thiazole moiety of thiamine.
Fig. 1. Predicted genes involved in thiamine biosynthesis by A. nidulans.
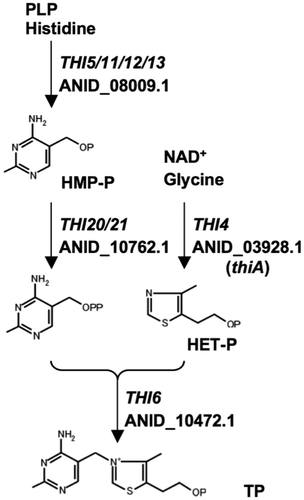
The purified recombinant ThiA (rThiA) resolved as a single band on SDS-PAGE with a molecular mass of 37 kDa (Fig. (A)). Recombinant ThiA degraded NAD+ to produce ADP-ribose and nicotinamide (Fig. (B)) but none of NADH, NADP+, and NADPH (data not shown). When incubating rThiA with ADP-ribose, we detected another compound (peak 4 in Fig. (C)), which a longer incubation of rThiA with NAD+ also generated. This peak disappeared after glycine was added to the reaction (data not shown). These properties of the compound detected as peak 4 coincided with those of ADP-ribulose, which is a reaction intermediate of yeast Thi4p and NAD+ that chemically reacts with glycine to generate the thiamine precursor ADP-thiazole.Citation15) These results indicated that A. nidulans ThiA uses NAD+ as a substrate for synthesizing thiamine precursors as well as S. cerevisiae Thi4p.
Fig. 2. Recombinant ThiA hydrolyzes NAD+ in vitro.
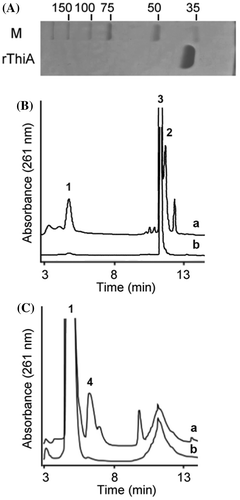
A thiA gene disruptant was constructed by double crossing over with the plasmid and the fungal chromosome at the 5′- and 3′-regions of thiA (Fig. (A)). Southern blotting identified a specific 2.8-kb signal for PstI-digested total DNA from the transformant (ThiAΔ) instead of the 2.2-kb signal that corresponded to intact thiA in the wild-type strain (WT). These findings indicated that the gene was deleted from the transformant. Gene disruption resulted in the loss of specific ThiA spots on 2D-PAGE images of cellular proteins of ThiAΔ (Fig. (B), Supplementary Fig. and Table S2), confirming that ThiA production is impaired in this strain. Furthermore, ThiAΔ did not grow on minimum medium. By adding thiamine or HET (10 μM each) to the medium, the growth rate of ThiAΔ equaled that of the WT (Fig. (C)). These results indicated that thiA is associated with thiamine synthesis in A. nidulans.
Fig. 3. A. nidulans thiA gene is essential for thiamine synthesis.
Hypoxic upregulation of thiamine synthesis
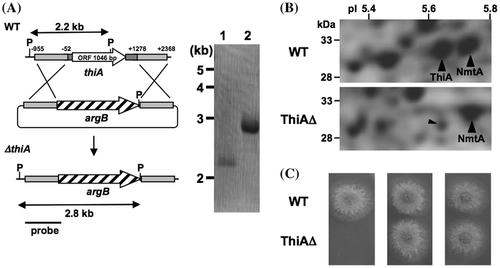
The contents of thiamine including its phosphoesters were measured in mycelia that had been cultured in the presence of exogenous thiamine followed by thiamine-depleted minimum medium for 12 h. When incubated under aerobic conditions, WT mycelia contained 34 ± 5 pmol mg−1 of thiamine (Fig. (A)), whereas ThiAΔ contained 30% of this amount, confirming that thiA functions in thiamine synthesis. Any remaining thiamine is likely to be incorporated into the cells during the initial culture. Under these conditions, WT and ThiAΔ growth did not significantly differ (data not shown), indicating that the low level of thiamine detected in ThiAΔ did not limit fungal cell growth in the presence of O2.
Fig. 4. Hypoxic role of thiamine synthesis.
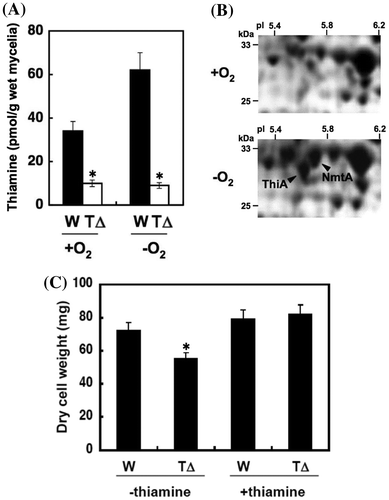
Proteomic analysis of A. nidulans identified cellular proteins for thiA and ANIA_08009.1 (nmtA) that were upregulated 8.1- and 5.0-fold, respectively, under hypoxic conditions (Fig. (B)).Citation2) Wild-type mycelia accumulated 1.9-fold more thiamine after incubation under hypoxic, than aerobic conditions (Fig. (A)), which did not occur in ThiAΔ. In contrast to that under aerobic conditions, the dry cell weight of ThiAΔ grown under hypoxia was 80% of that of the WT (Fig. (C)). This indicates that A. nidulans fungal cells produced more thiamine to support growth when incubated under hypoxic conditions.
Stress responses of thiamine riboswitch for thiA expression
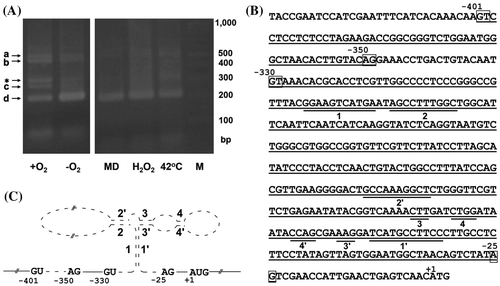
Reverse transcription-PCR analysis of WT cultured in minimal medium amplified a 200-bp DNA fragment corresponding to the 5′-untranslated region of thiA (Fig. (A)). Nucleotide sequencing of this fragment revealed 53- and 307-bp introns (Fig. (B)) located in the region as suggested.Citation22) Nucleotide sequencing other amplified 510- and 250-bp DNA fragments indicated that they correspond to pre-mRNA in which the first and second introns, respectively, remained unspliced (Fig. (B)). A 300-bp fragment (asterisk in Fig. (A)), which previous study did not findCitation22), was detected and remained uncharacterized in this study. The second intron (−330 to −25 in Fig. (B)) contained inverted repeats (1/1′ to 4/4′ in Fig. (B) and (C)) that were similar to those in the fungal and plant thiamine riboswitches that form TPP aptamers upon binding with TPP.Citation21,22) The presence of substantial levels of unspliced mRNA in the absence of thiamine (+O2 in Fig. (A)) indicated that such absence did not fully derepress the thiamine riboswitch and resultant thiA gene expression. The fungal cells probably contained substantial amounts of thiamine acquired during the initial culture in medium containing thiamine, and then retained some of it under partial thiamine-limiting conditions. We investigated the effects of environmental stressors on the thiA riboswitch under thiamine-limiting conditions. Adding hydrogen peroxide and menadione to the culture medium or heating cultures to 42 °C increased intron splicing (Fig. (A)), indicating that these stressors derepressed thiA expression. Hypoxia increased the splicing of both introns, which is consistent with increased thiamine accumulation in the hypoxic cells (Fig. (A)). Transcripts of the thiA gene quantified using real-time PCR showed that the fungus generated 1.2-fold more thiA transcripts grown under hypoxia than that of normoxia. These results indicated that hypoxia and stressors in addition to thiamine depletion are factors involved in the maximal induction of thiA and cellular thiamine production.
Fig. 5. Post-transcriptional regulation of thiA by thiamine riboswitch.
Thiamine upregulation for metabolic adaptation to hypoxia
We found that the mechanism of hypoxic BCAA production is more prominent in A. nidulans when the carbon source is ethanol rather than glucose.Citation6) The WT strain cultured using ethanol as the sole carbon source under hypoxic conditions, produced 9.7, 18.5 and 15.3 μmol of Val, Leu, and Ile in culture supernatants, respectively (Fig. (A)), and < 0.01 μmol/g of wet weight under aerobic conditions. ThiAΔ cells produced lower levels of respective BCAAs (1.3, 2.7, and 2.3 μmol), and exogenous thiamine rescued the impaired BCAA production (Fig. (A)). These results indicated that thiamine synthesis limits BCAA production under hypoxic conditions. The biosynthesis of BCAA requires the TPP-containing enzyme, acetohydroxy acid synthase, the production of which is upregulated under hypoxic conditions.Citation6) The upregulated thiamine production under hypoxia could be a mechanism that shifts cellular metabolism to dissimilatory BCAA production by supplying TPP to this enzyme for adaptation into hypoxia.
Fig. 6. Hypoxic thiamine production enhances cellular metabolism.
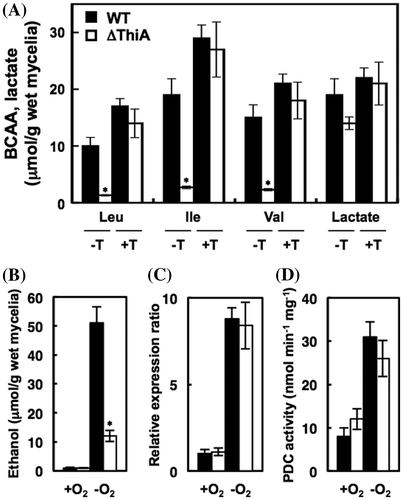
Ethanol and lactate fermentation are hypoxic mechanisms for regenerating cellular NAD+. A. nidulans upregulated ethanol production under hypoxic conditions, and ThiAΔ generated 4.3-fold less ethanol than WT (Fig. (B)), indicating that thiamine regulates this mechanism. The enzyme pyruvate decarboxylase (PDC) requires TPP to convert pyruvate to acetaldehyde, a precursor of ethanol.Citation27) Gene transcripts of PDC (pdcA, ANIA_04888.1) in hypoxic A. nidulans cells were quantified using real-time PCR. A. nidulans generated 8.8-fold more pdcA transcripts under hypoxia, compared with normoxia (Fig. (C)). Cellular PDC activity was 3.9-fold higher in the hypoxic cells (Fig. (D)), indicating that PDC upregulated by hypoxia contributes to ethanol production. The activity of PDC was not defective in ThiAΔ cell extracts (Fig. (D)) probably due to the adding of saturated amounts of TPP in reactions that are required for reconstituting enzyme activity. Lactate fermentation, which is not dependent upon PDC, responded to hypoxia to a lesser extent and was minimally affected by ThiA (Fig. (A)). These findings indicated that A. nidulans synthesizes more thiamine under hypoxic, than aerobic conditions (Fig. (B)), increases the catalytic rate of the TPP-enzymes and upregulates BCAA and ethanol fermentation.
Discussion
Most published studies of the regulation of thiamine synthesis have focused on available thiamine, and information about regulation by environmental stressors is scarce.Citation10–12) The present study found that hypoxia upregulates a post-translational mechanism for thiamine synthesis by A. nidulans. This mechanism is important for the fungus to ferment BCAA and ethanol by providing TPP for acetohydroxy acid synthase and PDC. The fungal mechanisms of BCAA and ethanol fermentation are significant for regenerating NAD+ under hypoxic conditions.Citation6) Thus, the hypoxic regulation of thiamine synthesis contributes to energy conservation by A. nidulans and constitutes an adaptive mechanism to low oxygen levels.
Another role of hypoxic thiamine upregulation can be considered. We reported that hypoxic A. nidulans cells incompletely reduce exogenous nitrate (as a nitrogen source) and accumulate nitrite, which deaminates and damages purine bases in nucleotides, and accumulate cellular deaminated DNA.Citation2) Under these circumstances, proteins for nucleotide synthesis are upregulated to activate cellular nucleotide turnover.Citation2) Such enzymes include transketolase, which contains a TPP cofactor and is a key enzyme for synthesizing C5 nucleotide precursors in the PPP. The hypoxic induction of ThiA might be important for supplying TPP to transketolase and hence for DNA damage tolerance. Further physiological significance can be supposed. Increased thiamine production and transketolase activity might lead to upregulated NADPH production by the PPP, which is a major source of cellular NADPH, a crucial substrate for glutathione and thioredoxin reductases and several dehydrogenases that detoxify damaged cellular components, and thus is critical for growth in the presence of these stressors.Citation26,28,29)
The present study found that the thiamine riboswitch on thiA pre-mRNA becomes derepressed in response to various environmental stresses. Thiamine riboswitch of N. crassa thi-4 are credited with playing a dual role in mitochondrial DNA damage tolerance, and being responsive to environmental stressors, respectively.Citation12,30) Taken together with the present findings, these results suggest that the stress response of the thiamine riboswitch is ubiquitous among fungi although further investigations are required to substantiate this notion.
We applied culture conditions that limited but did not absolutely exclude cellular thiamine, which was evident from the finding that ThiAΔ contained 30% of the amount of thiamine produced by the WT (Fig. (A)). This implies that a minimal level of endogenous thiamine substantially represses the thiamine riboswitch (Fig. (A)). Full derepression by stressors such as reactive oxygen species and heat shock might be a consequence of decreased cellular thiamine levels caused by the degrading thiamine or an unknown action of the stressors. However, this seems unlikely, at least in the hypoxic response because hypoxic cells accumulated more thiamine despite full derepression. The hypoxic derepression mechanism is considered to be independent of the amount of intracellular thiamine.
Industrial pyruvate and ethanol fermentation by fungi is important, and controlling cellular thiamine levels is prerequisite for their maximal production. Thiamine limitation minimizes the activities of TPP-containing PDC and pyruvate dehydrogenase and increases the fermentative production of pyruvate by the industrially important yeast Torulopsis glabrata as well as laboratory yeast.Citation31,32) Our finding of stress-dependent derepression of thiA expression indicates that several factors imposed by fungal fermentation processes affect thiamine production and hence fermented products. Further investigations are required to determine whether stress-dependent thiamine derepression is conserved among A. nidulans and industrial fungi.
Authors contribution
MS and NT planned the studies and prepared the manuscript. MS, SM, and MK designed and performed experiments. EI and SZ performed the experiments. All authors reviewed the results, contributed to the writing, and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental materials
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2016.1158631.
TBBB_1158631_Supplemental.pdf
Download PDF (1.1 MB)Acknowledgments
We thank Norma Foster for critical reading of the manuscript.
Additional information
Funding
References
- Masuo S, Terabayashi Y, Shimizu M, et al. Takaya N Global gene expression analysis of Aspergillus nidulans reveals metabolic shift and transcription suppression under hypoxia. Mol. Genet. Genomics. 2010;284:15–24.
- Shimizu M, Fujii T, Masuo S, et al. Proteomic analysis of Aspergillus nidulans cultured under hypoxic conditions. Proteomics. 2009;9:7–19.10.1002/pmic.v9:1
- Terabayashi Y, Shimizu M, Kitazume T, et al. Conserved and specific responses to hypoxia in Aspergillus oryzae and Aspergillus nidulans determined by comparative transcriptomics. Appl. Microbiol. Biotechnol. 2012;93:305–317.10.1007/s00253-011-3767-4
- Vödisch M, Scherlach K, Winkler R, et al. Analysis of the Aspergillus fumigatus proteome reveals metabolic changes and the activation of the pseurotin A biosynthesis gene cluster in response to hypoxia. J. Proteome Res. 2011;10:2508–2524.10.1021/pr1012812
- Takasaki K, Shoun H, Yamaguchi M, et al. Fungal ammonia fermentation-A novel metabolic mechanism that couples the dissimilatory and assimilatory pathways of both nitrate and ethanol. J. Biol. Chem. 2004;279:12414–12420.10.1074/jbc.M313761200
- Shimizu M, Fujii T, Masuo S, et al. Mechanism of de novo branched-chain amino acid synthesis as an alternative electron sink in hypoxic Aspergillus nidulans cells. Appl. Environ. Microbiol. 2010;76:1507–1515.10.1128/AEM.02135-09
- Friedrich W. Vitamins. Berlin: Walter de Gruyter; 1988.10.1515/9783110859188
- Butterworth RF. Thiamin deficiency and brain disorders. Nutr. Res. Rev. 2003;16:277–283.10.1079/NRR200367
- Jordan F. Current mechanistic understanding of thiamin diphosphate-dependent enzymatic reactions. Nat. Prod. Rep. 2003;20:184–201.10.1039/b111348h
- Settembre E, Begley TP, Ealick SE. Structural biology of enzymes of the thiamin biosynthesis pathway. Curr. Opin. Struct. Biol. 2003;13:739–747.10.1016/j.sbi.2003.10.006
- Begley TP, Downs DM, Ealick SE, et al. Thiamin biosynthesis in prokaryotes. Arch. Microbiol. 1999;171:293–300.10.1007/s002030050713
- Spenser ID, White RL. Biosynthesis of vitamin B1 (thiamin): an instance of biochemical diversity. Angew. Chem. Int. Ed. Engl. 1997;36:1032–1046.10.1002/(ISSN)1521-3773
- Godoi PHC, Galhardo RS, Luche DD, et al. Structure of the thiazole biosynthetic enzyme THI1 from Arabidopsis thaliana. J. Biol. Chem. 2006;281:30957–30966.10.1074/jbc.M604469200
- Chatterjee A, Jurgenson CT, Schroeder FC, et al. Thiamin biosynthesis in eukaryotes: characterization of the enzyme-bound product of thiazole synthase from Saccharomyces cerevisiae and its implications in thiazole biosynthesis. J. Am. Chem. Soc. 2006;128:7158–7159.10.1021/ja061413o
- Chatterjee A, Jurgenson CT, Schroeder FC, et al. Biosynthesis of thiamin thiazole in eukaryotes: Conversion of NAD to an advanced intermediate. J. Am. Chem. Soc. 2007;129:2914–2922.10.1021/ja067606t
- Chatterjee A, Abeydeera ND, Bale S, et al. Saccharomyces cerevisiae THI4p is a suicide thiamine thiazole synthase. Nature. 2011;478:542–546.10.1038/nature10503
- Nosaka K. Recent progress in understanding thiamin biosynthesis and its genetic regulation in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2006;72:30–40.10.1007/s00253-006-0464-9
- Li M, Petteys BJ, McClure JM, et al. Thiamine biosynthesis in Saccharomyces cerevisiae is regulated by the NAD+-dependent histone deacetylase Hst1. Mol. Cell Biol. 2010;30:3329–3341.10.1128/MCB.01590-09
- Miranda-Ríos J. The THI-box riboswitch, or how RNA binds thiamin pyrophosphate. Structure. 2007;15:259–265.10.1016/j.str.2007.02.001
- Cressina E, Chen L, Moulin M, et al. Identification of novel ligands for thiamine pyrophosphate (TPP) riboswitches. Biochem. Soc. Trans. 2011;39:652–657.10.1042/BST0390652
- Cheah MT, Wachter A, Sudarsan N, et al. Control of alternative RNA splicing and gene expression by eukaryotic riboswitches. Nature. 2007;447:497–500.10.1038/nature05769
- Kubodera T, Watanabe M, Yoshiuchi K, et al. Thiamine-regulated gene expression of Aspergillus oryzae thiA requires splicing of the intron containing a riboswitch-like domain in the 5′-UTR. FEBS Lett. 2003;555:516–520.10.1016/S0014-5793(03)01335-8
- Shimizu M, Masuo S, Fujita T, et al. Hydrolase controls cellular NAD, sirtuin, and secondary metabolites. Mol. Cell. Biol. 2012;32:3743–3755.10.1128/MCB.00032-12
- Sambrook J, Fritch EF, Maniatis T. In molecular cloning: a laboratory manual. vol. 2. Cold spring harbor, NY: Cold Spring harbor laboratory press; 1989.
- Shimizu M, Takaya N. Nudix hydrolase controls nucleotides and glycolytic mechanisms in hypoxic Aspergillus nidulans. Biosci. Biotechnol. Biochem. 2013;77:1888–1893.10.1271/bbb.130334
- Sato I, Shimizu M, Hoshino T, et al. The glutathione system of Aspergillus nidulans involves a fungus-specific glutathione S-transferase. J. Biol. Chem. 2009;284:8042–8053.10.1074/jbc.M807771200
- Romagnoli G, Luttik MA, Kotter P, et al. Substrate specificity of thiamine pyrophosphate-dependent 2-oxo-Acid decarboxylases in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2012;78:7538–7548.10.1128/AEM.01675-12
- de Vries RP, Flitter SJ, van de Vondervoort PJ, et al. Glycerol dehydrogenase, encoded by gldB is essential for osmotolerance in Aspergillus nidulans. Mol. Microbiol. 2003;49:131–141.10.1046/j.1365-2958.2003.03554.x
- Thon M, Al-Abdallah Q, Hortschansky P, et al. The thioredoxin system of the filamentous fungus Aspergillus nidulans: impact on development and oxidative stress response. J. Biol. Chem. 2007;282:27259–27269.10.1074/jbc.M704298200
- Machado CR, Praekelt UM, de Oliveira RC, et al. Dual role for the yeast THI4 gene in thiamine biosynthesis and DNA damage tolerance. J. Mol. Biol. 1997;273:114–121.10.1006/jmbi.1997.1302
- Hua Q, Yang C, Shimizu K. Metabolic flux analysis for efficient pyruvate fermentation using vitamin-auxotrophic yeast of Torulopsis glabrata. J. Biosci. Bioeng. 1999;87:206–213.10.1016/S1389-1723(99)89014-8
- Xu G, Hua Q, Duan N, et al. Regulation of thiamine synthesis in Saccharomyces cerevisiae for improved pyruvate production. Yeast. 2012;29:209–217.10.1002/yea.v29.6
