Abstract
Because plants are continually exposed to various environmental stresses, they possess numerous transcription factors that regulate metabolism to adapt and acclimate to those conditions. To clarify the gene regulation systems activated in response to photooxidative stress, we isolated 76 high light and heat shock stress-inducible genes, including heat shock transcription factor (Hsf) A2 from Arabidopsis. Unlike yeast or animals, more than 20 genes encoding putative Hsfs are present in the genomes of higher plants, and they are categorized into three classes based on their structural characterization. However, the multiplicity of Hsfs in plants remains unknown. Furthermore, the individual functions of Hsfs are also largely unknown because of their genetic redundancy. Recently, the developments of T-DNA insertion knockout mutant lines and chimeric repressor gene-silencing technology have provided effective tools for exploring the individual functions of Hsfs. This review describes the current knowledge on the individual functions and activation mechanisms of Hsfs.
Graphical abstract
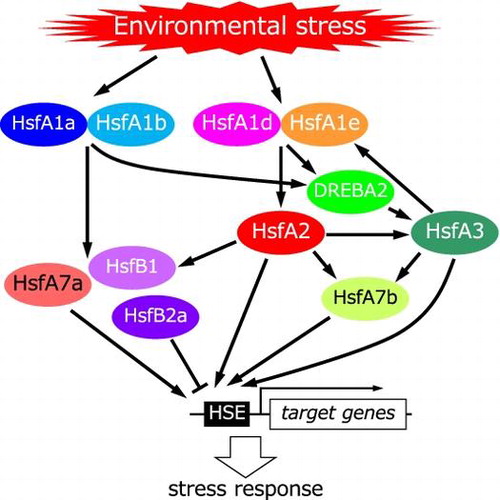
Model for heat shock transcription factor (Hsf) signaling via several Hsfs in response to environmental stress in Arabidopsis.
Plants as sessile organisms are continually exposed to various environmental stresses such as high light (HL), heat shock (HS) stresses, UV irradiation, drought, salt, and cold. Therefore, plants have developed complicated defense mechanisms to acclimate and to adapt to various types of environmental stresses.Citation1–3) Genome-wide transcriptome analyses clearly demonstrated that responses to various types of environmental stresses were activated by the induction or repression of many genes encoding proteins involved in numerous biological processes, indicating that transcription factors play crucial roles in regulating gene expression in these responses.Citation4–6)
At least 1533 transcription factors are encoded in the Arabidopsis genome.Citation7) Recently, it has been reported that 5.0 and 5.4% of the genes in monocots and eudicots encode transcription factors, respectively, on the basis of their genomic sequences.Citation8) These values are higher than the values in Drosophila melanogaster (4.7%) and Caenorhabditis elegans (3.6%), although it is comparable with those in humans (6.0%).Citation7) These findings suggest the possibility that plants, unlike animals, are unable to move and therefore have many transcription factors for acclimating and adapting to various environmental stresses. To understand the complicated molecular mechanism in response to environmental stresses, many researchers have explored the functions of numerous transcription factors and their target genes.Citation1–3)
Although light is essential for photosynthesis, excess light energy to CO2 fixation capacity can lead to a depression of photosynthetic efficiency because of the oxidative damage by reactive oxygen species (ROS), such as superoxide, hydrogen peroxide, singlet oxygen, and hydroxyl radicals.Citation9–11) Because various environmental stresses suppress CO2 fixation capacity by stomatal closure, the inhibition of the Calvin cycle, and other mechanisms, excess light energy easily leads to an over-reduction of photosynthetic electron transport and enhances the production of ROS. Thus, the light-dependent generation of ROS is termed photooxidative stress, and much of the injury to plants imposed by stress exposure is associated with oxidative damage at the cellular level.Citation9–11)
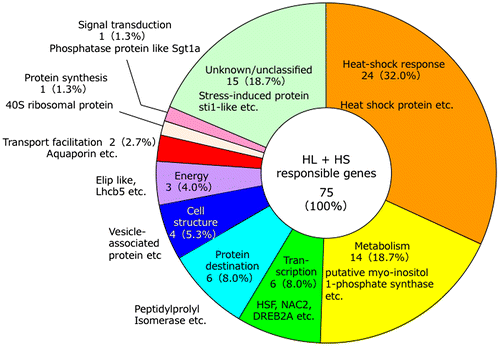
To clarify the gene regulation systems activated in response to photooxidative stresses, we isolated HL and HS (HL + HS) stress-inducible genes by suppression-subtractive hybridization from Arabidopsis.Citation12) HL + HS stress-inducible genes were captured through the construction of a subtractive cDNA library from mRNAs isolated from 2-week-old Arabidopsis plants exposed to a combination of HL + HS stress (800 μE m−2 sec−1, 40 °C) for 1 h. The subtracted cDNA clones (768 clones) were isolated and screened with reverse Northern analysis, then 611 positive clones were sequenced. The sequences of clones were evaluated by comparing them with the nucleotide sequences deposited in National Center for Biotechnology Information (NCBI) databases using the BLAST program, and as a result 76 genes were identified (Fig. ). Two genes encoding ROS-scavenging enzymes, namely glutathione S-transferase 6 and peroxiredoxin type 2B, were also induced under HL + HS stress conditions, suggesting that these proteins scavenge the elevated ROS under stress conditions. HL + HS stress-inducible genes included three transcription factors encoding dehydration-responsive element-binding protein (DREB) 2A, an NAC transcription factor designated ANAC078, and heat shock transcription factor (Hsf) A2. ANAC078 was found to participate in the regulation of flavonoid biosynthesis and 20S and 26S proteasomes in response to photooxidative stresses.Citation13–15) Furthermore, several genes encoding heat shock proteins (Hsps) were included in the HL + HS stress-inducible genes, suggesting that HsfA2 plays an important role in inducing these genes in response to photooxidative stresses.
Fig. 1. Annotation of high light (HL) + heat shock (HS)-responsive genes from Arabidopsis.
In eukaryotes, conserved Hsfs are key components in the signal transduction pathways involved in the induction of Hsps in response to several environmental stresses and chemical stressors.Citation16–19) Hsf acts through a highly conserved heat shock element (HSE; 5′-AGAAnnTTCT-3′) in the promoter region of HS stress-inducible genes in all eukaryotes.Citation18,19)
In yeast, the disruption of the Hsf gene is lethal even at normal growth temperatures.Citation20,21) Although the Hsf protein in D. melanogaster was dispensable for cell growth or viability, the protein was required for oogenesis and early larval development.Citation22) Mutation of the DNA-binding domain of human Hsf4 was revealed to be related to autosomal dominant lamellar cataract, indicating that Hsf4 plays a crucial role in lens development.Citation23) Xiao et al. reported that homozygous Hsf1 knockin mice can survive to adulthood.Citation24) However, these mice exhibit serious phenotypes such as defects of the chorioallantoic placenta and prenatal lethality, growth retardation, and female infertility. Although basal Hsp expression was not altered in homozygous Hsf1 knockin mice, HS stress responsive gene expression was not detected. These findings clearly illustrated that Hsfs in yeast and animals are important for both stress responses and development.
There are four and three Hsfs in mice and humans, respectively, only one Hsf each in D. melanogaster and C. elegans and one Hsf plus three Hsf-related proteins in yeast.Citation25–27) In contrast, 21, 24, 25, 30, and 52 open reading frames encoding Hsfs were identified in the Arabidopsis, tomato, rice, maize, and soybean genomes, respectively.Citation18,19) However, the individual functions of plants Hsfs are unknown because of their genetic redundancy.
Recently, the individual functions of Hsfs have gradually been disclosed because of the developments of T-DNA insertion knockout mutant lines and chimeric repressor gene-silencing technology, indicating that higher plants have developed complicated Hsf signaling networks composed of many Hsfs that cooperate in response to environmental stresses. This review focuses on recent studies concerning the individual functions and activation mechanisms of Hsfs in plants.
I. Structure of plant Hsfs
Plant Hsf family is assigned to three classes
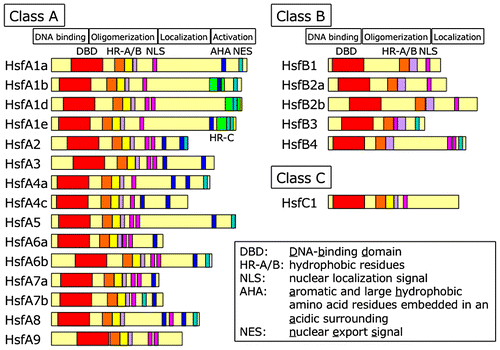
Hsfs contain a DNA-binding domain (DBD), oligomerization domain (OD or HR-A/B regions), nuclear localization signal, nuclear export signal, and activation motif (AHA motif). Based on these structural characteristics, plant Hsfs have been allocated into three major classes (classes A, B, and C) (Fig. ).Citation18) Furthermore, they have been subclassified into 16 groups (A1–9, B1–5, and C1–2).Citation19)
Only class A Hsfs contain AHA motifs, which are essential for transactivation.Citation18,19) Unlike class A Hsfs, class B and C Hsfs lack AHA motifs. Class B Hsfs contain a repression domain instead of an AHA motif (Fig. ).Citation28,29)
II. Physiological roles of plant Hsfs
Class A1 Hsfs act as master regulators in response to environmental stresses
The first report on the functional analysis of Hsfs in planta focused on Solanum lycopersicum (syn. Lycopersicon esculentum, tomato) HsfA1a.Citation16) In the SlHsfA1a co-suppressing tomato plants (CS-SlHsfA1a), the expression levels of Hsps were extremely decreased under normal conditions and HS stress, and these plants exhibited increased sensitivity to HS stress. Interestingly, the expression of SlHsfA2 and SlHsfB2 was also suppressed in the CS-SlHsfA1a plants. Based on these findings, Mishra et al. concluded that HsfA1 is the master regulator in response to HS stress.Citation16) Similar observations have been reported for the dominant-negative AtHsfA1a-expressing or AtHsfA1a and AtHsfA1b double-knockout Arabidopsis mutants (KO-AtHsfA1a/A1b), in which the induction of Hsps and Hsfs expression was suppressed during HS stress.Citation30,31) Furthermore, it has been reported that AtHsfA1a and AtHsfA1b regulate the expression not only of many Hsps but also of ascorbate peroxidase 2 (Apx2), and galactinol synthases (GolSs).Citation32–34) However, the KO-AtHsfA1a/A1b mutants did not exhibit a strong loss of thermotolerance. Because four members of class A1 Hsfs (AtHsfA1a, AtHsfA1b, AtHsfA1d, and AtHsfA1e) with remarkable homology are conserved in Arabidopsis, it appears likely that AtHsfA1d and AtHsfA1e complement the function of AtHsfA1a and AtHsfA1b in these mutants. Liu et al. generated one quadruple HsfA1 knockout (QK) and four triple knockout Arabidopsis mutants, of which the latter mutants contain only one intact HsfA1 (named aTK, bTK, dTK, and eTK, where the prefixed letters represent the remaining functional HsfA1).Citation35) The QK mutants displayed extremely decreased thermotolerance as well as remarkably altered morphologies and growth retardation. The expression of many HS stress-responsible genes, including Hsps and Hsfs, was significantly decreased in the QK mutants under HS stress. Indeed, the QK mutants also exhibited sensitivity to H2O2, mannitol, or NaCl treatments compared with wild-type plants. These findings strongly indicate that HsfA1s act as master regulators of genes expression in response to several environmental stresses, including photooxidative stresses. In addition, these findings revealed that HsfA1s are involved in growth and development under non-stress conditions.
Although the growth of aTK, bTK, dTK, and eTK mutants was similar to that of the wild-type plants, the eTK mutants displayed decreased thermotolerance compared with the aTK, bTK, and dTK mutants.Citation35) In the eTK mutants, but not in the aTK, bTK, and dTK mutants, the HS stress-inducible expression of DREB2A, which plays an important role in response to HS and osmotic stresses,Citation36–39) was suppressed.Citation35,39) Similar findings were obtained regarding the expression of some Hsps and class A Hsfs.Citation35,39) Although the expression level of AtHsfA1a, AtHsfA1b, and AtHsfA1d was not changed under normal and stress conditions, that of AtHsfA1e was increased under stress conditions.Citation12) These findings suggest that AtHsfA1a, AtHsfA1b, and AtHsfA1d activate in response to several types of environmental stresses in Arabidopsis plants.
HsfA2 functions as a signal enhancer of HsfA1s
HsfA2 is one of the most characterized Hsfs. Although the expression of AtHsfA2 was not detected under normal conditions in Arabidopsis, several types of environmental stresses, including HS, HL, oxidative stresses, and salinity, dramatically induced the expression of AtHsfA2.Citation12,40–43) The expression level of AtHsfA2 was higher than that of all class A Hsfs.Citation12) Furthermore, AtHsfA2 expression was rapidly induced within 30 min under HL stress.Citation12,44) Similar findings that HsfA2s expression was rapidly and strongly induced in response to HS stress in rice, maize, wheat, pepper, lily, and pondweeds have been reported.Citation45–51)
The overexpression of HsfA2 in many plant species conferred increased stress tolerance.Citation12,43,48,50) In contrast, AtHsfA2-knockout Arabidopsis mutants and Arabidopsis plants overexpressing the dominant-negative form of AtHsfA2 displayed decreased stress tolerance,Citation12,42,43) suggesting that AtHsfA2 is a key regulator in response to several types of environmental stresses. Moreover, AtHsfA2 is required for acquired thermotolerance and anoxia tolerance in Arabidopsis.Citation42,52)
AtHsfA2 regulates the expression of Hsps, Apx2, and GolSs, and their AtHsfA2-target genes are considerably overlapped with those of AtHsfA1s.Citation12,34,35,42,53,54) The expression of AtHsfA2 is regulated by AtHsfA1s.Citation35,39,44) In particular, we found that AtHsfA1d and AtHsfA1e are important for the induction of AtHsfA2 expression.Citation44) On the other hand, it has been reported that tomato SlHsfA1 and SlHsfA2 interact and form heterooligomers.Citation55,56) This interaction was illustrated to be essential for nuclear import of SlHsfA2 and the superactivation of the expression of HS stress-responsible genes.Citation55,57) These findings suggest that HsfA2 functions as a signal enhancer of HsfA1s.
The overexpression of AtHsfA2 in QK mutants (A2QK) rescued the developmental defects of the QK mutant, indicating that AtHsfA2 can compensate for the function of AtHsfA1s.Citation58) However, Liu and Charng reported that genes that are differentially regulated by AtHsfA1s or AtHsfA2 exist.Citation58) The genes involved in redox homeostasis were preferentially regulated by AtHsfA2, whereas AtHsfA1s-preferring genes were enriched in transcription function. It is interesting that these different gene regulation systems can induce such differences in gene expression.
HsfA3 functions in response to HS or osmotic stress depending on DREB
In tomato, SlHsfA3 is constitutively expressed under normal conditions or HS stress.Citation59) On the contrary, the expression of Arabidopsis AtHsfA3 was induced under HS or drought stresses.Citation36–38) In Arabidopsis, the expression of AtHsfA3 was regulated by AtDREB2A.Citation36–38) The expression of AtDREB2A was rapidly induced by HS stress within 30 min, after which it drastically decreased.Citation38) The expression of AtDREB2A was also gradually induced by drought and salt stresses. Interestingly, AtHsfA1s regulated HS stress-inducible AtDREB2A expression but not its drought-inducible expression.Citation39) AtHsfA3 also regulates many Hsps, and its deficiency leads to decreased thermotolerance.Citation37,38) Furthermore, AtDREB2C was also involved in the regulation of AtHsfA3.Citation60) Thus, the expression of AtHsfA3 is regulated by AtDREB2A and/or AtDREB2C, and AtHsfA3 functions to induce the expression of several genes in response to HS or osmotic stresses.
The expression of AtHsfA3 is induced later than that of AtHsfA2, suggesting the possibility that AtHsfA3 functions to induce the expression of many Hsps during prolonged HS stress or recovery from HS stress.Citation12)
HsfA4s have various functions and HsfA5 acts as a specific repressor of HsfA4
A rice spl7 mutant displayed spontaneous lesions under a natural summer field condition, and spl7 encoded OsHsfA4d with a Trp to Cys transition in ß1 strand of DBD.Citation61) Although the effects of this transition on the transactivation or DNA-binding activity of OsHsfA4d was not analyzed, the spl7 phenotype was complemented by transformation of Spl7, suggesting the possibility that spl4 was inactive as an Hsf. Therefore, such lesion development in the spl4 mutants was caused by increased stress sensitivity. These findings suggest that OsHsfA4d plays an important role in protecting against HS or HL stress or UV radiation in rice.
In the cadmium (Cd)-hypersensitive yeast, the expression of wheat (Triticum aestivum) TaHsfA4a improved Cd tolerance.Citation62) OsHsfA4a also improved Cd tolerance in yeast, but OsHsfA4d did not have such an effect. Interestingly, an analysis of TaHsfA4a via domain swapping with OsHsfA4d revealed that two amino acid residues (Ala31 and Leu42) in the DBD of TaHsfA4a were critical for Cd tolerance in yeast. In rice plants, the overexpression of TaHsfA4a improved Cd tolerance and upregulated metallothionein expression, which acts as a chelator of Cd and other heavy metals. Moreover, OsHsfA4a-knockout rice mutants displayed Cd hypersensitivity.Citation62) These finding suggests that HsfA4a plays an important role in responding to Cd stress and that two amino acids in the DBD of HsfA4a are involved in this mechanism. However, AtHsfA4a or AtHsfA4c did not improve Cd tolerance in yeast.Citation62) The function of Hsf4a as a Cd tolerance factor is limited to only monocots.
In the AtApx1 (key enzyme for ROS metabolism)-knockout Arabidopsis mutants (KO-AtApx1), the expression of AtHsfA4a was constitutively and markedly induced under normal conditions and photooxidative stress.Citation63,64) The expression of AtApx1 was regulated by the zinc finger protein Zat12, the expression of which was induced under photooxidative stresses.Citation65) The overexpression of the dominant-negative form of AtHsfA4a suppressed the induction of Zat12 expression.Citation64) Based on these findings, Davletova et al. demonstrated that AtHsfA4a acts as a redox sensor.Citation64)
Arabidopsis root handedness 1 (rha1) is a null mutant for AtHsfA4c, and its roots exhibited minimal right-handed slanting, decreased gravitropic response, and notable resistance to 2,4-dichlorophenoxy-acetic acid but scarce resistance to indole-3-acetic acid and naphthaleneacetic acid.Citation66) The roots of rha1 mutants also displayed clear resistance to auxin transport inhibitors and ethylene. However, the mechanism by which AtHsfA4c is involved in this phenotype is unclear.
Thus, HsfA4s regulates various metabolic pathways and stress response. Interestingly, it has been reported that AtHsfA5 acts as a specific repressor of the transactivation activity of AtHsfA4a or AtHsfA4c.Citation67) The oligomerization domain of AtHsfA5 alone was required and sufficient to repress the transactivation activity of AtHsfA4s. Therefore, it appears likely that HsfA5 participates in the regulation of transactivation activity of HsfA4s. Further analyses are required to clarify the role of HsfA5 in the regulation of metabolic pathways and stress responses by HsfA4s.
HsfA6 functions in response to drought, salt, and osmotic stresses via abscisic acid (ABA) signaling
The expression of Arabidopsis AtHsfA6a was induced under drought and salt stresses.Citation41) Recently, AtHsfA6a was demonstrated to induce stress-responsible genes under salt or drought stresses via the ABA-dependent signaling pathway.Citation68) The expression of AtHsfA6a was regulated by ABA-responsive element binding proteins (AREBs). In particular, AREB3 most strongly activated the AtHsfA6a promoter among AREBs. In the AtHsfA6a-overexpressing Arabidopsis plants, the expression of many genes was altered. Most of these genes were responsive to abiotic stresses, including drought, salt, and cold stresses. Hwang et al. reported that VOZ1, a negative regulator of ABA signaling, interacted with AtHsfA6a.Citation68) However, the effect of this interaction on the expression of AtHsfA6a-target genes was not explored.
HsfA9 is involved in embryogenesis and seed development
It has been reported that HsfA9 was specifically expressed during embryogenesis and seed development in sunflower (Helianthus annuus) and Arabidopsis.Citation69,70) Arabidopsis AtHsfA9 was found to regulate the expression of small Hsps and Hsp101.Citation69) Sunflower HaHsfA9 was not detected in the seedlings, leaves, and stems of sunflower under normal or stress conditions.Citation70) Similar observations have been reported for Arabidopsis plants.Citation69) Seed-specific overexpression of HaHsfA9 in tobacco plants enhanced the accumulation of Hsps in seeds and improved seed longevity.Citation71) Recently, it was reported that the overexpression of grape (Vitis vinifera) VvHsfA9 in Arabidopsis plants increased seed germination under normal conditions.Citation72) These findings suggest that HsfA9 plays an important role in embryogenesis and seed development. Seed-specific overexpression of its dominant-negative form (HaHsfA9-SRDX) in tobacco plants decreased the accumulation of Hsps and seed longevity.Citation73) However, negative effects on seed development and germination were not observed in the dominant-negative lines, suggesting the possibility that the function of HsfA9 is not essential for seed development.Citation19) Scharf et al. provided interesting information that monocots (O. sativa, Brachypodium distachyon, Sorghum bicolor, and Zea mays) lack HsfA9 and that the transcript level of rice OsHsfA1a was extremely high in seeds.Citation19) These lines of evidence suggest the possibility that HsfA1 functions instead of HsfA9 in monocots seeds.
HaHsfA9 interacts with HaIAA27, an auxin/indole acetic acid protein, and HaIAA27 represses the transactivation activity of HaHsfA9.Citation74) Meanwhile, the expression of AtHsfA9 is regulated by the seed-specific transcription factor ABA-Insensitive3.Citation69) These findings suggest that the auxin and ABA signaling is involved in the regulation of embryogenesis and seed development via HsfA9.
HaHsfA9 also interacts with HaHsfA4a, and their co-overexpression synergistically activates the HaHsp17.7 G4 promoter in sunflower embryos and leaves.Citation75) Moreover, tobacco plants co-overexpressing these genes exhibited enhanced seed longevity and synergistic effects on seedling tolerance to severe dehydration and oxidative stresses compared with plants overexpressing either gene alone.Citation76) Interestingly, this synergic effect of HaHsfA9 and HaHsfA4 was canceled by the co-expression of HaIAA27.Citation75) It has been reported that HaHsfA9 also interacts with HaDREB2 and that their co-overexpression in tobacco plants confirmed enhanced basal thermotolerance and seed longevity, although the overexpression of HaDREB2 had no effect on basal thermotolerance.Citation77) However, the detailed molecular mechanisms of these synergistic effects remain unknown.
Functions of Hsf7 and HsfA8
Because the expression of Arabidopsis AtHsfA8 was induced similarly as that of AtHsfA4a in the KO-AtApx1 mutants, it appears likely that AtHsfA8 also acts as a redox sensor.Citation65) Most recently, Giesguth et al. reported that AtHsfA8 may function as a redox sensor in plants.Citation78) The AtHsfA8-YFP fusion protein was localized in the cytosol of Arabidopsis protoplasts under reducing conditions, whereas the protein translocated to the nucleus under an oxidizing condition. Site-directed mutagenesis of Cys24 and/or Cys269 blocked the translocation of AtHsfA8-YFP protein to the nucleus. These findings suggest the possibility that disulfide bond formation between Cys24 and Cys269 regulates the partitioning of AtHsfA8 between the cytosol and nucleus. It is noteworthy that these Cys residues were not conserved in monocots and that Cys269 is conserved in only Brassicaceae.
In Arabidopsis, the expression of AtHsfA7a and AtHsfA7b was strongly induced by HS stressCitation12,79) and were regulated by HsfA1s,Citation39,44) suggesting the possibility that AtHsfA7a and AtHsfA7b have similar functions as HsfA2. However, little is known about the function of HsfA7s in plants.
Class B Hsfs act as repressors
Although class B Hsfs lack AHA motifs, all Arabidopsis class B Hsfs were localized in the nucleus under normal conditions, and they exhibited no activator function in tobacco protoplasts and yeast cells.Citation17) It has been reported that class B Hsfs participate in the repression or downregulation of HS stress responses.Citation80) Ikeda and Ohme-Takagi reported that Arabidopsis AtHsfB1 contain an R/KLFGV motif, which acts as a transcriptional repressor.Citation28) This motif is conserved in the orthologs of class B Hsfs, excluding HsfB5, in various plants.Citation19,28) The overexpression of AtHsfB1 in Arabidopsis plants suppresses the induction of HS stress-inducible genes compared with their expression in the wild-type plants under HS stress.Citation29) The expression of many HS stress-inducible genes in AtHsfB1 and AtHsfb2b double-knockout Arabidopsis plants (KO-AtHsfB1/B2b) was upregulated compared with that in the wild-type plants under normal conditions.Citation29) Furthermore, the KO-AtHsfB1/B2b plants displayed increased basal thermotolerance compared with the wild-type plants. However, the acquired thermotolerance in the KO-AtHsfB1/B2b plants was lower than that in the wild-type plants. The expression of most class B Hsfs in Arabidopsis was induced under several types of environmental stresses.Citation28,41) Based on these findings, Ikeda et al. proposed a model of class B Hsf function in the HS stress response.Citation29) Under normal conditions, AtHsfB1 and AtHsfB2b suppress the expression of stress-inducible Hsfs (AtHsfA2, AtHsfA7a, AtHsfB1, and AtHsfB2b) and some Hsps. When plants encounter HS stress, the expression of AtHsfB1 and AtHsfB2b is induced. During the attenuation of the HS stress response, AtHsfB1 and AtHsfB2b again suppress the induced expression of AtHsfA2 and Hsps. It appears likely that class B Hsfs play an important role in attenuating the stress response.
Furthermore, it has been reported that AtHsfB1 and AtHsfB2b negatively regulated the expression of defensin genes (AtPdf1.2a and AtPdf1.2b), and their Arabidopsis mutants displayed increased disease resistance compared with that of the wild-type plants.Citation81) These findings suggest that AtHsfB1 and AtHsfB2b also regulate the disease response. It is noteworthy that Pdf1.2a and Pdf1.2b are HS stress-inducible genes.Citation19)
On the contrary, tomato SlHsfB1 was revealed to function as a coactivator cooperating with class A Hsfs.Citation82) SlHsfA1 and SlHsfB1 assembled into an enhanceosome-like complex, resulting in strong synergistic activation of reporter and exogenous gene expression. Furthermore, SlHsfB1 activated the expression of housekeeping genes.Citation82)
Recently, it was reported that Arabidopsis AtHsfB4 is involved in root developmentCitation83) and that AtHsfB2b regulated the control of the circadian clock through the repression of Pseudo Response Regulator 7, a component of the circadian clock.Citation84)
Physiological function of class C Hsfs
Although the widespread occurrence of class C Hsfs in plants suggests possibility that class C Hsfs may serves an important role in plants, the function of class C Hsfs remains largely unknown. Kotak et al. reported that Arabidopsis AtHsfC1 is located in the nucleus.Citation17) Recently, the functional analyses of rice OsHsfC1b were demonstrated.Citation85) The expression of OsHsfC1b was induced by salt, mannitol, and ABA treatments, but not by H2O2 treatment. Knockout and knockdown OsHsfC1b rice mutants exhibited decreased tolerance to salt and osmotic stresses and increased sensitivity to ABA. The growth of knockout and knockdown OsHsfC1b rice mutants was retarded compared with that of control plants under normal conditions. It appears likely that OsHsfC1b has an important role in the response to salt and osmotic stresses and in the regulation of development. Interestingly, the expression of salt-responsive genes involved in signaling and ion homeostasis was altered in the knockout and knockdown OsHsfC1b rice mutants compared with that in the wild-type plants. These findings suggest the possibility that OsHsfC1b regulates the expression of these genes via direct or an indirect machinery. The transcript levels of some small Hsps in these mutants were upregulated under salt stress compared with those in the wild-type plants, suggesting that OsHsfC1b repress the expression of small Hsps under normal and stress conditions. However, many researchers have reported that the overexpression of small Hsps leads to enhanced tolerance to several types of stresses, including salt and osmotic stresses.Citation86–89) Further studies are needed to clarify the physiological function of class C Hsfs.
III. Hsf signaling network and activation mechanism of HsfA1s
As previously described, HsfA1s trigger in response to several types of environmental stresses, and HsfA2 amplify the signal from HsfA1s. AtHsfA1s also regulate the induction of AtHsfA7a, AtHsfA7b, AtHsfB1, and AtHsfB2a expression under stresses in Arabidopsis plants.Citation35,39,44) The expression of AtHsfA3 is regulated by AtDREB2A through AtHsfA1s.Citation39) AtHsfA3 regulates the expression of AtHsfA1e,Citation38) and AtHsfA2 induces the AtHsfA3 expression.Citation43) Based on the data reported till date, we have proposed a model of Hsf signaling under various environmental stresses (Fig. ). Thus, higher plants appeared to have developed a complicated Hsf signaling network to respond and adapt to various environmental stresses, and HsfA1s play pivotal roles in responses to environmental stress.
Fig. 3. Model for heat shock transcription factor (Hsf) signaling via several Hsfs in response to environmental stress in Arabidopsis.
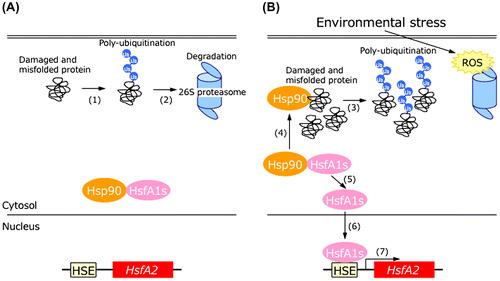
The activation or inactivation mechanisms of human Hsf1 are well studied. These mechanisms include phosphorylation, sumoylation, and acetylation of Hsf1 and interactions with Hsp90 or Hsp70. Treatment of Arabidopsis plants with geldanamycin (GDA) or radicicol, inhibitors of Hsp90, induced the expression of HS stress-inducible Hsps in the absence of stress.Citation90) AtHsp90 was revealed to interact with AtHsfA1a, AtHsfA1b, and AtHsfA1d.Citation39,90) In addition to GDA, we found that treatment with MG132, a 26S proteasome inhibitor, induced the expression of AtHsfA2, AtHsp18.1-CI, and AtApx2 in Arabidopsis.Citation91) Interestingly, the levels of polyubiquitinated proteins as well as those of AtHsfA2 transcripts were rapidly increased under oxidative stress, whereas they were completely suppressed by pre-treatment with ascorbate, a scavenger of ROS, under oxidative stress.Citation91) These findings suggest that cytosolic Hsp90 activity and 26S proteasome function are involved in the activation of HsfA1s under stress conditions.
Generally, it is well known that ROS are important signaling molecules for regulating plant responses to abiotic and biotic stresses.Citation63–65,92,93) Volkov et al. demonstrated that the induction of small Hsps and Apxs expression in Arabidopsis cell suspension cultures during HS stress was required to increase intracellular ROS levels.Citation94) However, we found that treatment with MG132 or GDA did not cause an increase of intracellular ROS levels and that the induction of AtHsfA2 by these treatments was not suppressed by ascorbate treatment.Citation91) Taken together, we demonstrated the signal transduction pathway involved in the regulation of AtHsfA2 expression (Fig. ).Citation91) Under normal conditions, HsfA1s are constitutively expressed as inactive forms bound to Hsp90 in the cytosol, and the damaged and misfolded proteins are polyubiquitinated (step 1) and then degraded by the 26S proteasome (step 2). However, the accumulation of intracellular ROS under environmental stresses causes the accumulation of damaged and misfolded proteins and polyubiquitinated proteins as a result of inhibition of 26S proteasome activity (step 3). Because the accumulated misfolded and damaged proteins sequester Hsp90 (step 4), the HsfA1s-Hsp90 complex dissociates and releases HsfA1s (step 5). Then, free HsfA1s released from Hsp90 induces the expression of target genes, including HsfA2 (steps 6 and 7). HsfA2 induces the expression of Hsps and several defense genes to adapt or acclimate to stress. To attenuate the stress response, the induced class B Hsfs act as suppressers of several defense genes. Thus, the induction of several genes is achieved via a complicated Hsf signaling network in response to several types of environmental stresses.
Fig. 4. A model of the signal transduction pathway involved in the regulation of HsfA2 expression under normal conditions (A) or oxidative stress (B) in Arabidopsis.
It has been reported that Hsfs interact with other classes of Hsfs or other proteins such as Hsf-binding proteinCitation95,96) and calmodulin-binding protein kinase 3.Citation97) These interactions change the transcriptional activity of Hsfs. Furthermore, it has been reported that the transcriptional activity of AtHsfA2 was regulated by sumoylation.Citation98) Jung et al. reported that the translocation of AtHsfA1d from the cytoplasm to the nucleus regulated the induction of AtApx2 expression during HL irradiation and that the disulfide bound between Cys153 and Cys357 in AtHsfA1d is critical for the transcriptional activity of AtHsfA1d.Citation99) Liu et al. (2013) revealed that AtHsfA1a directly sensed HS stress, pH changes, and hydrogen peroxide via a change of its redox status and that its HSE binding activity was activated by these stresses.Citation100) However, such modifications mechanisms of plant Hsfs are not fully clear. To understand the regulatory system in response to environmental stress in plants, it is an important to clarify the activation mechanism of Hsfs.
IV. Conclusion and future perspectives
Within the past 20 years, the individual functions of plants Hsfs have been elucidated. Although many Hsfs regulate the expression of common genes, individual Hsfs have unique functions as parts of different signal transduction pathways operating in response to environmental stress and during development. Furthermore, higher plants have developed Hsf signaling networks composed of many Hsfs that cooperate in response to environmental stresses. It is likely that the acquisition of such complicated gene regulation systems is one of the primary factors permitting plants to adapt and acclimate to various environmental stresses. However, many of these analyses focused on HS stress responses, and there is limited information about Hsf signaling systems in photooxidative stress responses. Moreover, the molecular mechanisms involved in the activation of HsfA1s are largely unknown. Further analyses are required to clarify whether the activation of Hsfs is regulated by ROS or individual stress-specific molecular mechanisms.
Funding
This work was supported by JSPS KAKENHI [grant number 21780310], [grant number 24780331] and [grant number 26450503].
Acknowledgments
I express my sincere gratitude to Professor Shigeru Shigeoka (Kinki University), Professor Fumio Watanabe (Tottori University), Emeritus Professor Akiho Yokota (Nara Institute of Science and Technology), Emeritus Professor Akira Wadano (Osaka Prefecture University), and Emeritus Professor Yoshihisa Nakano (Osaka Prefecture University) for their encouragement, support, and discussion. I am deeply grateful to Dr Ayako Nishizawa-Yokoi, Dr Takanori Maruta, Dr Teruyuki Morishita, Dr Kazuya Yoshimura, Dr Masahiro Tamoi, Dr Takahisa Ogawa, Dr Noriaki Tanabe, and former and current co-workers in our laboratory for their help and cooperation. I would also like to express thanks to the Japanese Society for Bioscience, Biotechnology, and Agrochemistry Society Award for the Encouragement of Young Scientists.
Disclosure statement
No potential conflict of interest was reported by the author.
Notes
This review was written in response to the author’s receipt of the JSBBA Award for Young Scientists in 2015.
References
- Kreps JA, Wu Y, Chang HS, et al. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002;130:2129–2141.10.1104/pp.008532
- Rossel JB, Wilson IW, Pogson BJ. Global changes in gene expression in response to high light in Arabidopsis. Plant Physiol. 2002;130:1109–1120.10.1104/pp.005595
- Seki M, Narusaka M, Ishida J, et al. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA micro array. Plant J. 2002;31:279–292.10.1046/j.1365-313X.2002.01359.x
- Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006;57:781–803.10.1146/annurev.arplant.57.032905.105444
- Nakashima K, Takasaki H, Mizoi J, et al. NAC transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta. 2012;1819:97–103.10.1016/j.bbagrm.2011.10.005
- Hua J. From freezing to scorching, transcriptional responses to temperature variations in plants. Curr. Opin. Plant Biol. 2009;12:568–573.10.1016/j.pbi.2009.07.012
- Riechmann JL, Heard J, Martin G, et al. Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science. 2000;290:2105–2110.10.1126/science.290.5499.2105
- Jin J, Zhang H, Kong L, et al. PlantTFDB 3.0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res. 2014;42:D1182–D1187.10.1093/nar/gkt1016
- Foyer CH, Lelandais M, Kunert kJ. Photooxidative stress in plants. Physiol. Plant. 1994;92:696–717.10.1111/ppl.1994.92.issue-4
- Alscher RG, Donahue JL, Cramer CL. Reactive oxygen species and antioxidants: relationships in green cells. Physiol. Plant. 1997;100:224–233.10.1111/ppl.1997.100.issue-2
- Shigeoka S, Ishikawa T, Tamoi M, et al. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002;53:1305–1319.10.1093/jexbot/53.372.1305
- Nishizawa A, Yabuta Y, Yoshida E, et al. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 2006;48:535–547.10.1111/tpj.2006.48.issue-4
- Morishita T, Kojima Y, Maruta T, et al. Arabidopsis NAC transcription factor, ANAC078, regulates flavonoid biosynthesis under high-light. Plant Cell Physiol. 2009;50:2210–2222.10.1093/pcp/pcp159
- Yabuta Y, Morishita T, Kojima Y, et al. Identification of recognition sequence of ANAC078 protein by the cyclic amplification and selection of targets technique. Plant Signal. Behav. 2010;5:695–697.10.4161/psb.5.6.11577
- Yabuta Y, Osada R, Morishita T, et al. Involvement of Arabidopsis NAC transcription factor in the regulation of 20S and 26S proteasomes. Plant Sci. 2011;181:421–427.10.1016/j.plantsci.2011.07.001
- Mishra SK, Tripp J, Winkelhaus S, et al. In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev. 2002;16:1555–1567.10.1101/gad.228802
- Kotak S, Port M, Ganguli A, et al. Characterization of C-terminal domains of Arabidopsis heat stress transcription factors (Hsfs) and identification of a new signature combination of plant class A Hsfs with AHA and NES motifs essential for activator function and intracellular localization. Plant J. 2004;39:98–112.10.1111/tpj.2004.39.issue-1
- Nover L, Bharti K, Döring P, et al. Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need? Cell Stress Chaperones. 2001;6:177–189.10.1379/1466-1268(2001)006<0177:AATHST>2.0.CO;2
- Scharf KD, Berberich T, Ebersberger I, et al. The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim. Biophys. Acta. 2012;1819:104–119.10.1016/j.bbagrm.2011.10.002
- Wiederrecht G, Seto D, Parker CS. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell. 1988;54:841–853.10.1016/S0092-8674(88)91197-X
- Sorger PK, Pelham HR. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988;54:855–864.10.1016/S0092-8674(88)91219-6
- Jedlicka P, Mortin MA, Wu C. Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J. 1997;16:2452–2462.10.1093/emboj/16.9.2452
- Bu L, Jin Y, Shi Y, et al. Mutant DNA-binding domain of HSF4 is associated with autosomal dominant lamellar and Marner cataract. Nat. Genet. 2002;31:276–278.10.1038/ng921
- Xiao X, Zuo X, Davis AA, et al. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18:5943–5952.10.1093/emboj/18.21.5943
- Nakai A. New aspects in the vertebrate heat shock factor system: Hsf3 and Hsf4. Cell Stress Chaperones. 1999;4:86–93.10.1379/1466-1268(1999)004<0086:NAITVH>2.3.CO;2
- Nover L, Scharf KD, Gagliardi D, et al. The Hsf world: classification and properties of plant heat stress transcription factors. Cell Stress Chaperones. 1996;1:215–223.10.1379/1466-1268(1996)001<0215:THWCAP>2.3.CO;2
- Fujimoto M, Nakai A. The heat shock factor family and adaptation to proteotoxic stress. FEBS J. 2010;277:4112–4125.10.1111/j.1742-4658.2010.07827.x
- Ikeda M, Ohme-Takagi M. A novel group of transcriptional repressors in Arabidopsis. Plant Cell Physiol. 2009;50:970–975.10.1093/pcp/pcp048
- Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance. Plant Physiol. 2011;157:1243–1254.10.1104/pp.111.179036
- Wunderlich M, Werr W, Schöffl F. Generation of dominant-negative effects on the heat shock response in Arabidopsis thaliana by transgenic expression of a chimaeric HSF1 protein fusion construct. Plant J. 2003;35:442–451.10.1046/j.1365-313X.2003.01815.x
- Lohmann C, Eggers-Schumacher G, Wunderlich M, et al. Two different heat shock transcription factors regulate immediate early expression of stress genes in Arabidopsis. Mol. Genet. Genomics. 2004;271:11–21.10.1007/s00438-003-0954-8
- Panchuk II, Volkov RA, Schöffl F. Heat stress-and heat shock transcription factor-dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol. 2002;129:838–853.10.1104/pp.001362
- Panikulangara TJ, Eggers-Schumacher G, Wunderlich M, et al. Galactinol synthase1. a novel heat shock factor target gene responsible for heat-induced synthesis of raffinose family oligosaccharides in Arabidopsis. Plant Physiol. 2004;136:3148–3158.10.1104/pp.104.042606
- Busch W, Wunderlich M, Schöffl F. Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J. 2005;41:1–14.
- Liu HC, Liao HT, Charng YY. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 2011;34:738–751.10.1111/pce.2011.34.issue-5
- Sakuma Y, Maruyama K, Qin F, et al. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc. Natl. Acad. Sci. USA. 2006;103:18822–18827.10.1073/pnas.0605639103
- Schramm F, Larkindale J, Kiehlmann E, et al. A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. Plant J. 2008;53:264–274.
- Yoshida T, Sakuma Y, Todaka D, et al. Functional analysis of an Arabidopsis heat-shock transcription factor HsfA3 in the transcriptional cascade downstream of the DREB2A stress-regulatory system. Biochem. Biophys. Res. Commun. 2008;368:515–521.10.1016/j.bbrc.2008.01.134
- Yoshida T, Ohama N, Nakajima J, et al. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol. Genet. Genomics. 2011;286:321–332.10.1007/s00438-011-0647-7
- Schramm F, Ganguli A, Kiehlmann E, et al. The heat stress transcription factor HsfA2 serves as a regulatory amplifier of a subset of genes in the heat stress response in Arabidopsis. Plant Mol. Biol. 2006;60:759–772.10.1007/s11103-005-5750-x
- Miller G, Mittler R. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 2006;98:279–288.10.1093/aob/mcl107
- Charng YY, Liu HC, Liu NY, et al. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 2007;143:251–262.
- Ogawa D, Yamaguchi K, Nishiuchi T. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J. Exp. Bot. 2007;58:3373–3383.10.1093/jxb/erm184
- Nishizawa-Yokoi A, Nosaka R, Hayashi H, et al. HsfA1d and HsfA1e involved in the transcriptional regulation of HsfA2 function as key regulators for the Hsf signaling network in response to environmental stress. Plant Cell Physiol. 2011;52:933–945.10.1093/pcp/pcr045
- Yokotani N, Ichikawa T, Kondou Y, et al. Expression of rice heat stress transcription factor OsHsfA2e enhances tolerance to environmental stresses in transgenic Arabidopsis. Planta. 2008;227:957–967.10.1007/s00425-007-0670-4
- Chauhan H, Khurana N, Agarwal P, et al. Heat shock factors in rice (Oryza sativa L.): genome-wide expression analysis during reproductive development and abiotic stress. Mol. Genet. Genomics. 2011;286:171–187.10.1007/s00438-011-0638-8
- Lin YX, Jiang HY, Chu ZX, et al. Genome-wide identification, classification and analysis of heat shock transcription factor family in maize. BMC Genomics. 2011;12:76.10.1186/1471-2164-12-76
- Chauhan H, Khurana N, Agarwal P, et al. A seed preferential heat shock transcription factor from wheat provides abiotic stress tolerance and yield enhancement in transgenic Arabidopsis under heat stress environment. PLoS ONE. 2013;8:e79577.10.1371/journal.pone.0079577
- Guo M, Lu JP, Zhai YF, et al. Genome-wide analysis, expression profile of heat shock factor gene family (CaHsfs) and characterisation of CaHsfA2 in pepper (Capsicum annuum L.). BMC Plant Biol. 2015;15:151.10.1186/s12870-015-0512-7
- Gong B, Yi J, Wu J, et al. LlHSFA1, a novel heat stress transcription factor in lily (Lilium longiflorum), can interact with LlHSFA2 and enhance the thermotolerance of transgenic Arabidopsis thaliana. Plant Cell Rep. 2014;33:1519–1533.10.1007/s00299-014-1635-2
- Amano M, Iida S, Kosuge K. Comparative studies of thermotolerance: different modes of heat acclimation between tolerant and intolerant aquatic plants of the genus Potamogeton. Ann. Bot. 2012;109:443–452.10.1093/aob/mcr300
- Banti V, Mafessoni F, Loreti E, et al. The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant Physiol. 2010;152:1471–1483.10.1104/pp.109.149815
- Nishizawa A, Yabuta Y, Shigeoka S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008;147:1251–1263.10.1104/pp.108.122465
- Nishizawa-YOKOI A, Yoshida E, Yabuta Y, et al. Analysis of the regulation of target genes by an Arabidopsis heat shock transcription factor, HsfA2. Biosci. Biotechnol. Biochem. 2009;73:890–895.10.1271/bbb.80809
- Scharf KD, Heider H, Höhfeld I, et al. The tomato Hsf System: HsfA2 needs interaction with HsfA1 for efficient nuclear import and may be localized in cytoplasmic heat stress granules. Mol. Cell Biol. 1998;18:2240–2251.10.1128/MCB.18.4.2240
- Heerklotz D, Döring P, Bonzelius F, et al. The balance of nuclear import and export determines the intracellular distribution and function of tomato heat stress transcription factor HsfA2. Mol. Cell Biol. 2001;21:1759–1768.10.1128/MCB.21.5.1759-1768.2001
- Chan-Schaminet KY, Baniwal SK, Bublak D, et al. Specific interaction between tomato HsfA1 and HsfA2 creates hetero-oligomeric superactivator complexes for synergistic activation of heat stress gene expression. J Biol. Chem. 2009;284:20848–20857.10.1074/jbc.M109.007336
- Liu HC, Charng YY. Common and distinct functions of Arabidopsis class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant Physiol. 2013;163:276–290.10.1104/pp.113.221168
- Bharti K, Schmidt E, Lyck R, et al. Isolation and characterization of HsfA3, a new heat stress transcription factor of Lycopersicon peruvianum. Plant J. 2000;22:355–365.10.1046/j.1365-313x.2000.00746.x
- Chen H, Hwang JE, Lim CJ, et al. Arabidopsis DREB2C functions as a transcriptional activator of HsfA3 during the heat stress response. Biochem. Biophys. Res. Commun. 2010;401:238–244.10.1016/j.bbrc.2010.09.038
- Yamanouchi U, Yano M, Lin H, et al. A rice spotted leaf gene, Spl7, encodes a heat stress transcription factor protein. Proc. Natl. Acad. Sci. USA. 2002;99:7530–7535.10.1073/pnas.112209199
- Shim D, Hwang JU, Lee J, et al. Orthologs of the class A4 heat shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. Plant Cell. 2009;21:4031–4043.10.1105/tpc.109.066902
- Pnueli L, Liang H, Rozenberg M, et al. Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J. 2003;34:187–203.10.1046/j.1365-313X.2003.01715.x
- Davletova S, Rizhsky L, Liang H, et al. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell. 2005;17:268–281.10.1105/tpc.104.026971
- Rizhsky L, Davletova S, Liang H, et al. The zinc finger protein Zat12 Is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J. Biol. Chem. 2004;279:11736–11743.10.1074/jbc.M313350200
- Fortunati A, Piconese S, Tassone P, et al. A new mutant of Arabidopsis disturbed in its roots, right-handed slanting, and gravitropism defines a gene that encodes a heat-shock factor. J. Exp. Bot. 2008;59:1363–1374.10.1093/jxb/ern047
- Baniwal SK, Chan KY, Scharf KD, et al. Role of heat stress factor HsfA5 as specific repressor of HsfA4. J. Biol. Chem. 2007;282:3605–3613.
- Hwang SM, Kim DW, Woo MS, et al. Functional characterization of Arabidopsis HsfA6a as a heat-shock transcription factor under high salinity and dehydration conditions. Plant Cell Environ. 2014;37:1202–1222.10.1111/pce.12228
- Kotak S, Vierling E, Bäumlein H, et al. A novel transcriptional cascade regulating expression of heat stress proteins during seed development of Arabidopsis. Plant Cell. 2007;19:182–195.10.1105/tpc.106.048165
- Almoguera C, Rojas A, Díaz-Martín J, et al. A seed-specific heat-shock transcription factor involved in developmental regulation during embryogenesis in sunflower. J. Biol. Chem. 2002;277:43866–43872.10.1074/jbc.M207330200
- Prieto-Dapena P, Castaño R, Almoguera C, et al. Improved resistance to controlled deterioration in transgenic seeds. Plant Physiol. 2006;142:1102–1112.10.1104/pp.106.087817
- Li Z, Tian Y, Zhao W, et al. Functional characterization of a grape heat stress transcription factor VvHsfA9 in transgenic Arabidopsis. Acta Physiol. Plant. 2015;37:133.10.1007/s11738-015-1884-x
- Tejedor-Cano J, Prieto-Dapena P, Almoguera C, et al. Loss of function of the HSFA9 seed longevity program. Plant Cell Environ. 2010;33:1408–1417.
- Carranco R, Espinosa JM, Prieto-Dapena P, et al. Repression by an auxin/indole acetic acid protein connects auxin signaling with heat shock factor-mediated seed longevity. Proc. Natl. Acad. Sci. USA. 2010;107:21908–21913.10.1073/pnas.1014856107
- Tejedor-Cano J, Carranco R, Personat JM, et al. A passive repression mechanism that hinders synergic transcriptional activation by heat shock factors involved in sunflower seed longevity. Mol. Plant. 2014;7:256–259.10.1093/mp/sst117
- Personat JM, Tejedor-Cano J, Prieto-Dapena P, et al. Co-overexpression of two heat shock factors results in enhanced seed longevity and in synergistic effects on seedling tolerance to severe dehydration and oxidative stress. BMC Plant Biol. 2014;14:56.10.1186/1471-2229-14-56
- Almoguera C, Prieto-Dapena P, Díaz-Martín J, et al. The HaDREB2 transcription factor enhances basal thermotolerance and longevity of seeds through functional interaction with HaHSFA9. BMC Plant Biol. 2009;9:75.10.1186/1471-2229-9-75
- Giesguth M, Sahm A, Simon S, et al. Redox-dependent translocation of the heat shock transcription factor AtHSFA8 from the cytosol to the nucleus in Arabidopsis thaliana. FEBS Lett. 2015;589:718–725.10.1016/j.febslet.2015.01.039
- von Koskull-Döring P, Scharf KD, Nover L. The diversity of plant heat stress transcription factors. Trends Plant Sci. 2007;12:452–457.10.1016/j.tplants.2007.08.014
- Czarnecka-Verner E, Yuan CX, Scharf KD, et al. Plants contain a novel multi-member class of heat shock factors without transcriptional activator potential. Plant Mol. Biol. 2000;43:459–471.10.1023/A:1006448607740
- Kumar M, Busch W, Birke H, et al. Heat shock factors HsfB1 and HsfB2b are involved in the regulation of Pdf1.2 expression and pathogen resistance in Arabidopsis. Mol. Plant. 2009;2:152–165.10.1093/mp/ssn095
- Bharti K, Von Koskull-Döring P, Bharti S, et al. Tomato heat stress transcription factor HsfB1 represents a novel type of general transcription coactivator with a histone-like motif interacting with the plant CREB binding protein ortholog HAC1. Plant Cell. 2004;16:1521–1535.10.1105/tpc.019927
- Begum T, Reuter R, Schöffl F. Overexpression of AtHsfB4 induces specific effects on root development of Arabidopsis. Mech. Dev. 2013;130:54–60.10.1016/j.mod.2012.05.008
- Kolmos E, Chow BY, Pruneda-Paz JL, et al. HsfB2b-mediated repression of PRR7 directs abiotic stress responses of the circadian clock. Proc. Natl. Acad. Sci. USA. 2014;111:16172–16177.10.1073/pnas.1418483111
- Schmidt R, Schippers JH, Welker A, et al. Transcription factor OsHsfC1b regulates salt tolerance and development in Oryza sativa ssp. japonica. AoB Plants. 2012;2012:pls0110.
- Malik MK, Slovin JP, Hwang CH, et al. Modified expression of a carrot small heat shock protein gene, hsp17. 7, results in increased or decreased thermotolerancedouble dagger. Plant J. 1999;20:89–99.10.1046/j.1365-313X.1999.00581.x
- Sun W, Bernard C, van de Cotte B, et al. At-HSP17.6A, encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression. Plant J. 2001;27:407–415.10.1046/j.1365-313X.2001.01107.x
- Mu C, Zhang S, Yu G, et al. Overexpression of small heat shock protein LimHSP16.45 in Arabidopsis enhances tolerance to abiotic stresses. PLoS ONE. 2013;8:e82264.10.1371/journal.pone.0082264
- Ham DJ, Moon JC, Hwang SG, et al. Molecular characterization of two small heat shock protein genes in rice: their expression patterns, localizations, networks, and heterogeneous overexpressions. Mol. Biol. Rep. 2013;40:6709–6720.10.1007/s11033-013-2786-x
- Yamada K, Fukao Y, Hayashi M, et al. Cytosolic HSP90 regulates the heat shock response that is responsible for heat acclimation in Arabidopsis thaliana. J. Biol. Chem. 2007;282:37794–37804.10.1074/jbc.M707168200
- Nishizawa-Yokoi A, Tainaka H, Yoshida E, et al. The 26S proteasome function and Hsp90 activity involved in the regulation of HsfA2 expression in response to oxidative stress. Plant Cell Physiol. 2010;51:486–496.10.1093/pcp/pcq015
- Yabuta Y, Maruta T, Yoshimura K, et al. Two distinct redox signaling pathways for cytosolic APX induction under photooxidative stress. Plant Cell Physiol. 2004;45:1586–1594.10.1093/pcp/pch181
- Shigeoka S, Maruta T. Cellular redox regulation, signaling, and stress response in plants. Biosci. Biotechnol. Biochem. 2014;78:1457–1470.10.1080/09168451.2014.942254
- Volkov RA, Panchuk II, Mullineaux PM, et al. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 2006;61:733–746.10.1007/s11103-006-0045-4
- Fu S, Rogowsky P, Nover L, et al. The maize heat shock factor-binding protein paralogs EMP2 and HSBP2 interact non-redundantly with specific heat shock factors. Planta. 2006;224:42–52.10.1007/s00425-005-0191-y
- Hsu SF, Lai HC, Jinn TL. Cytosol-localized heat shock factor-binding protein, AtHSBP, functions as a negative regulator of heat shock response by translocation to the nucleus and is required for seed development in Arabidopsis. Plant Physiol. 2010;153:773–784.10.1104/pp.109.151225
- Liu HT, Gao F, Li GL, et al. The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction in Arabidopsis thaliana. Plant J. 2008;55:760–773.10.1111/tpj.2008.55.issue-5
- Cohen-Peer R, Schuster S, Meiri D, et al. Sumoylation of Arabidopsis heat shock factor A2 (HsfA2) modifies its activity during acquired thermotholerance. Plant Mol. Biol. 2010;74:33–45.10.1007/s11103-010-9652-1
- Jung HS, Crisp PA, Estavillo GM, et al. Subset of heat-shock transcription factors required for the early response of Arabidopsis to excess light. Proc. Natl. Acad. Sci. USA. 2013;110:14474–14479.10.1073/pnas.1311632110
- Liu Y, Zhang C, Chen J, et al. Arabidopsis heat shock factor HsfA1a directly senses heat stress, pH changes, and hydrogen peroxide via the engagement of redox state. Plant Physiol. Biochem. 2013;64:92–98.10.1016/j.plaphy.2012.12.013