Abstract
The gene expression pattern of the glucose transporters (GLUT1, GLUT3, GLUT8, and GLUT12) among pectoralis major and minor, biceps femoris, and sartorius muscles from newly hatched chicks was examined. GLUT1 mRNA level was higher in pectoralis major muscle than in the other muscles. Phosphorylated AKT level was also high in the same muscle, suggesting a relationship between AKT and GLUT1 expression.
Glucose is an essential metabolic substrate that is transported into animal cells by the glucose transporter (GLUT) family protein. Skeletal muscle is considered to be an important tissue for glucose uptake in the whole body.Citation1,2) Among the 12 identified GLUT isoforms in mammals,Citation3) GLUT4 is well known for its major contribution to insulin-stimulated glucose uptake into the skeletal muscle.Citation4) On the other hand, GLUT1 is considered as a major transporter of basal glucose uptake into the skeletal muscle.Citation5,6)
Chickens maintain higher blood plasma glucose levels compared to most of the vertebrates.Citation7–10) In chicken embryo, plasma insulin was detected at 13 days of incubation, and its level gradually increased during the perinatal period.Citation11) Insulin plays a significant role in the regulation of plasma glucose level in the chickens as well as mammals, i.e. insulin stimulation enhanced the glucose uptake into skeletal musclesCitation10) and decreased plasma glucose concentration in chickens.Citation9,10)
It has been reported that chickens lack the homologous gene of GLUT4Citation12); however, studies have reported that mRNA expressions of GLUT1, GLUT3, GLUT8, and GLUT12 are expressed in the skeletal muscles of chicken.Citation13,14) In response to insulin stimulation, GLUT1 mRNA expression was found to be increased in the skeletal muscleCitation15) and chicken myoblasts.Citation16) Recently, Coudert et al.Citation14) reported that gene expression encoding SLC2A12, which is a chicken GLUT12 ortholog, was lowered in chickens subjected to insulin immuno-neutralization. Thus, among GLUT isoforms, GLUT1 and GLUT12 are suggested to play a significant role in insulin-stimulated glucose uptake in the skeletal muscle of chicken. However, a few studies have reported that GLUT isoforms are responsible for basal glucose uptake into the skeletal muscle of chickens.
Therefore, the aim of this study was to examine the mRNA expression levels of the GLUT isoforms (i.e. GLUT1, GLUT3, GLUT8, and GLUT12) in four different muscles, i.e. the pectoralis major muscle, which is composed of only glycolytic fibers; the pectoralis minor muscle; the biceps femoris muscle; and the sartorius muscle, which is composed of both glycolytic and oxidative fibers.Citation17) Moreover, since GLUT1 mRNA expression was increased by insulin-induced AKT phosphorylation in mammals,Citation18) we examined the protein level of phosphorylated AKT in the four different muscles. To eliminate the effects of feed nutrients and feeding-induced insulin secretion on the GLUT expression in the skeletal muscles, we prepared the tissues from newly hatched chicks before feeding.
All experimental protocols and procedures were reviewed and approved by the Animal Care and Use Committee of Kagoshima University (Kagoshima, Japan). Sixteen newly hatched broiler male chicks (Gallus gallus domesticus), weighing 45.33 ± 1.44 g, were supplied by a commercial hatchery (Kumiai Hina Center, Kagoshima, Japan). The chicks were housed in an electrically heated battery brooder in which the temperature was maintained at 35 °C. All chicks were sacrificed by cervical dislocation using carbon dioxide anesthesia. The pectoralis major and minor muscles, biceps femoris muscle, and sartorius muscle were collected and immediately frozen in liquid nitrogen and stored at −80 °C until further use.
The total RNA extraction with ISOGEN II (Nippon Gene, Tokyo, Japan) and reverse transcription reaction were performed as described in a previous study.Citation19) Real-time PCR was performed using the 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) with SYBR Select Master Mix (Applied Biosystems, Foster City, CA, USA). In this study, the following primers were used: GLUT1 (5′-GATGGCTTTGTCCTTTGAGATGC-3′ and 5′-CAAAGATGCTGGTGGAGTAGTAG-3′), GLUT3 (5′-TTAGCTGGGTGTCGGTGCTGG-3′ and 5′-CCATGAGAGCACCCCCAGCAA-3′), GLUT8 (5′-CCAAATGGGAACAACTCATCAA-3′ and 5′-GGGCAAAACCAGCAACAAA -3′), GLUT12 (5′-TCTCTCTGGCCAGCCTACTT-3′ and 5′-TTCACTCAGGACGAGCCAAG-3′), hexokinase 2 (HK2) (5′-AAGCTCTCTTCGGATCTGCG-3′ and 5′-CTTGGTGAGGATCTCGTGGG-3′), peroxisome proliferative-activated receptor, gamma, and coactivator-1 alpha (PGC-1α) (5′-GCAATTGAAGAGCGTCGTGT-3′ and 5′-CCATAGCTGTCTCCATCATC-3′), insulin receptor (INSR) (5′-GACTCTCCAACGAACAGGTG-3′ and 5′-TCAGCATCTCAATGACCTCAA-3′), Insulin-like growth factor-I (IGF-I) (5′-CTTCAGTTCGTATGTGGAGACA-3′ and 5′-GATTTAGGTGGCTTTATTGGAG-3′), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (5′-CCTCTCTGGCAAAGTCCAAG-3′ and 5′-CATCTGCCCATTTGATGTTG-3′). The gene expression results are shown as a ratio to the value of the pectoralis major muscle.
Western blot analysis was performed as described previously.Citation20) In the present study, the following antibodies were used: AKT (♯9272; Cell Signaling Technology, Beverly, MA, USA) and phospho-AKT (Ser473) (♯9271; Cell Signaling Technology).
The data were expressed as means ± SEM. Statistical comparisons were performed using the analysis of variance followed by Tukey’s multiple comparisons test. The differences were considered to be significant if p < 0.05. All analyses were performed using the general linear model procedure of the Statistical Analysis System software package (SAS/STAT Version 9.3, Statistical Analysis Systems Institute Inc., Cary, NC, USA).
In this study, we found that the GLUT1 mRNA expression in pectoralis major muscle was higher than that in pectoralis minor muscle, biceps femoris muscle, and sartorius muscle (Fig. (A)). GLUT3 mRNA expression in the pectoralis major muscle was higher than that in the biceps femoris and sartorius muscles, while GLUT8 mRNA expression was higher in the pectoralis major and minor muscles as compared to the biceps femoris muscle and sartorius muscle, respectively (Fig. (B) and (C)). On the other hand, there was no significant difference in GLUT12 mRNA expression among the pectoralis major and minor muscles and the biceps femoris muscle, while that in the pectoralis minor muscle was higher than that in the sartorius muscle (Fig. (D)). This expression pattern of GLUT12 is in agreement with the study by Coudert et al.Citation14) in which they found that the expressions of both mRNA and protein levels of GLUT12 were similar between the pectoralis major and leg muscles. These results suggest that the mRNA expressions of GLUT1, GLUT3, and GLUT8 (but not GLUT12) in the pectoralis major muscle were comparatively high among the examined skeletal muscles of the newly hatched chicks.
Fig. 1. Gene expressions of GLUT isoforms in the skeletal muscles of the newly hatched chicks. The mRNA expressions of GLUT1 (A), GLUT3 (B), GLUT8 (C), and GLUT12 (D).
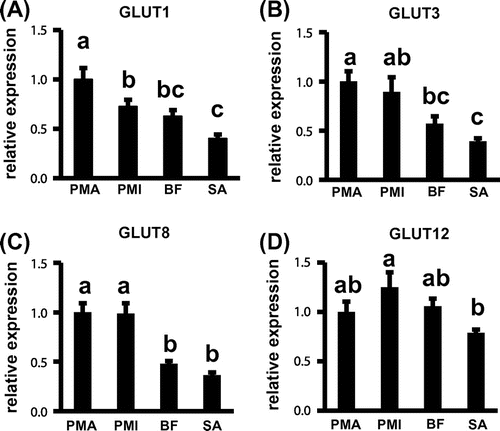
Although the reason behind the pectoralis major muscle showing higher expressions of genes encoding GLUT1, GLUT3, and GLUT8 remains unclear, one possible explanation may be the muscle fiber composition of these skeletal muscles. In chicken, the pectoralis major muscle is composed of only glycolytic fibers, while these two muscles are composed of both glycolytic and oxidative fibers.Citation17) In addition, the activities of enzymes involved in glucose metabolism were remarkably higher in glycolytic fiber-rich muscle compared to those in the oxidative fiber-rich muscle of one-day-old chicks.Citation20) In this study, we found that the expression of HK2 mRNA was higher in the pectoralis major muscle compared to the other three muscles (supplemental Fig. 1(A)). These results suggest that the high levels of mRNA expressions of GLUT1, GLUT3, and GLUT8 in the pectoralis major muscle might contribute toward basal glucose uptake into the muscle cells.
In this study, the phosphorylated AKT protein level in pectoralis major muscle was higher than that in the other three muscles. However, the total AKT protein level was also higher in the pectoralis major and minor muscles as compared to the biceps femoris muscle and sartorius muscle, respectively (Fig. (A)–(C)). It has been reported that phosphorylated AKT protein level induces GLUT1 mRNA expression in response to insulin in mouse hepatoma cells.Citation18) These results suggest that GLUT1 mRNA expression is related to phosphorylated AKT protein level in the skeletal muscles of newly hatched chicks. Moreover, in the skeletal muscles, the AKT protein phosphorylation is induced by either insulin or IGF-I signaling.Citation21,22) Indeed, in this study, INSR and IGF-I mRNA expressions in the pectoralis major muscle were found to be the highest in the skeletal muscles (Fig. (D) and (E)). Therefore, it was suggested that either the INSR or IGF-I mRNA expressions might also be related to the expression level of GLUT1 mRNA in the skeletal muscle of the chicks.
Fig. 2. Protein and gene expressions in the skeletal muscles of the newly hatched chicks. A, The representative western blot data of phosphorylated AKT and total AKT. Protein levels of phosphorylated AKT (B) and total AKT (C) in these skeletal muscles. The mRNA expressions of INSR (D) and IGF-I (E).
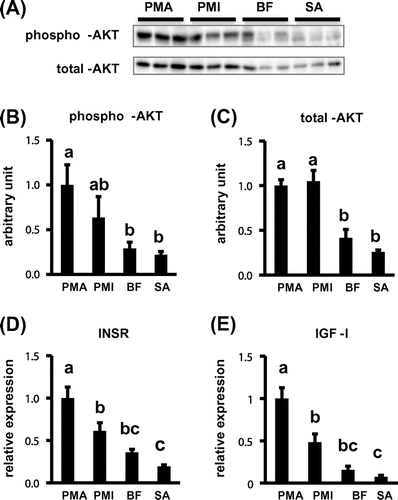
Furthermore, studies have reported that PGC-1α plays a role as the regulator of hepatic gluconeogenesisCitation23) and activates transcription of the GLUT4 gene in mammalian myogenic cells culture.Citation24) In addition, Wende et al.Citation25) found that PGC-1α increased gene expressions of GLUT1 and HK in the skeletal muscle of mice. In this study, we found that the expression pattern of PGC-1α mRNA was similar to that of GLUT1 and HK2 in the skeletal muscles of newly hatched chicks (supplemental Fig. 1(B)). Since the gene expression of PGC-1α was induced by activated AKT signaling in human umbilical vein endothelial cells,Citation26) these results suggest that PGC-1α might be related to the expression level of GLUT1 in the skeletal muscle of newly hatched chicks. Although the newly hatched chicks are adapted to metabolize lipid and protein substrate from egg yolk and albumen, the first feeding leads them to drastic alterations in glucose metabolism. The findings of this study may help to understand how the first feeding reflects gene expressions involved in glucose metabolism in the skeletal muscle of the newly hatched chicks in the future.
The findings of our study showed that, among the four GLUT isoforms, the expression pattern of GLUT1 mRNA was similar to that of phosphorylated AKT protein level in the skeletal muscle of the newly hatched chicks before feeding.
Author contributions
Saki Shimamoto, Daichi Ijiri, Kazuki Nakashima, and Akira Ohtsuka conceived and designed the experiments. Saki Shimamoto, Daichi Ijiri, and Mana Kawaguchi performed the experiments and contributed reagents/materials/analysis tools. Saki Shimamoto and Daichi Ijiri wrote the paper.
Disclosure statement
The authors declare that there are no conflicts of interest that would prejudice the impartiality of this scientific work.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2016.1162088.
TBBB_1162088_Supplemental_Material.docx
Download MS Word (52.6 KB)Notes
Abbreviations: GLUT, glucose transporter; INSR, insulin receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HK2, hexokinase 2.
References
- DeFronzo RA, Jacot E, Jequier E, et al. The Effect of insulin on the disposal of intravenous glucose: results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007.10.2337/diab.30.12.1000
- DeFronzo RA, Gunnarsson R, Björkman O, et al. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J. Clin. Invest. 1985;76:149–155.10.1172/JCI111938
- Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br. J. Nutr. 2003;89:3–9.10.1079/BJN2002763
- Watson RT, Pessin JE. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog. Horm. Res. 2001;56:175–193.10.1210/rp.56.1.175
- Kraegen EW, Sowden JA, Halstead MB, et al. Glucose transporters and in vivo glucose uptake in skeletal and cardiac muscle: fasting, insulin stimulation and immunoisolation studies of GLUT1 and GLUT4. Biochem. J. 1993;295:287–293.10.1042/bj2950287
- Buse MG, Robinson KA, Marshall BA, et al. Enhanced O-GlcNAc protein modification is associated with insulin resistance in GLUT1-overexpressing muscles. Am. J. Physiol. Endocrinol. Metab. 2002;283:E241–E250.10.1152/ajpendo.00060.2002
- Hazelwood RL, Lorenz FW. Effects of fasting and insulinon carbohydrate metabolism of the domestic fowl. Am. J. Physiol. 1959;197:47–51.
- Belo PS, Romsos DR, Leville GA. Blood metabolites and glucose metabolism in the fed and fasted chicken. J. Nutr. 1976;106:1135–1143.
- Akiba Y, Chida Y, Takahashi T, et al. Persistent hypoglycemia induced by continuous insulin infusion in broiler chickens. Br. Poult. Sci. 1999;40:701–705.10.1080/00071669987124
- Tokushima Y, Takahashi K, Sato K, et al. Glucose uptake in vivo in skeletal muscles of insulin-injected chicks. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2005;141:43–48.10.1016/j.cbpc.2005.01.008
- Lu JW, McMurtry JP, Coon CN. Developmental changes of plasma insulin, glucagon, insulin-like growth factors, thyroid hormones, and glucose concentrations in chick embryos and hatched chicks. Poult. Sci. 2007;86:673–683.10.1093/ps/86.4.673
- Seki Y, Sato K, Kono T, et al. Broiler chickens (Ross strain) lack insulin-responsive glucose transporter GLUT4 and have GLUT8 cDNA. Gen. Comp. Endocrinol. 2003;133:80–87.10.1016/S0016-6480(03)00145-X
- Kono T, Nishida M, Nishiki Y, et al. Characterisation of glucose transporter (GLUT) gene expression in broiler chickens. Br. Poult. Sci. 2005;46:510–515.10.1080/00071660500181289
- Coudert E, Pascal G, Dupont J, et al. Phylogenesis and biological characterization of a new glucose transporter in the chicken (Gallus gallus), GLUT12. PLoS One. 2015;10:e0139517.10.1371/journal.pone.0139517
- Kono T, Nishiki Y, Seki Y, et al. Insulin stimulates glucose transporter 1 (GLUT1) and hexokinase II (HK II) gene expression in chicken skeletal muscle. EPC 2006 - 12th European Poultry Conference, Verona, Italy; 2006 Sep 10–14. Paper 138.
- Zhao JP, Bao J, Wang XJ, et al. Altered gene and protein expression of glucose transporter1 underlies dexamethasone inhibition of insulin-stimulated glucose uptake in chicken muscles. J. Anim. Sci. 2012;90:4337–4345.10.2527/jas.2012-5100
- Nishida J, Machida NW, Tagome M, et al. Distribution of parvalbumin in specific fibre types of chicken skeletal muscles. Br. Poult. Sci. 1995;36:585–597.10.1080/00071669508417804
- Barthel A, Okino ST, Liao J, et al. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J. Biol. Chem. 1999;274:20281–20286.10.1074/jbc.274.29.20281
- Ijiri D, Higuchi A, Saegusa A, et al. Role of prolactin-like protein (PRL-L) in cold-induced increase of muscle mass in chicks. Gen. Comp. Endocrinol. 2013;186:94–100.10.1016/j.ygcen.2013.03.007
- Bass A, Lusch G, Pette D. Postnatal differentiation of the enzyme activity pattern of energy-supplying metabolism in slow (red) and fast (white) muscles of chicken. Eur. J. Biochem. 1970;13:289–292.10.1111/ejb.1970.13.issue-2
- Mackenzie RW, Elliott BT. Akt/PKB activation and insulin signaling: a novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab. Syndr. Obes. 2014;13:55–64.10.2147/DMSO
- Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen. Comp. Endocrinol. 2010;167:344–351.10.1016/j.ygcen.2010.04.009
- Puigserver P, Rhee J, Donovan J, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature. 2003;423:550–555.
- Michael LF, Wu Z, Cheatham RB, et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl. Acad. Sci. USA. 2001;98:3820–3825.10.1073/pnas.061035098
- Wende AR, Schaeffer PJ, Parker GJ, et al. A role for the transcriptional coactivator PGC-1α in muscle refueling. J. Biol. Chem. 2007;282:36642–36651.10.1074/jbc.M707006200
- Beeson CC, Beeson GC, Buff H, et al. Integrin-dependent Akt1 activation regulates PGC-1 expression and fatty acid oxidation. J. Vasc. Res2012;49:89–100.10.1159/000332326
