Abstract
Aspergillus species are among the most important filamentous fungi in terms of industrial use and because of their pathogenic or toxin-producing features. The genomes of several Aspergillus species have become publicly available in this decade, and genomic analyses have contributed to an integrated understanding of fungal biology. Stress responses and adaptation mechanisms have been intensively investigated using the accessible genome infrastructure. Mitogen-activated protein kinase (MAPK) cascades have been highlighted as being fundamentally important in fungal adaptation to a wide range of stress conditions. Reverse genetics analyses have uncovered the roles of MAPK pathways in osmotic stress, cell wall stress, development, secondary metabolite production, and conidia stress resistance. This review summarizes the current knowledge on the stress biology of Aspergillus species, illuminating what we have learned from the genomic data in this “post-genomic era.”
Graphical abstract
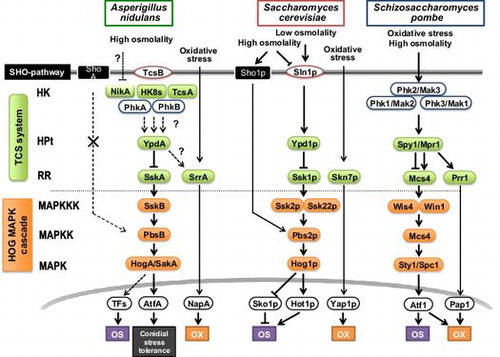
Comparison of signaling components in HOG pathway among Aspergillus nidulans, Saccharomyces cerevisiae, and Schizosaccharomyces pombe.
Filamentous fungi are ubiquitous in nature and are commonly found in and on decaying woods and plants, as well as in the soil. Their saprophytic characteristics are ecologically important as they play a crucial role in carbon and nitrogen recycling in nature. As well as being important components of the ecosystem, many species of filamentous fungi affect human life either beneficially or detrimentally. For instance, some fungi produce a variety of enzymes and organic acids that are used in food and pharmaceutical industries, and in producing fermented foods. A small but significant number of filamentous fungi cause diseases in animals and plants.
Growing on solid substrates seems to be the predominant mode during the lifecycle of filamentous fungi in nature. These fungi generally exist in a mycelial form in plant debris, compost piles, dead insects, and even immunocompromised humans and animals. In such habitats, the fungi must cope with several abiotic and biotic stressors including low or high temperature, dryness, low nutrient levels, oxygen limitation, ultraviolet (UV) radiation, and oxidative stress. Therefore, fungi have evolved mechanisms to sense and respond to the harsh conditions to survive in their environment. When grown on solid substrates, some fungi produce asexual or sexual spores that serve as stress-resistant genetic vehicles.Citation1) Spore production is triggered by environmental cues such as light, nutrient conditions, temperature, and oxygen levels.Citation2,3) Spore production is one of the most important ways in which filamentous fungi survive in their surroundings and prosper in their environmental niches.Citation4)
The genomes of many filamentous fungi have been sequenced and are now available publicly.Citation5–8) This has led to a better understanding of filamentous fungal biology and improvements in the use of these organisms in various industries. In particular, the wealth of genetic data has enriched our knowledge of fungal stress responses and adaptation mechanisms. In this review, we summarize the latest findings on the stress response and adaptation mechanisms of fungi during culturing. We focus on the genus Aspergillus because full genome sequences have been determined for several Aspergillus species. Much can be learned about stress biology from genomic analyses of multiple species in the same genus. Such information will lead to further advances in the general biology of fungi and their evolutionary relationships.
I. Signaling pathways related to stress response and adaptation
The genome sequences of A. oryzae, Aspergillus nidulans, and Aspergillus fumigatus were published in 2005.Citation5–7) One of the first findings was that almost all of the stress signaling components identified in yeasts are encoded in the genomes of filamentous fungi. Comparative genomics studies have shown that filamentous fungi have targets of rapamycin (TOR) signaling and protein kinase A (PKA) signaling pathways, mitogen-activated protein kinase cascades, and calcineurin signaling, as well as upstream signaling machinery (G-protein coupled receptors, G-protein subunits, and two-component signaling (TCS) system). All of these pathways and proteins have been well studied in Saccharomyces cerevisiae.Citation9‒13) Notably, some components of the signaling systems were found to be duplicated or diversified in filamentous fungi, reflecting their complex multicellular organization. The existence of duplicated/diversified signaling components suggested that filamentous fungi have evolved extensive sensing and signaling mechanisms to adapt to their surrounding environments. Based on the results of comparative studies, stress response mechanisms were further studied using a reverse genetic approach. The following section focuses on MAPK cascades and the upstream TCS system because their roles in the stress response have been clarified in several reverse genomics studies over the last decade.
I.i. Mitogen-activated protein kinase cascades
The MAPK cascade is a highly conserved signaling unit found in fungi, plants, and animals.Citation14,15) The prototypical MAPK cascade consists of three different modules: MAPK, MAPK kinase (MAPKK), and MAPK kinase kinase (MAPKKK). MAPKKK activation is followed by phosphorylation of MAPKK, which in turn phosphorylates MAPK.Citation9–11,15) Typically, the phosphorylated MAPK translocates from the cytosol into the nucleus, where it activates (in most case, phosphorylates) target proteins.Citation16) The serial activation transmits certain environmental signals to the nucleus and regulates transcription therein leading to appropriate cellular responses. There are five functionally distinct MAPKs in S. cerevisiae: (1) Fus3p (mating-pheromone response pathway), (2) Kss1p (pseudohyphal development pathway), (3) Hog1p (high osmolality glycerol (HOG) pathway), (4) Slt2/Mpk1p (protein kinase C or cell wall integrity pathway), and (5) Smk1p (spore wall assembly pathway).Citation10,11,17) Four of these MAPKs (all except Smk1p) are controlled by MAPKKs (Ste7p, Pbs2p, and redundant Mkk1p and Mkk2p), and MAPKKKs (Ste11p, Bck1p, and redundant Ssk2p and Ssk22p) (Fig. ).
Fig. 1. Schematic diagrams of MAPK signaling pathways in Saccharomyces cerevisiae.
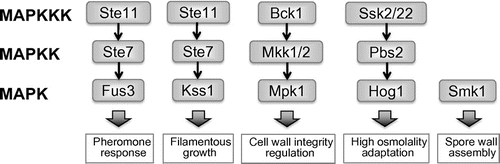
Homology searches revealed that there are three types of MAPK proteins and three MAPKK and MAPKKK proteins encoded in the genomes of filamentous fungi, including A. nidulans, A. oryzae, A. fumigatus, and Aspergillus niger (Table ). This finding suggested that while the Kss1 (pseudohyphal development) pathway and Smk1 (spore wall assembly) pathway are absent, three MAPK cascades function in the stress responses of filamentous fungi: (1) the Fus3 (pheromone) pathway, (2) the Hog1 (HOG) pathway, and (3) the Mpk1 (cell wall integrity) pathway (Fig. ). One component of the HOG pathway, a MAPK protein, is duplicated in A. nidulans and A. fumigatus, (each has HogA/SakA and MpkC) and triplicated in A. oryzae and A. niger (each has HogA/SakA, MpkC, and MpkD). This suggests that the signal transduction cascade of A. oryzae and A. niger might be more complex than that of A. nidulans and A. fumigatus.
Table 1. Proteins of Aspergillus MAPK cascades.
Fig. 2. Components of three MAPK cascades and associated mechanisms in Aspergillus nidulans.
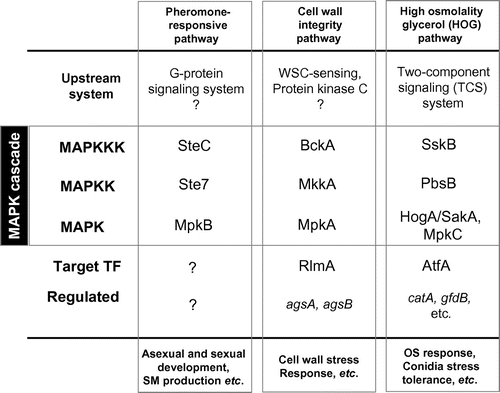
The physiological roles of the three MAPK cascades in Aspergillus species have been investigated. The MpkB MAPK in the pheromone-responsive pathway was reported to play a role in hyphal growth, conidiation, conidia viability, sexual development, and autolysis in A. nidulans.Citation18,19) MpkB was also required for the control of secondary metabolite production and growth upon micafungin treatment.Citation20,21) The upstream component SteC MAPKKK, a homolog of S. cerevisiae Ste11, has been characterized.Citation22) Compared with the wild type, the deletion mutant of steC showed a slower growth rate, formation of more branched hyphae, altered conidiophore morphology, and inhibited heterokaryon formation and cleistothecium development. Recently, Bayram et al.Citation23) demonstrated that the SteC–Ste7–MpkB complex with an adaptor protein Ste50 mediates development and secondary metabolism in A. nidulans. Together, these findings indicated that the pheromone-responsive pathway in Aspergillus is responsible for multiple biological processes.
The second MAPK cascade is the cell wall integrity pathway. This pathway is activated in response to several environmental stimuli in S. cerevisiae, resulting in increased expressions of genes encoding integral cell wall proteins and enzymes involved in cell wall biogenesis.Citation24) Based on the results of studies on this pathway in S. cerevisiae, the corresponding Aspergillus MpkA MAPK cascade was characterized by several groups. The A. nidulans mpkA deletion mutant showed significant defects in conidia germination and in polarized growth, and markedly delayed colony growth.Citation25) The mutant was sensitive to two cell wall perturbing reagents; micafungin and calcofluor white (CFW).Citation26) These phenotypes suggested that MpkA is involved in maintaining the cell wall and promoting cell wall remodeling.
Notably, the genes that are regulated in the cell wall integrity pathway differ between A. nidulans and S. cerevisiae. Whereas the Mpk1p cascade controls the expressions of FKS1 (encoding β-1,3-glucan synthase) and CHS3 (encoding chitin synthase) in S. cerevisiae, the expressions of the corresponding genes in A. nidulans (fksA encoding β-1,3-glucan synthase and a gene-encoding chitin synthase) are independent of MpkA.Citation24,26) Another intriguing finding was that MpkA regulated the expressions of two α-1,3-glucan synthase genes, agsA and agsB.Citation26) This may be because α-1,3-glucan is one of the major cell wall polysaccharides in Aspergillus, but not in S. cerevisiae. The evolutionarily different functional roles of the cell wall integrity pathways are key factors in understanding the physiology of filamentous fungi.
The third MAPK cascade is the HOG pathway. This pathway plays a pivotal role in the osmotic stress response in S. cerevisiae.Citation9) The S. cerevisiae HOG pathway consists of Ssk2p/Ssk22p MAPKKKs, Pbs2p MAPKK, and Hog1p MAPK, and is regulated by two different upstream branches; the TCS system and the Sho1p pathway (Fig. ). Signaling of the Sho1p branch depends on an interaction with Pbs2p MAPKK to regulate Hog1p MAPK. Hence, the two upstream routes converge at Pbs2p MAPKK. In contrast, the A. nidulans PbsB protein, an ortholog of Pbs2p, lacks the Pro-rich motif that is required for binding to the Src-homology3 (SH3) domain of Sho1p. This suggests that PbsB does not interact with the ShoA branch (which corresponds to the Sho1p branch). In fact, PbsB was unable to transduce the signal from the Sho1p branch in a complementation experiment in S. cerevisiae, but it transduced the signal from the TCS branch.Citation27) Taken together, these results showed that the connection between the Sho1p branch and Pbs2p is not conserved in A. nidulans.
Fig. 3. Comparison of signaling components in HOG pathway among Aspergillus nidulans, Saccharomyces cerevisiae, and Schizosaccharomyces pombe.
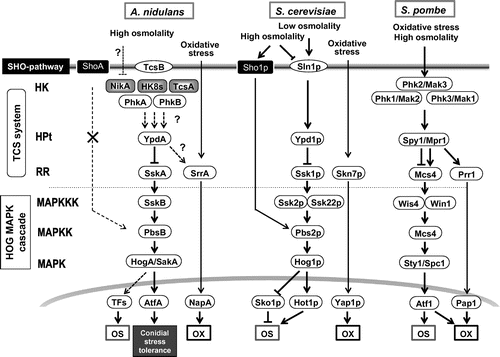
The physiological functions of the HOG pathway have been intensively analyzed by a genetic deletion approach in wide variety of filamentous fungi.Citation28‒30) A. nidulans possesses SskB MAPKKK, PbsB MAPKK, and HogA/SakA MAPK in the HOG pathway (Fig. and ). The sskB-, pbsB-, and hogA-deletion mutants showed growth inhibition under high osmolality. In response to osmotic shock, the HogA MAPK was phosphorylated in an SskB- and PbsB-dependent manner.Citation27) The hogA mutant also showed a reduction in conidia viability.Citation31) Intriguingly, HogA was shown to be highly phosphorylated in conidia, even after prolonged incubation (up to 7 days) in water at 4 °C.Citation32) These results suggested that the HOG pathway is responsible for conidial stress tolerance in A. nidulans.
The A. fumigatus deletion mutant of SakA (HogA) also showed growth inhibition under osmotic stress. SakA was shown to be phosphorylated (activated) in response to osmotic shock and certain types of fungicide in a TCS system-dependent manner.Citation33) A. fumigatus SakA regulates germination in response to nitrogen availability, and was shown to be phosphorylated upon nitrogen or carbon starvation during vegetative growth.Citation34) Recently, sskB expression in A. fumigatus was shown to be regulated directly by the calcium-responsive transcription factor (TF) CrzA.Citation35) The deletion mutant of sskB was sensitive to excess calcium, suggesting that the calcium signaling pathway is involved in regulating the HOG pathway. Interestingly, the sskB deletion mutant showed attenuated virulence in a mouse infection model, supporting the view that the HOG pathway plays a role in the pathogenicity of A. fumigatus.
Besides HogA/SakA, an additional MAPK, MpkC, is present in the Aspergillus HOG pathway. The deletion mutant of MpkC in A. fumigatus showed growth defects on minimal medium with sorbitol or mannitol as the sole carbon source.Citation36) Recently, Hagiwara et al.Citation37) reported that the sakA deletion mutant produced conidia with normal stress resistance (unlike the stress-sensitive conidia produced by the A. nidulans hogA mutant) and that the mpkCsakA double deletion mutant produced stress-labile conidia with reduced trehalose content. Collectively, these results showed that the HOG pathway of Aspergillus species functions in various physiological processes to adapt to the surrounding environment.
I.ii. TCS system
The TCS (also known as His-Asp phosphorelay signaling) system, which was first described in bacteria, is a common signal transduction mechanism that exists in organisms ranging from bacteria to fungi and plants, but not in animals.Citation38–41) The prototypical system in bacteria consists of two types of common signal transducers (hence, “two-component”); a histidine kinase (HK) and a response regulator (RR). Eukaryotes have an additional component, a histidine-containing phosphor-transmitter (HPt); hence, their signaling system has three components (HK-HPt-RR). Each component has a conserved motif with an invariant amino acid residue, His or Asp, and phosphor-groups are transferred (relayed) between the components at these amino acid residues (His to Asp). The eukaryotic HK has both a kinase domain (with a conserved His) and a receiver domain (with a conserved Asp), and is known as a hybrid-HK. The kinase domain is autophosphorylated from ATP, most likely via conformational changes in response to certain stimuli. In turn, the phosphor-group is transferred to the Asp residue in the receiver domain. The HPt contains a conserved His and the RR has a receiver domain with a conserved Asp. Hence, the fungal TCS system consists of a multi-step phosphorelay (His-Asp-His-Asp) among the three components.Citation40,41)
The TCS systems in S. cerevisiae and Schizosaccharomyces pombe have been well characterized. S. cerevisiae has one HK (Sln1p), three RRs (Ssk1p, Skn7p, and Rim15p), and one HPt (Ypd1p). S. pombe has three HKs (Phk1–Phk3), three RRs (Mcs4, Prr1, and Cek1), and one HPt (Mpr1) (Fig. ).Citation9) In yeasts, these systems are involved in osmotic and oxidative stress responses and in regulating the mitotic cell cycle and sexual development.Citation42–44) Genomic analyses of A. nidulans, A. oryzae, A. niger, and A. fumigatus revealed that they contain 15, 15, 11, and 13 HK genes, respectively (Table ). Thus, HK genes are more abundant in filamentous fungi genomes than in yeast genomes,Citation45) suggesting that signaling networks might be more complex in filamentous fungi than in yeasts.
Table 2. Two-component signaling (TCS) system proteins.
As stated above, the TCS system regulates the HOG MAPK cascade in yeast. A comparative genomics analysis suggested that one of the Aspergillus RRs, SskA (corresponding to S. cerevisiae Ssk1p), likely activates SskB MAPKKK by direct interaction, and consequently activates the HOG pathway.Citation46) Genetic analyses of A. nidulans and A. fumigatus revealed that SskA is indispensable for phosphorylation of the HogA/SakA MAPK. The deletion of SskA resulted in retarded growth under high osmolality conditions.Citation27,33)
The other characterized RR is an Skn7-type RR (SrrA in A. nidulans and Skn7 in A. fumigatus) that is not involved in regulating the HOG pathway in Aspergillus species.Citation47) The Skn7-type RRs were shown to be required for the oxidative stress response, as is the case in S. cerevisiae and S. pombe.Citation46–49) According to the findings of studies on yeasts,Citation50,51) the Skn7-type RR of filamentous fungi may function in association with an AP-1-type TF, a highly conserved regulator of the oxidative stress response. The deletion mutant of A. fumigatus skn7 showed remarkable sensitivity to oxidative stress in vitro; however, virulence of the mutant was comparable to that of the parental strain in a mouse infection model.Citation52) This study suggested that the oxidative stress response is not necessarily related to the pathogenicity of A. fumigatus. In future studies, nonetheless, it will be important to elucidate whether upstream components such as HKs and HPt are involved in the oxidative stress response and in the activation of the Skn7-type RR in filamentous fungi.
Other types of RR have been found and characterized in A. nidulans (SskB and SskC) and A. oryzae (AoRim15) (Table ). The A. nidulans mutants of SskB and SskC showed no distinguishable phenotypes.Citation46) In A. oryzae, AoRim15 was shown to be involved in the resistance to heat and oxidative stresses in conidia.Citation53) The Aorim15 mutant showed reduced conidiation and sclerotia formation, suggesting that AoRim15 plays a role in the development of A. oryzae. Although AoRim15 is regarded as an RR because it has a receiver domain similar to that of Ssk1-type or Skn7-type RRs, the Asp residue that is required to accept the phosphor-group is replaced with Glu in the receiver domain of AoRim15. Instead, it contains PAS and protein kinase domains. These bioinformatics data raise the possibility that AoRim15 does not function in a TCS system.
The genomes of Aspergillus species encode 11–15 HKs, which have been investigated to some extent.Citation45) First, the HKs of four aspergilli were classified into nine families (HK1–HK9) based on amino acid sequence similarity (Table ). The HK1 family is orthologous to S. cerevisiae Sln1p, and the HK4 and HK5 families are orthologous to S. pombe Phk1/Phk2 and Phk3, respectively. This comparative genomic evidence suggests that the TCS system of filamentous fungi has evolved from that of yeasts in a somewhat reciprocal fashion. Homology searches revealed that HK6 is the prevalent HK among filamentous fungi, and it is also found in basidiomycetes.Citation41,54) Together, these findings suggested that TCS systems may have evolved both differentially and conservatively in each species to adapt to their environmental niche.
The HKs in the HK1 family are thought to play a role in osmotic stress responses because of their similarity to the S. cerevisiae Sln1p. Thus, the A. nidulans HK1 protein TcsB has been investigated in detail. Heterologous expression of the A. nidulans tcsB gene complemented the lethality of a S. cerevisiae strain defective in sln1.Citation55) Contrary to expectations, however, the tcsB deletion mutant did not show any detectable phenotypic defects on standard and osmotic stress media, and TcsB was not required for the phosphorylation of the HogA MAPK in response to osmotic and oxidative stresses.Citation27,55) In a recent report on A. fumigatus, TcsB was shown to be involved in phosphorylation of the SakA MAPK in response to a cold shock, and was required for growth under high-temperature conditions.Citation56) Although TcsB plays a certain role in the stress responses of filamentous fungi, another HK may contribute to the response and adaptation to osmotic changes.
The HK3 family contains A. nidulans TcsA and A. fumigatus Fos1. The tcsA mutant did not produce conidia on standard medium, but the defect in conidiation was suppressed by addition of 1 M sorbitol to the medium.Citation57) TcsA is required for the nuclear localization of the basic helix–loop–helix TF, DevR, which is involved in conidia formation under standard growth conditions.Citation58) Conversely, the fos1 deletion mutant did not show detectable defects in either hyphal growth or morphology on solid medium.Citation33) However, the expression of fos1 was increased in a SakA-dependent manner in response to osmotic stress, suggesting that Fos1 may be involved in regulating the HOG pathway in A. fumigatus. This is an interesting example of how HKs in the same family can show different roles in different Aspergillus species.
The HK4 and HK5 families are orthologous to the HKs of S. pombe that are involved in the oxidative stress response, mitotic cell cycle control, and sexual development.Citation42,59) The transcriptions of A. fumigatus phkA and phkB (corresponding to HK4 and HK5 proteins, respectively) increased in response to osmotic shock.Citation33) However, the growth of the deletion mutants of phkA or phkB genes under high osmolarity stress conditions was comparable to that of the wild-type (WT) A. fumigatus strain.Citation33) In both A. fumigatus and A. nidulans,Citation60) the phkA and phkB deletion mutants did not show reduced growth under oxidative stress conditions, compared with that of the parental strains. The physiological roles of the HKs in Aspergillus species remain unknown.
The HK in the HK6 family has a characteristic motif, a repeated HAMP domain, at its N-terminus. Although the function of the HAMP domain is elusive, some reports have suggested that this domain plays a role in the perception of osmotic conditions.Citation61,62) Indeed, both in A. nidulans and A. fumigatus, disruption of nikA encoding a HK6 family protein resulted in a retarded growth on high osmolality medium.Citation33,63) This result led to the hypothesis that instead of HK1, HK6 might regulate the HOG pathway in the response and adaptation to osmotic conditions. This idea was partly supported by experiments in which NikA orthologous genes of other fungi (i.e. Magnaporthe grisea and Alternaria brassicicola) were heterologously expressed in S. cerevisiae.Citation64,65) However, phosphorylation of the SakA MAPK in response to osmotic stress depended on SskA RR in A. fumigatus, and was independent of NikA. This result suggested that other HKs may compensate for the lack of NikA in the regulation of the HOG pathway in A. fumigatus.Citation33)
Besides its role in the osmotic stress response, the HK in the HK6 family has been implicated in the response to phenylpyrrole and dicarboximide classes of fungicides.Citation41) Two representatives of these fungicides, fludioxonil and iprodione, are widely used to protect crops from range of plant pathogenic fungi. Deletion of the gene encoding the HK in the HK6 family resulted in resistance to these fungicides in both A. nidulansCitation66) and A. fumigatus,Citation33) as well as in some plant pathogenic fungi.Citation67‒69) Upon treatment with these fungicides, the HOG pathway is activated in an SskA-dependent manner in A. fumigatus and A. nidulans.Citation33,66) In the A. fumigatus nikA deletion mutant, the fludioxonil (0.1–1 μg/mL)-induced SakA MAPK phosphorylation level was lower than that in the wild type.Citation33) However, the level of SakA MAPK phosphorylation was similar in the nikA mutant and wild type when treated with a relatively high concentration (10 μg/mL) of fludioxonil. These data suggested that an excessive dose of fludioxonil may affect the HOG pathway through HKs other than NikA. Considering all of the evidence for the roles of NikA in the responses to fungicides and osmotic stress, it appears that NikA plays a major role in regulating the HOG pathway in Aspergillus species, although there remain many open questions.
The HK7 family is composed of phytochrome proteins that play a major role in red light perception. The A. nidulans phytochrome FphA has red light-dependent autophosphorylation activity, and functions in the balance between asexual and sexual development and in mycotoxin production in response to red light.Citation70,71) FphA forms a complex consisting of the developmental regulator VeA and white collar-like, blue light-signaling proteins LreA (WC-1) and LreB (WC-2).Citation72) Thus, FphA plays a central role in photosensory systems.
In the TCS system circuitry of yeasts, HPt is an intermediate in signaling between HK and RR, where all HKs (Sln1p in S. cerevisiae and Phk1, Phk2, and Phk3 in S. pombe) are thought to function upstream of HPt (Fig. ). In contrast, Aspergillus species have 11–15 HKs and only a single HPt, which raises the question as to whether all of the HKs interact with HPt to transmit their signals. One possible explanation for the high multiplicity of the upstream HK proteins in Aspergillus species is that HK functions may be specialized in each developmental stage or in response to certain environmental stimuli. This was partly proven by comprehensive transcriptional analyses of an array of HKs expressed at various developmental stages and during stress responses in both A. nidulans and A. fumgiatus. For instance, A. nidulans nikA was upregulated during the asexual stage and preferentially expressed in conidial heads and stalksCitation60,73); hysA was upregulated in response to osmotic shock and fludioxonil treatment and was expressed in conidiaCitation73,74); A. fumigatus fos-1, phkA, phkB, and fhk6 were upregulated in response to osmotic shockCitation33); A. fumigatus fhk1 was upregulated during the asexual stageCitation37); and A. fumigatus fhk5 was upregulated under oxygen-limited conditions.Citation75)
The HPt is found in all fungal species, which highlights its importance in the TCS system. Indeed, S. cerevisiae has a single HPt, Ypd1p, whose gene YPD1 is an essential gene; mutants lacking this gene showed hyper-phosphorylation of the Hog1p MAPK, which was lethal to cells.Citation76) In contrast, the S. pombe HPt, Mpr1/Spy1, is not essential for their growth but is involved in transmitting signals for mitotic cell cycle regulation and oxidative stress responses.Citation77,78) The Candida albicans HPt, Ypd1, is also dispensable for viability, but its deletion led to constitutive filamentous growth under conditions that favor the yeast growth form.Citation79) Cells of the ypd1 mutant showed increased levels of phosphorylated Hog1 MAPK. Some HPts in filamentous fungi have been investigated. Vargas-Pérez et al.Citation47) reported that A. nidulans mutants lacking the HPt protein YpdA were obtained only as heterokaryons, and thus the homokaryon lacking ypdA was likely non-viable. This result indicated that the HPt protein is essential in A. nidulans. Likewise, genetic analyses confirmed that the HPt gene in N. crassa, hpt-1, is essential for viability.Citation80) In A. fumigatus, repression of ypdA expression resulted in impaired growth, suggesting that it plays an essential role in viability (unpublished data, Hagiwara et al.). In contrast, the HPt gene in the rice blast pathogenic fungus Magnaporthe oryzae, Moypd1, was shown to be dispensable for viability.Citation81) These recent data show that the importance of HPt in viability varies among different yeasts and filamentous fungi.
I.iii. TF downstream of MAPK
Studies on yeast MAPK cascades have also revealed that several TFs downstream of the MAPKs play an important role in cellular responses through transcriptional changes. In this decade, the TFs of filamentous fungi have been intensively investigated in several species. Comparisons of studies of TFs between yeasts and filamentous fungi have provided insights into evolutionarily differentiated functions of the MAPK cascade. This section summarizes the accumulated knowledge on TFs, especially those involved in the three MAPK cascades that exist in Aspergillus fungi, gained from comparative genomics analyses.
S. cerevisiae Ste12p is a target of Fus3p MAPK in the mating–pheromone response pathway, and of Kss1p MAPK in the pseudohyphal development pathway.Citation82) The Ste12-like protein SteA in A. nidulans lacks the Kss1p-interacting domain present in Ste12p, and instead has C-terminal C2/H2 Zn2+ finger domains that are not present in Ste12p.Citation83) The A. nidulans deletion mutant of steA showed normal conidiation but did not produce cleistothecia (fruiting bodies), suggesting that SteA is an important regulator of sexual development. In contrast, the A. oryzae steA deletion mutant showed no detectable phenotypes.Citation84) However, overexpression of steA resulted in defective hyphal growth and conidiation, and over-secretion of cell wall degrading enzymes. Hence, SteA may function as a regulator of cell wall metabolism in A. oryzae. It should be noted that any physical interaction of SteA with the pheromone-responsive pathway MAPK, MpkB, is yet to be determined in the aspergilla (Fig. ). Thus, the output mechanism for the MpkB MAPK cascade remains unknown.
The S. cerevisiae HOG pathway has several TFs including Hot1p and Sko1p which interact with Hog1p MAPK.Citation85‒88) Sko1p is a bZIP-type TF in the ATF/CREB family that represses the expressions of a set of osmotic stress-responsive genes under low-osmolarity conditions. In response to high osmotic stress, Hog1p phosphorylates Sko1p, causing it to switch forms. This results in the derepression and activation of osmotic stress-responsive genes. Hot1p, a positive regulator that interacts with Hog1p, is also involved in modulating the expressions of osmotic stress-responsive genes (Fig. ). In S. pombe, phosphorylated Spc1 MAPK in the HOG pathway translocates from the cytoplasm to the nucleus to phosphorylate and activate a bZIP TF, Atf1 (Fig. ).Citation89,90) Based on the results of these studies, researchers searched for candidate TFs in the HogA MAPK cascade in Aspergillus genomes. A comparative transcriptome analysis revealed that AtfA, an ortholog of S. cerevisiae Sko1p and S. pombe Atf1, functions in the A. nidulans HOG pathway, where most of osmotic stress-responsive gene expressions were shown to be dependent on AtfA.Citation74) Furthermore, Lara-Rojas et al.Citation32) showed that HogA MAPK interacts with the AtfA protein upon oxidative and osmotic stresses, as well as in conidia. However, the atfA mutant did not show distinguishable growth defects under strong osmotic and oxidative stress conditions.Citation74,91) This suggests that AtfA has a minor role in adaptation to osmotic and oxidative stresses, and that unidentified TFs may function in the HOG pathway (Fig. ). Importantly, AtfA was shown to play as crucial a role as that of HogA in conidia viability, suggesting that HogA–AtfA is involved in conidia stress tolerance (Fig. ).Citation91) Involvement of AtfA in the HOG pathway and in conidia stress tolerance was also found in A. fumigatus.Citation37)
In the cell wall integrity pathway, S. cerevisiae Mpk1p phosphorylates and activates Rlm1p TF, which regulates at least 25 genes involved in cell wall biogenesis.Citation24) The filamentous fungi orthologs of these TFs were investigated in A. nidulans and A. niger. Disruption of rlmA in A. nidulans resulted in sensitivity to the cell wall perturbing reagent CFW, suggesting that RlmA plays a role in the cell wall stress response.Citation26) Regulation of the expression of α-1,3-glucan synthase genes, agsA and agsB, was shown to be dependent on both RlmA and MpkA MAPK. Similarly, the A. niger rlmA deletion mutant was sensitive to CFW, and RlmA was required for upregulation of the expressions of agsA and gfaA (encoding glutamine:fructose-6-phophate amidotransferase).Citation92) Taken together, these results showed that RlmA functions downstream of the MpkA MAPK cascade and is responsible for adaptation to cell wall stress conditions in the aspergilli.
II. Stress biology of hyphae
The most obvious morphological features of filamentous fungi are their hyphal form and multicellularity. The hyphae are highly polarized and branched to broaden their habitat and to obtain sufficient nutrients for survival. In general, the multicellular hyphal form is the predominant form of Aspergillus fungi when grown in natural environments including soil and compost. Hence, Aspergillus fungi would have evolved sophisticated stress adaptation mechanisms to allow hyphae to survive under various environmental stress conditions encountered in their surroundings. This section summarizes recent discoveries on the response and adaptation mechanisms of the hyphal form of Aspergillus fungi against abiotic stresses.
II.i. Osmotic stress response
Some fungi synthesize glycerol as the main osmolyte to adapt to high-osmolality conditions.Citation93‒95) In S. cerevisiae, glycerol production begins with the conversion of dihydroxyacetone phosphate (DHAP) through a two-step reaction catalyzed by glycerol-3-phosphate dehydrogenase (Gpd1p, Gpd2p) and glycerol-3-phosphatase (Gpp1p, Gpp2p).Citation9) As described above, hyperosmotic stress activates the S. cerevisiae HOG pathway. This in turn increases the transcriptions of GPD1 and GPP2 and facilitates glycerol production. In A. nidulans, the homologs for glycerol biosynthesis-related genes are GfdA, GfdB, and GppA.Citation96) The gfdA mutant showed decreased glycerol accumulation during growth under osmotic stress conditions, but it did not show greater sensitivity to high osmolality stress.Citation97) The expression of another A. nidulans GPD homolog, GfdB, was shown to increase in response to osmotic shock in a HogA-MAPK-cascade-dependent manner, but its functional role remains undetermined.Citation98) In A. fumigatus, glycerol was accumulated under a NaCl-derived osmotic stress treatment in a SakA MAPK- and PbsB MAPKK-dependent manner.Citation37) The mutants lacking sakA or pbsB showed stronger growth inhibition under high-NaCl conditions, suggesting that accumulated glycerol in A. fumigatus hyphae plays a role in stress adaptation. For other Aspergillus species, polyols such as glycerol, erythritol, arabitol, or mannitol were found in hyphae as compatible solutes under osmotic stress conditions.Citation99‒103) In such conditions, whereas A. niger mainly produces glycerol, Aspergillus repens and Aspergillus flavus produce a mixture of polyols. The main osmolyte accumulated in hyphae seems to vary among different Aspergilli species.
II.ii. Oxidative stress response
Aerobic organisms must maintain a reduced cellular environment. Reactive oxygen species are spontaneously generated from oxygen metabolism in the cells. Hence, living organisms constantly sense and adapt to changes in the redox balance. One of the central fungal regulators of antioxidant responses is AP-1 TF. As mentioned above, in S. cerevisiae and S. pombe, the Skn7-type RRs (Skn7p and Prr1, respectively) interact with AP-1 TFs (Yap1p and Pap1, respectively). This mediates the expressions of genes encoding several antioxidants and antioxidant enzymes, including cytosolic catalase (Ctt1p and Ctt1), superoxide dismutase (Sod1p and Sod1), thioredoxin (Trx2p and Trx1), thioredoxin reductase (Trr1p and Trr1), and thioredoxin peroxidase (Tsa1p and Tpx1).Citation104‒106) The AP-1 family TF and Skn7-type RR have been well characterized in A. nidulans and A. fumigatus. All of the proteins are required for growth under oxidative stress conditions.Citation46, 52,107,108) Notably, their mutants showed oxidative stress sensitivity at the hyphal stage but not in conidia, suggesting that another mechanism against oxidative stress exists in conidia.
II.iii. Response to hypoxic conditions
Molecular oxygen plays an essential role in respiratory ATP synthesis and is also critical for the biosynthesis of sterols and hemes. Studies on S. cerevisiae showed that cells sense oxygen availability at least partly through cellular heme and sterol levels in the hypoxic response.Citation109,110) Filamentous fungi require oxygen to proliferate, but the mechanisms of hypoxia adaptation remain largely unstudied. Some genome-wide analyses of Aspergillus species have revealed that glycolysis is upregulated under hypoxic conditions.Citation111,112) This upregulation of glycolysis may contribute to the generation of ATP via substrate-level phosphorylation.
Although the details of the signaling mechanism remain unclear, recent studies on A. fumigatus have provided evidence that a sterol response element binding protein (SREBP), SrbA, is a key regulator for adaptation to hypoxic conditions.Citation113,114) SrbA is a TF containing a bHLH motif and plays a role in ergosterol biosynthesis by regulating various metabolic genes. A strain lacking SrbA showed impaired growth under hypoxic conditions. As is the case in S. cerevisiae, ergosterol biosynthesis is likely associated with the oxygen level in A. fumigatus. SrbA proteins are widely conserved among filamentous fungi. In fact, SrbA is required for growth under hypoxic conditions in A. oryzae (unpublished data, Gomi et al).
Recent studies have demonstrated that adaptation to hypoxic conditions is intimately associated with the pathogenicity of two human pathogenic fungi, A. fumigatus and Cryptococcus neoformans.Citation115) The SREBP proteins (SrbA of A. fumigatus and Sre1 of C. neoformans) are required for virulence and growth under hypoxic conditions.Citation113,116) In the mice infection model for invasive mycoses, fungal cells encounter hypoxic microenvironments at the sites where they penetrate the epithelial cell layers of the lung and invade the lung tissues. Thus, adaptation to oxygen-limited conditions is a requirement for the survival of fungal pathogens in vivo. Several groups have conducted genome-wide transcriptional analyses of A. fumgiatus during the hypoxia response.Citation75,117) Together, the results of those studies showed that a wide array of cellular mechanisms (i.e. cell wall metabolism, ergosterol biosynthesis, amino acid transport, nitrogen metabolism, and glycolysis) are affected by the transition from normoxic to hypoxic conditions. Notably, the expression of fhk5 encoding an HK8 family HK strongly increased under hypoxic conditions.Citation75) The fhk5 null mutant showed significant altered morphology only when ethanol was the sole carbon source in hypoxic culture, suggesting that Fhk5 plays a specific role in ethanol utilization under hypoxic conditions.
II.iv. Response to elevated temperature
Temperature is one of the most important factors that fungi must cope with in the natural environment. For instance, the temperature increases to more than 50 °C in compost piles due to fermentation heat. Although Aspergillus species are generally considered to be mesophiles, A. fumigatus is characterized by its moderate thermotolerance and is able to grow at 50 °C.Citation118) Because human pathogenic fungi must adapt to the elevated body temperature during infection, A. fumigatus may have evolved to grow at higher temperatures.
The heat-shock response is mediated by a highly conserved molecular chaperone known as the heat shock protein (HSP). This protein has been intensively investigated in S. cerevisiae.Citation119) The HSP family consists of five evolutionarily conserved groups, i.e. HSP100, HSP90, HSP70, HSP60, and a class of small HSPs.Citation120) In Aspergillus, some HSPs are known to be involved in responses to various stresses such as pH, antifungal drugs, osmotic, and oxidative stresses, as well as temperature shift.Citation121‒123) However, the detailed functions of each HSP in Aspergillus fungi in response to high temperature remain obscure because the adaptation to temperature seems to be multifactorial. Another key molecule in high-temperature adaptation is trehalose, which is a potential stress protectant. Trehalose accumulates in response to heat shock in A. nidulans and A. fumigatus. Like in S. cerevisiae, trehalose-6-phosphate synthase (TpsA) was shown to be essential for trehalose accumulation under high-temperature conditions, and trehalose was shown to contribute to high-temperature resistance in Aspergillus species.Citation124,125) Thus, a strategy to produce a thermo-tolerant Aspergillus strain is to develop a trehalose-overproducing line. In one case, an improved thermo-tolerant S. cerevisiae strain was obtained by overexpressing the trehalose-6-phosphate synthase gene.Citation126)
To further explore the heat shock response, transcriptomic and proteomic analyses were conducted in A. fumigatus.Citation127,128) Albrecht et al. demonstrated that 54 proteins were upregulated following a shift from 30 °C to 48 °C, 12 of which were assigned as chaperones (HSP60, HSP70, and HSP90, etc.).Citation128) Additionally, enzymes involved in the oxidative and nitrosative stress response (AFUA_4G09110: cytochrome c peroxidase Ccp1; AFUA_4G03410: flavohemoprotein; AFUA_5G09910: nitroreductase family protein) were upregulated in the temperature shift. It is notable that many proteins involved in carbohydrate metabolism (glycolysis and pentose phosphate pathways) were found to be upregulated by heat shock. This suggested that de novo energy production and balancing the redox state are necessary in the heat shock response of A. fumgiatus. A recent transcriptome study was conducted on Aspergillus kawachii, in which the culture temperature was decreased from 40 to 30 °C to mimic the industrial culture conditions for fermentation of the Japanese distilled spirit, shochu.Citation129) Upon lowering of the temperature, the expression levels of genes involved in the glycerol, trehalose, and pentose phosphate pathways, as well as HSP genes, were downregulated. This trend is opposite to that of A. fumigatus under heat shock treatment described above.
III. Stress biology of conidia
Formation of conidia is thought to be one strategy for sessile fungi to adapt to the limited resources at a given place. Conidia are, in general, stress-tolerant propagules, and Aspergillus fungi vigorously produce air-borne conidia under appropriate conditions. The conidia of A. fumigatus are etiological agents for aspergillosis, in which inhaled conidia colonize and germinate in the lungs of immunocompromised patients. Contamination of harvested crops and food products by conidia of some mycotoxigenic species such as A. flavus and Aspergillus parasiticus has led to enormous economic losses around the world. Therefore, the stress biology of conidia is a topic that should attract great attention in the next decade. In this section, we focus on the molecular mechanisms of conidial stress tolerance (summarized in Fig. ).
Fig. 4. Summary of stress biology of hyphae and conidia in Aspergillus fungi.
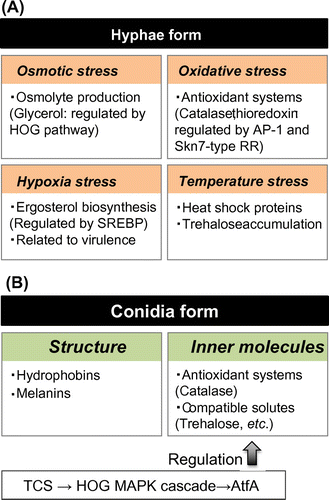
III.i. Conidia structure for stress resistance
The conidia of Aspergillus species have thick cell walls composed of α-glucan, β-glucan, and chitin. The conidia cell walls are further covered with hydrophobin on the outermost layer. The rodlet layer of hydrophobins confers hydrophobicity to the conidia, and masks the cell wall components to prevent immune cells from immediately detecting the inhaled conidia inside lungs.Citation130,131) A recent study on the hydrophobins of A. nidulans showed that six hydrophobins (RodA, and DewA–E) are involved in the hydrophobicity of the conidia and that RodA plays a major role in rodlet layer formation. Both dewD and dewE are expressed in vegetative hyphae, but the other four hydrophobin genes are not. Instead, they are strongly induced during the asexual stage.
Melanin, which is incorporated into the cell walls, gives the conidia of filamentous fungi better rigidity and resistance to certain environmental stresses.Citation132) For example, in the conidia of A. niger, dihydroxynaphthalene (DHN)-melanin confers strength against heat and UV stresses.Citation133,134) The conidia of A. fumigatus also contain DHN-melanin, which is thought to provide protection from environmental stresses such as UV radiation and heat.Citation135) Furthermore, DHN-melanin protects A. fumigatus conidia against phagocytic killing by inhibiting the acidification mechanism of phagolysosomes.Citation136,137) More intriguing observations were reported recently by Brakhage’s group, who demonstrated that the pigment-less, white conidia of the pksP mutant were ingested more frequently than WT conidia by the soil-living amoeba Dictyostelium discoideum.Citation138) Hence, DHN-melanin not only plays an essential role in infections of mammalian hosts, but also contributes to avoidance of amoeba predation.
These data on conidial structure support the idea that fungi might have evolutionally strengthened the shield on the surface of their conidia against enemies encountered in their environment. Despite their significance, however, the molecular mechanisms regulating hydrophobin production and melanin formation remain elusive.
III.ii Stress resistance-conferring molecules inside conidia
A conidia-specific catalase (CatA) was shown to play crucial role in tolerance against oxidative and thermal stress in A. nidulans and A. fumigatus conidia.Citation139,140) A. oryzae also has a CatA ortholog that is specifically expressed in conidia.Citation141) The expression of catA genes is dependent on AtfA TF and its upstream pathway, the TCS–HOG pathway, in Aspergillus species.Citation31,37,46,91,142) Besides AtfA, there are some paralogs in each fungus, and only A. oryzae AtfB has been characterized. Disruption of atfB resulted in conidia sensitivity to oxidative stress and decreased catA expression in conidia.Citation143) However, these phenotypes were moderate compared with those observed in the atfA deletion mutant.Citation142) These results suggested that AtfA plays a major role and AtfB plays an additional role in conidia stress tolerance in A. oryzae. The genomes of all Aspergillus species sequenced to date contain multiple atf genes (AspGD, http://www.aspergillusgenome.org/). For instance, A. oryzae has three atf genes (atfA: AO090003000685; atfB: AO090120000418; and atfC: AO090010000747), A. nidulans has three atf genes (atfA: AN2911; atfB: AN6849; and atfC: AN8643) and A. fumigatus has four aft genes (atfA: Afu3g11330; atfB: Afu5g12960; atfC: Afu1g17360; and atfD: Afu6g12150), whose encoded proteins share a bZIP-type DNA-binding domain that is highly conserved among atf genes. This implies that multiplication and functional differentiation of atf genes might be evolutionally important for these fungi to survive in their surrounding environments.
Another important determinant for stress tolerance of conidia is the accumulation of compatible solutes such as trehalose and mannitol. Trehalose and mannitol function as stress protectants in conidia of some Aspergillus species.Citation124,125,144) The atfA and atfB deletion mutants of A. oryzae showed decreased expressions of two trehalose-6-phophate synthase genes (tpsA and tpsB) during asexual development.Citation103) Indeed, trehalose is accumulated to lower levels in the mutant conidia than in the control conidia, likely resulting in stress sensitivity of the mutant conidia. As described above, A. nidulans SskA, SskB, PbsB, HogA, and AtfA are responsible for the stress tolerance of conidia, and SskA was shown to be involved in tpsA expression in conidia. These results indicated that the TCS–HOG pathway followed by AtfA TF play an important role in trehalose biosynthesis during conidiation (Fig. ).Citation27,46,91) In Aspergillus species, AtfA is essential for conidia viability in various environmental conditions.
IV. Concluding remarks
In this decade, in the post-genomic era, many studies have focused on interpreting the genomic information obtained for filamentous fungi. Consequently, a large body of data on genes and proteins is now available. Progress in molecular biology has allowed us to further understand how organisms live in, and adapt to, their natural habitats. These improvements in our fundamental understanding will further improve industrial processes including biomaterial production and food fermentation. In the next decade, next-generation sequencing technology promises enormous amounts of data for genomes, genes, and proteins, allowing for comparisons not only among species, but also among strains or among genera. This will undoubtedly result in further progress in several technologies that affect aspects of human life; crop protection, food preservation, food fermentation, chemical production, and prevention of infectious diseases.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Wyatt TT, Wösten HA, Dijksterhuis J. Fungal spores for dispersion in space and time. Adv. Appl. Microbiol. 2013;85:43–91.10.1016/B978-0-12-407672-3.00002-2
- Adams TH, Yu JH. Coordinate control of secondary metabolite production and asexual sporulation in Aspergillus nidulans. Curr. Opin. Microbiol. 1998;1:674–677.10.1016/S1369-5274(98)80114-8
- Etxebeste O, Garzia A, Espeso EA, et al. Aspergillus nidulans asexual development: making the most of cellular modules. Trends Microbiol. 2010;18:569–576.10.1016/j.tim.2010.09.007
- Fischer R, Kües U. Asexual sporulation in mycelial fungi. In: Kües U, Fischer R, editors. The mycota: growth, differentiation and sexuality. Vol. 1. Berlin: Springer; 2006. p. 263–292.
- Galagan JE, Calvo SE, Cuomo C, et al. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature. 2005;438:1105–1115.10.1038/nature04341
- Machida M, Asai K, Sano M, et al. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161.10.1038/nature04300
- Nierman WC, Pain A, Anderson MJ, et al. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2005;438:1151–1156.10.1038/nature04332
- Pel HJ, de Winde JH, Archer DB, et al. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat. Biotechnol. 2007;25:221–231.10.1038/nbt1282
- Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 2002;66:300–372.10.1128/MMBR.66.2.300-372.2002
- Chen RE, Thorner J. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2007;1773:1311–1340.10.1016/j.bbamcr.2007.05.003
- Elion EA. Pheromone response, mating and cell biology. Curr. Opin. Microbiol. 2000;3:573–581.10.1016/S1369-5274(00)00143-0
- Fitzgibbon GJ, Morozov IY, Jones MG, et al. Genetic analysis of the TOR pathway in Aspergillus nidulans. Eukaryotic Cell. 2005;4:1595–1598.10.1128/EC.4.9.1595-1598.2005
- Conrad M, Schothorst J, Kankipati HN, et al. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2014;38:254–299.10.1111/1574-6976.12065
- Taj G, Agarwal P, Grant M, et al. MAPK machinery in plants: recognition and response to different stresses through multiple signal transduction pathways. Plant Signal. Behav. 2010;5:1370–1378.10.4161/psb.5.11.13020
- Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 1999;19:2435–2444.10.1128/MCB.19.4.2435
- Reiser V, Ruis H, Ammerer G. Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of hog1 mitogen-activated protein kinase in the budding yeast Saccharomyces cerevisiae. Mol. Biol. Cell. 1999;10:1147–1161.10.1091/mbc.10.4.1147
- Krisak L, Strich R, Winters RS, et al. SMK1, a developmentally regulated MAP kinase, is required for spore wall assembly in Saccharomyces cerevisiae. Genes Dev. 1994;8:2151–2161.10.1101/gad.8.18.2151
- Jun SC, Lee SJ, Park HJ, et al. The MpkB MAP kinase plays a role in post-karyogamy processes as well as in hyphal anastomosis during sexual development in Aspergillus nidulans. J. Microbiol. 2011;49:418–430.10.1007/s12275-011-0193-3
- Kang JY, Chun J, Jun SC, et al. The MpkB MAP kinase plays a role in autolysis and conidiation of Aspergillus nidulans. Fungal Genet. Biol. 2013;61:42–49.10.1016/j.fgb.2013.09.010
- Atoui A, Bao D, Kaur N, et al. Aspergillus nidulans natural product biosynthesis is regulated by MpkB, a putative pheromone response mitogen-activated protein kinase. Appl. Environ. Microbiol. 2008;74:3596–3600.10.1128/AEM.02842-07
- Yoshimi A, Fujioka T, Mizutani O, et al. Mitogen-activated protein kinases MpkA and MpkB independently affect micafungin sensitivity in Aspergillus nidulans. Biosci. Biotechnol. Biochem. 2015;79:836–844.10.1080/09168451.2014.998619
- Wei H, Requena N, Fischer R. The MAPKK kinase SteC regulates conidiophore morphology and is essential for heterokaryon formation and sexual development in the homothallic fungus Aspergillus nidulans. Mol. Microbiol. 2003;47:1577–1588.10.1046/j.1365-2958.2003.03405.x
- Bayram Ö, Bayram ÖS, Ahmed YL, et al. The Aspergillus nidulans MAPK module AnSte11-Ste50-Ste7-Fus3 controls development and secondary metabolism. PLoS Genet. 2012;8:e1002816.10.1371/journal.pgen.1002816
- Jung US, Levin DE. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 1999;34:1049–1057.10.1046/j.1365-2958.1999.01667.x
- Bussink HJ, Osmani SA. A mitogen-activated protein kinase (MPKA) is involved in polarized growth in the filamentous fungus, Aspergillus nidulans. FEMS Microbiol. Lett. 1999;173:117–125.10.1111/fml.1999.173.issue-1
- Fujioka T, Mizutani O, Furukawa K, et al. MpkA-dependent and -independent cell wall integrity signaling in Aspergillus nidulans. Eukaryotic Cell. 2007;6:1497–1510.10.1128/EC.00281-06
- Furukawa K, Hoshi Y, Maeda T. et al. Aspergillus nidulans HOG pathway is activated only by two-component signalling pathway in response to osmotic stress. Mol. Microbiol. 2005;56:1246–1261.10.1111/j.1365-2958.2005.04605.x
- Bahn YS. Master and commander in fungal pathogens: the two-component system and the HOG signaling pathway. Eukaryotic Cell. 2008;7:2017–2036.10.1128/EC.00323-08
- Rispail N, Soanes DM, Ant C, et al. Comparative genomics of MAP kinase and calcium-calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet. Biol. 2009;46:287–298.10.1016/j.fgb.2009.01.002
- Hamel LP, Nicole MC, Duplessis S, et al. Mitogen-activated protein kinase signaling in plant-interacting fungi: distinct messages from conserved messengers. Plant Cell. 2012;24:1327–1351.10.1105/tpc.112.096156
- Kawasaki L, Sanchez O, Shiozaki K. et al. SakA MAP kinase is involved in stress signal transduction, sexual development and spore viability in Aspergillus nidulans. Mol. Microbiol. 2002;45:1153–1163.10.1046/j.1365-2958.2002.03087.x
- Lara-Rojas F, Sánchez O, Kawasaki L. et al. Aspergillus nidulans transcription factor AtfA interacts with the MAPK SakA to regulate general stress responses, development and spore functions. Mol. Microbiol. 2011;80:436–454.10.1111/mmi.2011.80.issue-2
- Hagiwara D, Takahashi-Nakaguchi A, Toyotome T, et al. NikA/TcsC histidine kinase is involved in conidiation, hyphal morphology, and responses to osmotic stress and antifungal chemicals in Aspergillus fumigatus. PLoS One. 2013;8:e80881.10.1371/journal.pone.0080881
- Xue T, Nguyen CK, Romans A. et al. A mitogen-activated protein kinase that senses nitrogen regulates conidial germination and growth in Aspergillus fumigatus. Eukaryotic Cell. 2004;3:557–560.10.1128/EC.3.2.557-560.2004
- de Castro PA, Chen C, de Almeida RS, et al. ChIP-seq reveals a role for CrzA in the Aspergillus fumigatus high-osmolarity glycerol response (HOG) signalling pathway. Mol. Microbiol. 2014;94:655–674.10.1111/mmi.2014.94.issue-3
- Reyes G, Romans A, Nguyen CK, et al. Novel mitogen-activated protein kinase MpkC of Aspergillus fumigatus is required for utilization of polyalcohol sugars. Eukaryotic Cell. 2006;5:1934–1940.10.1128/EC.00178-06
- Hagiwara D, Suzuki S, Kamei K, et al. The role of AtfA and HOG MAPK pathway in stress tolerance in conidia of Aspergillus fumigatus. Fungal Genet. Biol. 2014;73:138–149.10.1016/j.fgb.2014.10.011
- Hoch JA, Silhavy TJ. Two-component signal transduction. Washington DC: ASM Press; 1995.
- Mizuno T. His-Asp phosphotransfer signal transduction. J. Biochem. 1998;123:555–563.10.1093/oxfordjournals.jbchem.a021972
- Shor E, Chauhan N. A case for two-component signaling systems as antifungal drug targets. PLoS Pathog. 2015;11:e1004632.10.1371/journal.ppat.1004632
- Defosse TA, Sharma A, Mondal AK, et al. Hybrid histidine kinases in pathogenic fungi. Mol. Microbiol. 2015;95:914–924.10.1111/mmi.12911
- Nakamichi N, Yamada H, Aoyama K, et al. His-to-Asp phosphorelay circuitry for regulation of sexual development in Schizosaccharomyces pombe. Biosci. Biotechnol. Biochem. 2002;66:2663–2672.10.1271/bbb.66.2663
- Nakamichi N, Yanada H, Aiba H, et al. Characterization of the Prr1 response regulator with special reference to sexual development in Schizosaccharomyces pombe. Biosci. Biotechnol. Biochem. 2003;67:547–555.10.1271/bbb.67.547
- Morigasaki S, Shiozaki K. Two-component signaling to the stress MAP kinase cascade in fission yeast. Methods Enzymol. 2010;471:279–289.10.1016/S0076-6879(10)71015-6
- Kobayashi T, Abe K, Asai K, et al. Genomics of Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2007;71:646–670.10.1271/bbb.60550
- Hagiwara D, Asano Y, Marui J, et al. The SskA and SrrA response regulators are implicated in oxidative stress responses of hyphae and asexual spores in the phosphorelay signaling network of Aspergillus nidulans. Biosci. Biotechnol. Biochem. 2007;71:1003–1014.10.1271/bbb.60665
- Vargas-Pérez I, Sánchez O, Kawasaki L. et al. Response regulators SrrA and SskA are central components of a phosphorelay system involved in stress signal transduction and asexual sporulation in Aspergillus nidulans. Eukaryotic Cell. 2007;6:1570–1583.10.1128/EC.00085-07
- Krems B, Charizanis C, Entian KD. The response regulator-like protein Pos9/Skn7 of Saccharomyces cerevisiae is involved in oxidative stress resistance. Curr. Genet. 1996;29:327–334.10.1007/BF02208613
- Ohmiya R, Kato C, Yamada H, et al. A fission yeast gene (prr1(+)) that encodes a response regulator implicated in oxidative stress response. J. Biochem. 1999;125:1061–1066.10.1093/oxfordjournals.jbchem.a022387
- Lee J, Godon C, Lagniel G, et al. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J. Biol. Chem. 1999;274:16040–16046.10.1074/jbc.274.23.16040
- Kuge S, Arita M, Murayama A, et al. Regulation of the yeast Yap1p nuclear export signal is mediated by redox signal-induced reversible disulfide bond formation. Mol. Cell Biol. 2001;21:6139–6150.10.1128/MCB.21.18.6139-6150.2001
- Lamarre C, Ibrahim-Granet O, Du C, et al. Characterization of the SKN7 ortholog of Aspergillus fumigatus. Fungal Genet. Biol. 2007;44:682–690.10.1016/j.fgb.2007.01.009
- Nakamura H, Kikuma T, Jin FJ, et al. AoRim15 is involved in conidial stress tolerance, conidiation and sclerotia formation in the filamentous fungus Aspergillus oryzae. J. Biosci. Bioeng. 2016. doi: 10.1016/j.jbiosc.2015.08.011. Epub ahead of print.
- Lavín JL, García-Yoldi A, Ramírez L, et al. Two-component signal transduction in Agaricus bisporus: a comparative genomic analysis with other basidiomycetes through the web-based tool BASID2CS. Fungal Genet. Biol. 2013;55:77–84.10.1016/j.fgb.2012.09.012
- Furukawa K, Katsuno Y, Urao T, et al. Isolation and functional analysis of a gene, tcsB, encoding a transmembrane hybrid-type histidine kinase from Aspergillus nidulans. Appl. Environ. Microbiol. 2002;68:5304–5310.10.1128/AEM.68.11.5304-5310.2002
- Ji Y, Yang F, Ma D, et al. HOG-MAPK signaling regulates the adaptive responses of Aspergillus fumigatus to thermal stress and other related stress. Mycopathologia. 2012;174:273–282.10.1007/s11046-012-9557-4
- Virginia M, Appleyard CL, McPheat WL, et al. A novel ‘two-component’ protein containing histidine kinase and response regulator domains required for sporulation in Aspergillus nidulans. Curr. Genet. 2000;37:364–372.
- Tüncher A, Reinke H, Martic G, et al. A basic-region helix-loop-helix protein-encoding gene (devR) involved in the development of Aspergillus nidulans. Mol. Microbiol. 2004;52:227–241.10.1111/j.1365-2958.2003.03961.x
- Aoyama K, Aiba H, Mizuno T. Genetic analysis of the His-to-Asp phosphorelay implicated in mitotic cell cycle control: involvement of histidine-kinase genes of Schizosaccharomyces pombe. Biosci. Biotechnol. Biochem. 2001;65:2347–2352.10.1271/bbb.65.2347
- Hayashi S, Yoshioka M, Matsui T, et al. Control of reactive oxygen species (ROS) production through histidine kinases in Aspergillus nidulans under different growth conditions. FEBS Open Bio. 2014;4:90–95.10.1016/j.fob.2014.01.003
- Meena N, Kaur H, Mondal AK. Interactions among HAMP domain repeats act as an osmosensing molecular switch in group III hybrid histidine kinases from fungi. J. Biol. Chem. 2010;285:12121–12132.10.1074/jbc.M109.075721
- El-Mowafy M, Bahgat MM, Bilitewski U. Deletion of the HAMP domains from the histidine kinase CaNik1p of Candida albicans or treatment with fungicides activates the MAP kinase Hog1p in S. cerevisiae transformants. BMC Microbiol. 2013;13:209.10.1186/1471-2180-13-209
- Hagiwara D, Mizuno T, Abe K. Characterization of NikA histidine kinase and two response regulators with special reference to osmotic adaptation and asexual development in Aspergillus nidulans. Biosci. Biotechnol. Biochem. 2009;73:1566–1571.10.1271/bbb.90063
- Motoyama T, Ohira T, Kadokura K. et al. An Os-1 family histidine kinase from a filamentous fungus confers fungicide-sensitivity to yeast. Curr. Genet. 2005;47:298–306.10.1007/s00294-005-0572-6
- Dongo A, Bataillé-Simoneau N, Campion C, et al. The group III two-component histidine kinase of filamentous fungi is involved in the fungicidal activity of the bacterial polyketide ambruticin. Appl. Environ. Microbiol. 2009;75:127–134.10.1128/AEM.00993-08
- Hagiwara D, Matsubayashi Y, Marui J, et al. Characterization of the NikA histidine kinase implicated in the phosphorelay signal transduction of Aspergillus nidulans, with special reference to fungicide responses. Biosci. Biotechnol. Biochem. 2007;71:844–847.10.1271/bbb.70051
- Izumitsu K, Yoshimi A, Tanaka C. Two-component response regulators Ssk1p and Skn7p additively regulate high-osmolarity adaptation and fungicide sensitivity in Cochliobolus heterostrophus. Eukaryotic Cell. 2007;6:171–181.10.1128/EC.00326-06
- Rispail N, Di Pietro A. The two-component histidine kinase Fhk1 controls stress adaptation and virulence of Fusarium oxysporum. Mol. Plant Pathol. 2010;11:395–407.10.1111/mpp.2010.11.issue-3
- Viaud M, Fillinger S, Liu W, et al. A class III histidine kinase acts as a novel virulence factor in Botrytis cinerea. Mol. Plant Microbe Interact. 2006;19:1042–1050.10.1094/MPMI-19-1042
- Brandt S, von Stetten D, Gunther M, et al. The fungal phytochrome FphA from Aspergillus nidulans. J. Biol. Chem. 2008;283:34605–34614.10.1074/jbc.M805506200
- Blumenstein A, Vienken K, Tasler R, et al. The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr. Biol. 2005;15:1833–1838.10.1016/j.cub.2005.08.061
- Purschwitz J, Müller S, Kastner C, et al. Functional and physical interaction of blue- and red-light sensors in Aspergillus nidulans. Curr. Biol. 2008;18:255–259.10.1016/j.cub.2008.01.061
- Suzuki A, Kanamaru K, Azuma N, et al. GFP-tagged expression analysis revealed that some histidine kinases of Aspergillus nidulans show temporally and spatially different expression during the life cycle. Biosci. Biotechnol. Biochem. 2008;72:428–434.10.1271/bbb.70543
- Hagiwara D, Asano Y, Marui J, et al. Transcriptional profiling for Aspergillus nidulans HogA MAPK signaling pathway in response to fludioxonil and osmotic stress. Fungal Genet. Biol. 2009;46:868–878.10.1016/j.fgb.2009.07.003
- Losada L, Barker BM, Pakala S, et al. Large-scale transcriptional response to hypoxia in Aspergillus fumigatus observed using RNAseq identifies a novel hypoxia regulated ncRNA. Mycopathologia. 2014;178:331–339.10.1007/s11046-014-9779-8
- Posas F, Wurgler-Murphy SM, Maeda T, et al. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1–YPD1–SSK1 “two-component” osmosensor. Cell. 1996;86:865–875.10.1016/S0092-8674(00)80162-2
- Aoyama K, Mitsubayashi Y, Aiba H, et al. Spy1, a histidine-containing phosphotransfer signaling protein, regulates the fission yeast cell cycle through the Mcs4 response regulator. J. Bacteriol. 2000;182:4868–4874.10.1128/JB.182.17.4868-4874.2000
- Nguyen AN, Lee A, Place W, et al. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol. Biol. Cell. 2000;11:1169–1181.10.1091/mbc.11.4.1169
- Mavrianos J, Desai C, Chauhan N. Two-component histidine phosphotransfer protein ypd1 is not essential for viability in Candida albicans. Eukaryotic Cell. 2014;13:452–460.10.1128/EC.00243-13
- Banno S, Noguchi R, Yamashita K, et al. Roles of putative His-to-Asp signaling modules HPT-1 and RRG-2, on viability and sensitivity to osmotic and oxidative stresses in Neurospora crassa. Curr. Genet. 2007;51:197–208.10.1007/s00294-006-0116-8
- Jacob S, Foster AJ, Yemelin A, et al. High osmolarity glycerol (HOG) signalling in Magnaporthe oryzae: identification of MoYPD1 and its role in osmoregulation, fungicide action, and pathogenicity. Fungal Biol. 2015;119:580–594.10.1016/j.funbio.2015.03.003
- Wong Sak Hoi J, Dumas B. Ste12 and Ste12-like proteins, fungal transcription factors regulating development and pathogenicity. Eukaryotic Cell. 2010;9:480–485.
- Vallim MA, Miller KY, Miller BL. Aspergillus SteA (Sterile12-like) is a homeodomain-C2/H2-Zn+2 finger transcription factor required for sexual reproduction. Mol. Microbiol. 2000;36:290–301.10.1046/j.1365-2958.2000.01874.x
- Morita H, Hatamoto O, Masuda T, et al. Function analysis of steA homolog in Aspergillus oryzae. Fungal Genet. Biol. 2007;44:330–338.10.1016/j.fgb.2006.10.009
- Proft M, Serrano R. Repressors and upstream repressing sequences of the stress-regulated ENA1 gene in saccharomyces cerevisiae: bZIP protein Sko1p confers HOG-dependent osmotic regulation. Mol. Cell. Biol. 1999;19:537–546.10.1128/MCB.19.1.537
- Rep M, Reiser V, Gartner U, et al. Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol. Cell. Biol. 1999;19:5474–5485.10.1128/MCB.19.8.5474
- Proft M, Struhl K. Hog1 kinase converts the Sko1-Cyc8-Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol. Cell. 2002;9:1307–1317.10.1016/S1097-2765(02)00557-9
- Rep M, Krantz M, Thevelein JM, et al. The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 2000;275:8290–8300.10.1074/jbc.275.12.8290
- Shiozaki K, Russell P. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 1996;10:2276–2288.10.1101/gad.10.18.2276
- Wilkinson MG, Samuels M, Takeda T, et al. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 1996;10:2289–2301.10.1101/gad.10.18.2289
- Hagiwara D, Asano Y, Yamashino T, et al. Characterization of bZip-type transcription factor AtfA with reference to stress responses of conidia of Aspergillus nidulans. Biosci. Biotechnol. Biochem. 2008;72:2756–2760.10.1271/bbb.80001
- Damveld RA, Arentshorst M, Franken A, et al. The Aspergillus niger MADS-box transcription factor RlmA is required for cell wall reinforcement in response to cell wall stress. Mol. Microbiol. 2005;58:305–319.10.1111/j.1365-2958.2005.04827.x
- Ji H, Lu X, Wang C, et al. Identification of a novel HOG1 homologue from an industrial glycerol producer Candida glycerinogenes. Curr. Microbiol. 2014;69:909–914.10.1007/s00284-014-0670-0
- Zajc J, Kogej T, Galinski EA, et al. Osmoadaptation strategy of the most halophilic fungus, Wallemia ichthyophaga, growing optimally at salinities above 15% NaCl. Appl. Environ. Microbiol. 2014;80:247–256.10.1128/AEM.02702-13
- Kayingo G, Wong B. The MAP kinase Hog1p differentially regulates stress-induced production and accumulation of glycerol and D-arabitol in Candida albicans. Microbiology. 2005;151:2987–2999.10.1099/mic.0.28040-0
- Miskei M, Karányi Z, Pócsi I. Annotation of stress-response proteins in the aspergilli. Fungal Genet. Biol. 2008;46 (Suppl 1):S105–S120.
- Fillinger S, Ruijter G, Tamás MJ, et al. Molecular and physiological characterization of the NAD-dependent glycerol 3-phosphate dehydrogenase in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 2001;39:145–157.10.1046/j.1365-2958.2001.02223.x
- Furukawa K, Yoshimi A, Furukawa T, et al. Novel reporter gene expression systems for monitoring activation of the Aspergillus nidulans HOG pathway. Biosci. Biotechnol. Biochem. 2007;71:1724–1730.10.1271/bbb.70131
- Witteveen CFB, Visser J. Polyol pools in Aspergillus niger. FEMS Microbiol. Lett. 1995;134:57–62.10.1111/fml.1995.134.issue-1
- Beever RE, Laracy EP. Osmotic adjustment in the filamentous fungus Aspergillus nidulans. J. Bacteriol. 1986;168:1358–1365.
- Redkar RJ, Locy RD, Singh NK. Biosynthetic pathways of glycerol accumulation under salt stress in Aspergillus nidulans. Exp. Mycol. 1995;19:241–246.10.1006/emyc.1995.1030
- Kelavkar UP, Chhatpar HS. Polyol concentrations in Aspergillus repens grown under salt stress. World J. Microbiol. Biotechnol. 1993;9:579–582.10.1007/BF00386298
- Mellon JE, Dowd MK, Cotty PJ. Time course study of substrate utilization by Aspergillus flavus in medium simulating corn (Zea mays) kernels. J. Agric. Food Chem. 2002;50:648–652.10.1021/jf011048e
- Ross SJ, Findlay VJ, Malakasi P, et al. Thioredoxin peroxidase is required for the transcriptional response to oxidative stress in budding yeast. Mol. Biol. Cell. 2000;11:2631–2642.10.1091/mbc.11.8.2631
- Moye-Rowley WS. Regulation of the transcriptional response to oxidative stress in fungi: similarities and differences. Eukaryotic Cell. 2003;2:381–389.10.1128/EC.2.3.381-389.2003
- Ikner A, Shiozaki K. Yeast signaling pathways in the oxidative stress response. Mutat. Res. 2005;569:13–27.10.1016/j.mrfmmm.2004.09.006
- Asano Y, Hagiwara D, Yamashino T, et al. Characterization of the bZip-type transcription factor NapA with reference to oxidative stress response in Aspergillus nidulans. Biosci. Biotechnol. Biochem. 2007;71:1800–1803.10.1271/bbb.70133
- Lessing F, Kniemeyer O, Wozniok I, et al. The Aspergillus fumigatus transcriptional regulator AfYap1 represents the major regulator for defense against reactive oxygen intermediates but is dispensable for pathogenicity in an intranasal mouse infection model. Eukaryotic Cell. 2007;6:2290–2302.10.1128/EC.00267-07
- Hon T, Dodd A, Dirmeier R, et al. A mechanism of oxygen sensing in yeast: Multiple oxygen-responsive steps in the heme biosynthetic pathway affect hap1 activity. J. Biol. Chem. 2003;278:50771–50780.10.1074/jbc.M303677200
- Davies BS, Rine J. A role for sterol levels in oxygen sensing in Saccharomyces cerevisiae. Genetics. 2006;174:191–201.10.1534/genetics.106.059964
- Masuo S, Terabayashi Y, Shimizu M, et al. Global gene expression analysis of Aspergillus nidulans reveals metabolic shift and transcription suppression under hypoxia. Mol. Genet. Genomics. 2010;284:415–424.10.1007/s00438-010-0576-x
- Vödisch M, Scherlach K, Winkler R, et al. Analysis of the Aspergillus fumigatus proteome reveals metabolic changes and the activation of the pseurotin a biosynthesis gene cluster in response to hypoxia. J. Proteome Res. 2011;10:2508–2524.10.1021/pr1012812
- Willger SD, Puttikamonkul S, Kim KH, et al. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008;4:e1000200.10.1371/journal.ppat.1000200
- Chung D, Barker BM, Carey CC, et al. ChIP-seq and in vivo transcriptome analyses of the Aspergillus fumigatus SREBP SrbA reveals a new regulator of the fungal hypoxia response and virulence. PLoS Pathog. 2014;10:e1004487.10.1371/journal.ppat.1004487
- Grahl N, Shepardson KM, Chung D, et al. Hypoxia and fungal pathogenesis: to air or not to air? Eukaryotic Cell. 2012;11:560–570.10.1128/EC.00031-12
- Chun CD, Liu OW, Madhani HD. A link between virulence and homeostatic responses to hypoxia during infection by the human fungal pathogen Cryptococcus neoformans. PLoS Pathog. 2007;3:e22.10.1371/journal.ppat.0030022
- Kroll K, Pähtz V, Hillmann F, et al. Identification of hypoxia-inducible target genes of Aspergillus fumigatus by transcriptome analysis reveals cellular respiration as an important contributor to hypoxic survival. Eukaryotic Cell. 2014;13:1241–1253.10.1128/EC.00084-14
- Kozakiewicsz Z, Smith D. Physiology of Aspergillus. In: Smith JE, editor. Biotechnology handbooks – 7: Aspergillus. New York, NY: Plenum Press; 1994. p. 23–40.
- Burnie JP, Carter TL, Hodgetts SJ, et al. Fungal heat-shock proteins in human disease. FEMS Microbiol. Rev. 2006;30:53–88.10.1111/j.1574-6976.2005.00001.x
- Li ZW, Li X, Yu QY, et al. The small heat shock protein (sHSP) genes in the silkworm, Bombyx mori, and comparative analysis with other insect sHSP genes. BMC Evol. Biol. 2009;9:215.10.1186/1471-2148-9-215
- Freitas JS, Silva EM, Leal J, et al. Transcription of the Hsp30, Hsp70, and Hsp90 heat shock protein genes is modulated by the PalA protein in response to acid pH-sensing in the fungus Aspergillus nidulans. Cell Stress Chaperones. 2011;16:565–572.10.1007/s12192-011-0267-5
- Lamoth F, Juvvadi PR, Fortwendel JR, et al. Heat shock protein 90 is required for conidiation and cell wall integrity in Aspergillus fumigatus. Eukaryotic Cell. 2012;11:1324–1332.10.1128/EC.00032-12
- Wu J, Wang M, Zhou L, et al. Small heat shock proteins, phylogeny in filamentous fungi and expression analyses in Aspergillus nidulans. Gene. 2016;575:675–679.10.1016/j.gene.2015.09.044
- Al-Bader N, Vanier G, Liu H, et al. Role of trehalose biosynthesis in Aspergillus fumigatus development, stress response, and virulence. Infect. Immun. 2010;78:3007–3018.10.1128/IAI.00813-09
- Fillinger S, Chaveroche MK, van Dijck P, et al. Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans. Microbiology. 2001;147:1851–1862.10.1099/00221287-147-7-1851
- An MZ, Tang YQ, Mitsumasu K, et al. Enhanced thermotolerance for ethanol fermentation of Saccharomyces cerevisiae strain by overexpression of the gene coding for trehalose-6-phosphate synthase. Biotechnol. Lett. 2011;33:1367–1374.10.1007/s10529-011-0576-x
- Albrecht D, Guthke R, Brakhage AA, et al. Integrative analysis of the heat shock response in Aspergillus fumigatus. BMC Genomics. 2010;11:32.10.1186/1471-2164-11-32
- Do JH, Yamaguchi R, Miyano S. Exploring temporal transcription regulation structure of Aspergillus fumigatus in heat shock by state space model. BMC Genomics. 2009;10:306.10.1186/1471-2164-10-306
- Futagami T, Mori K, Wada S, et al. Transcriptomic analysis of temperature responses of Aspergillus kawachii during barley koji production. Appl. Environ. Microbiol. 2015;81:1353–1363.10.1128/AEM.03483-14
- Aimanianda V, Bayry J, Bozza S, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117–1121.10.1038/nature08264
- Grünbacher A, Throm T, Seidel C, et al. Six hydrophobins are involved in hydrophobin rodlet formation in Aspergillus nidulans and contribute to hydrophobicity of the spore surface. PLoS One. 2014;9:e94546.10.1371/journal.pone.0094546
- Braga GU, Rangel DE, Fernandes ÉK, et al. Molecular and physiological effects of environmental UV radiation on fungal conidia. Curr. Genet. 2015;61:405–425.10.1007/s00294-015-0483-0
- Chiang YM, Meyer KM, Praseuth M, et al. Characterization of a polyketide synthase in Aspergillus niger whose product is a precursor for both dihydroxynaphthalene (DHN) melanin and naphtho-γ-pyrone. Fungal Genet. Biol. 2011;48:430–437.10.1016/j.fgb.2010.12.001
- Esbelin J, Mallea S, Ram AF, et al. Role of pigmentation in protecting Aspergillus niger conidiospores against pulsed light radiation. Photochem. Photobiol. 2013;89:758–761.10.1111/php.2013.89.issue-3
- Heinekamp T, Thywißen A, Macheleidt J, et al. Aspergillus fumigatus melanins: interference with the host endocytosis pathway and impact on virulence. Front. Microbiol. 2013;3:440.
- Thywißen A, Heinekamp T, Dahse HM, et al. Conidial dihydroxynaphthalene melanin of the human pathogenic fungus Aspergillus fumigatus interferes with the host endocytosis pathway. Front. Microbiol. 2011;2:96.
- Volling K, Thywissen A, Brakhage AA, et al. Phagocytosis of melanized Aspergillus conidia by macrophages exerts cytoprotective effects by sustained PI3K/Akt signalling. Cell. Microbiol. 2011;13:1130–1148.10.1111/cmi.2011.13.issue-8
- Hillmann F, Novohradská S, Mattern DJ, et al. Virulence determinants of the human pathogenic fungus Aspergillus fumigatus protect against soil amoeba predation. Environ. Microbiol. 2015;17:2858–2869.10.1111/1462-2920.12808
- Navarro RE, Stringer MA, Hansberg W. et al. catA, a new Aspergillus nidulans gene encoding a developmentally regulated catalase. Curr. Genet. 1996;29:352–359.
- Paris S, Wysong D, Debeaupuis JP, et al. Catalases of Aspergillus fumigatus. Infect. Immun. 2003;71:3551–3562.10.1128/IAI.71.6.3551-3562.2003
- Hisada H, Hata Y, Kawato A, et al. Cloning and expression analysis of two catalase genes from Aspergillus oryzae. J. Biosci. Bioeng. 2005;99:562–568.10.1263/jbb.99.562
- Sakamoto K, Iwashita K, Yamada O, et al. Aspergillus oryzae atfA controls conidial germination and stress tolerance. Fungal Genet. Biol. 2009;46:887–897.10.1016/j.fgb.2009.09.004
- Sakamoto K, Arima TH, Iwashita K, et al. Aspergillus oryzae atfB encodes a transcription factor required for stress tolerance in conidia. Fungal Genet. Biol. 2008;45:922–932.10.1016/j.fgb.2008.03.009
- Ruijter GJ, Bax M, Patel H, et al. Mannitol is required for stress tolerance in Aspergillus niger conidiospores. Eukaryotic Cell. 2003;2:690–698.10.1128/EC.2.4.690-698.2003
